By agreement with the publisher, this book is accessible by the search feature, but cannot be browsed.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Gilbert SF. Developmental Biology. 6th edition. Sunderland (MA): Sinauer Associates; 2000.

Developmental Biology. 6th edition.
Show detailsThe interaction of sperm and egg generally proceeds according to five basic steps (Figure 7.8; Vacquier 1998):

Figure 7.8
Summary of events leading to the fusion of egg and sperm plasma membranes in the sea urchin (A) and the mouse (B). (A) Sea urchin fertilization is external. (1) The sperm is activated by and chemotactically attracted to the egg. (2, 3) The egg jelly causes (more...)
- 1.
The chemoattraction of the sperm to the egg by soluble molecules secreted by the egg
- 2.
The exocytosis of the acrosomal vesicle to release its enzymes
- 3.
The binding of the sperm to the extracellular envelope (vitelline layer or zona pellucida) of the egg
- 4.
The passing of the sperm through this extracellular envelope
- 5.
Fusion of egg and sperm cell plasma membranes
Sometimes steps 2 and 3 are reversed (as in mammalian fertilization) and the sperm binds to the egg before releasing the contents of the acrosome. After these five steps are accomplished, the haploid sperm and egg nuclei can meet, and the reactions that initiate development can begin.
In many species, the meeting of sperm and egg is not a simple matter. Many marine organisms release their gametes into the environment. That environment may be as small as a tide pool or as large as an ocean. Moreover, it is shared with other species that may shed their sex cells at the same time. These organisms are faced with two problems: How can sperm and eggs meet in such a dilute concentration, and how can sperm be prevented from trying to fertilize eggs of another species? Two major mechanisms have evolved to solve these problems: species-specific attraction of sperm and species-specific sperm activation.
Sperm attraction: Action at a distance
Species-specific sperm attraction has been documented in numerous species, including cnidarians, molluscs, echinoderms, and urochordates (Miller 1985; Yoshida et al. 1993). In many species, sperm are attracted toward eggs of their species by chemotaxis, that is, by following a gradient of a chemical secreted by the egg. In 1978, Miller demonstrated that the eggs of the cnidarian Orthopyxis caliculata not only secrete a chemotactic factor but also regulate the timing of its release. Developing oocytes at various stages in their maturation were fixed on microscope slides, and sperm were released at a certain distance from the eggs. Miller found that when sperm were added to oocytes that had not yet completed their second meiotic division, there was no attraction of sperm to eggs. However, after the second meiotic division was finished and the eggs were ready to be fertilized, the sperm migrated toward them. Thus, these oocytes control not only the type of sperm they attract, but also the time at which they attract them.
The mechanisms of chemotaxis differamong species (see Metz 1978; Ward and Kopf 1993). One chemotactic molecule, a 14-amino acid peptide called resact, has been isolated from the egg jelly of the sea urchin Arbacia punctulata (Ward et al. 1985). Resact diffuses readily in seawater and has a profound effect at very low concentrations when added to a suspension of Arbacia sperm (Figure 7.9). When a drop of seawater containing Arbacia sperm is placed on a microscope slide, the sperm generally swim in circles about 50 μm in diameter. Within seconds after a minute amount of resact is injected into the drop, sperm migrate into the region of the injection and congregate there. As resact continues to diffuse from the area of injection, more sperm are recruited into the growing cluster. Resact is specific for A. punctulata and does not attract sperm of other species. A. punctulata sperm have receptors in their plasma membranes that bind resact (Ramarao and Garbers 1985; Bentley et al. 1986) and can swim up a concentration gradient of this compound until they reach the egg.

Figure 7.9
Sperm chemotaxis in Arbacia. One nanoliter of a 10-nM solution of resact is injected into a 20-μl drop of sperm suspension. The position of the micropipette is indicated in (A). (A) A 1-second photographic exposure showing sperm swimming in tight (more...)
Resact also acts as a sperm-activating peptide. Sperm-activating peptides cause dramatic and immediate increases in mitochondrial respiration and sperm motility (Tombes and Shapiro 1985; Hardy et al. 1994). The sperm receptor for resact is a transmembrane protein, and when it binds resact on the extracellular side, a conformational change on the cytoplasmic side activates the receptor’s enzymatic activity. This activates the mitochondrial ATP-generating apparatus as well as the dynein ATPase that stimulates flagellar movement in the sperm (Shimomura et al. 1986; Cook and Babcock 1993).
The acrosomal reaction in sea urchins
A second interaction between sperm and egg is the acrosomal reaction. In most marine invertebrates, the acrosomal reaction has two components: the fusion of the acrosomal vesicle with the sperm plasma membrane (an exocytosis that results in the release of the contents of the acrosomal vesicle) and the extension of the acrosomal process (Colwin and Colwin 1963). The acrosomal reaction in sea urchins is initiated by contact of the sperm with the egg jelly. Contact with egg jelly causes the exocytosis of the sperm’s acrosomal vesicle and the release of proteolytic enzymes that can digest a path through the jelly coat to the egg surface (Dan 1967; Franklin 1970; Levine et al. 1978). The sequence of these events is outlined in Figure 7.10.

Figure 7.10
Acrosomal reaction in sea urchin sperm. (A–C) The portion of the acrosomal membrane lying directly beneath the sperm plasma membrane fuses with the plasma membrane to release the contents of the acrosomal vesicle. (D) The actin molecules assemble (more...)
In sea urchins, the acrosomal reaction is thought to be initiated by a fucose-containing polysaccharide in the egg jelly that binds to the sperm and allows calcium to enter into the sperm head (Schackmann and Shapiro 1981; Alves et al. 1997; Vacquier and Moy 1997). The exocytosis of the acrosomal vesicle is caused by the calcium-mediated fusion of the acrosomal membrane with the adjacent sperm plasma membrane (Figures 7.10 and 7.11). The egg jelly factors that initiate the acrosomal reaction in sea urchins are often highly specific to each species* (Summers and Hylander 1975).

Figure 7.11
Acrosomal reaction in hamster sperm. (A) Transmission electron micrograph of hamster sperm undergoing the acrosomal reaction. The acrosomal membrane can be seen to form vesicles. (B) Interpre- tive diagram of electron micrographs showing the fusion of (more...)
The second part of the acrosomal reaction involves the extension of the acrosomal process (see Figure 7.10). This protrusion arises through the polymerization of globular actin molecules into actin filaments (Tilney et al. 1978).
Species-specific recognition in sea urchins
Once the sea urchin sperm has penetrated the egg jelly, the acrosomal process of the sperm contacts the surface of the egg (Figure 7.14A). A major species-specific recognition step occurs at this point. The acrosomal protein mediating this recognition is called bindin. In 1977, Vacquier and co-workers isolated this nonsoluble 30,500-Da protein from the acrosome of Strongylocentrotus purpuratus and found it to be capable of binding to dejellied eggs of the same species (Figure 7.14B; Vacquier and Moy 1977). Further, its interaction with eggs is relatively species-specific (Glabe and Vacquier 1977; Glabe and Lennarz 1979): bindin isolated from the acrosomes of S. purpuratus binds to its own dejellied eggs, but not to those of Arbacia punctulata. Using immunological techniques, Moy and Vacquier (1979) demonstrated that bindin is located specifically on the acrosomal process—exactly where it should be for sperm-egg recognition (Figure 7.15).

Figure 7.14
Species-specific binding of acrosomal process to egg cell surface in sea urchins. (A) Actual contact of a sea urchin sperm acrosomal process with an egg microvillus. (B) In vitro model of species-specific binding. The agglutination of dejellied eggs by (more...)

Figure 7.15
Localization of bindin on the acrosomal process. (A) Immunochemical technique used to localize bindin. Rabbit antibody was made to the bindin protein, and this antibody was incubated with sperm that had undergone the acrosomal reaction. If bindin was (more...)
Biochemical studies have shown that the bindins of closely related sea urchin species are indeed different.† This finding implies the existence of species-specific bindin receptors on the egg, vitelline envelope, or plasma membrane. Such receptors were also suggested by the experiments of Vacquier and Payne (1973), who saturated sea urchin eggs with sperm. As seen in Figure 7.16A, sperm binding does not occur over the entire egg surface. Even at saturating numbers of sperm (approximately 1500), there appears to be room on the ovum for more sperm heads, implying a limiting number of sperm-binding sites. The bindin receptor on the egg has recently been isolated (Giusti et al. 1997; Stears and Lennarz 1997). This 350-kDa protein may have several regions that interact with bindin. At least one of these sites recognizes only the bindin of the same species. The other site or sites appear to recognize a general bindin structure and can recognize the bindin of many species. The bindin receptors are thought to be aggregated into complexes on the egg cell surface, and hundreds of these complexes may be needed to tether the sperm to the egg (Figure 7.16B). Thus, species-specific recognition of sea urchin gametes occurs at the levels of sperm attraction, sperm activation, and sperm adhesion to the egg surface.
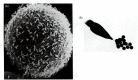
Figure 7.16
Bindin receptors on the egg. (A) Scanning electron micrograph of sea urchin sperm bound to the vitelline envelope of an egg. Although this egg is saturated with sperm, there appears to be room on the surface for more sperm, implying the existence of a (more...)
WEBSITE
7.5 The Lillie-Loeb dispute. In the early 1900s, fertilization research was framed by a dispute between F. R. Lillie and Jacques Loeb, who disagreed over whether the sperm recognized the egg through soluble factors or through cell-cell interactions. http://www.devbio.com/chap07/link0705.shtml
Gamete binding and recognition in mammals
ZP3: the sperm-binding protein of the mouse zona pellucida
The zona pellucida in mammals plays a role analogous to that of the vitelline envelope in invertebrates. This glycoprotein matrix, which is synthesized and secreted by the growing oocyte, plays two major roles during fertilization: it binds the sperm, and it initiates the acrosomal reaction after the sperm is bound (Saling et al. 1979; Florman and Storey 1982; Cherr et al. 1986). The binding of sperm to the zona is relatively, but not absolutely, species-specific. (Species-specific gamete recognition is not a major problem when fertilization occurs internally.)
The binding of mouse sperm to the mouse zona pellucida can be inhibited by first incubating the sperm with zona glycoproteins. Bleil and Wassarman (1980, 1986 1988) isolated an 83-kDa glycoprotein, ZP3, from the mouse zona that was the active competitor for binding in this inhibition assay. The other two zona glycoproteins they found, ZP1 and ZP2, failed to compete for sperm binding (Figure 7.17). Moreover, they found that radiolabeled ZP3 bound to the heads of mouse sperm with intact acrosomes. Thus, ZP3 is the specific glycoprotein in the mouse zona pellucida to which the sperm bind. ZP3 also initiates the acrosomal reaction after sperm have bound to it. The mouse sperm can thereby concentrate its proteolytic enzymes directly at the point of attachment at the zona pellucida.

Figure 7.17
Mouse ZP3 as the zona protein that binds sperm. (A) Diagram of the fibrillar structure of the mouse zona pellucida. The major strands of the zona are composed of repeating dimers of proteins ZP2 and ZP3. These strands are occasionally crosslinked together (more...)
The molecular mechanism by which the zona pellucida and the mammalian sperm recognize each other is presently being studied. The current hypothesis of mammalian gamete binding postulates a set of proteins on the sperm capable of recognizing specific carbohydrate regions of ZP3 (Figure 7.18A; Florman et al. 1984; Florman and Wassarman 1985; Wassarman 1987; Saling 1989). Removal of these threonine- or serine-linked carbohydrate groups from ZP3 abolishes its ability to bind sperm. Several proteins have been identified on the sperm cell surface that specifically bind to the ZP3 carbohydrates. Moreover, the deletion of these proteins from the sperm can inhibit or eliminate sperm-zona binding (see Kopf 1998).

Figure 7.18
Sperm ZP3-binding proteins at the zona pellucida. (A) ZP3-binding proteins on the mouse sperm are located in the plasma membrane, overlying the acrosome. In this confocal image, a ZP3-binding protein is stained red by antibody immunofluorescence. (B) (more...)
WEBSITE
7.6 Zona-binding proteins. There are numerous proteins on the sperm that bind to ZP3 on the zona pellucida. These proteins are important in mediating the activation signal to the sperm and for initiating the acrosomal reaction. http://www.devbio.com/chap07/link0706.shtml
Induction of the mammalian acrosomal reaction by ZP3
Unlike the sea urchin acrosomal reaction, the acrosomal reaction in mammals occurs only after the sperm has bound to the zona pellucida (Figure 7.8). The mouse sperm acrosomal reaction is induced by the crosslinking of ZP3 with the receptors for it on the sperm membrane (Endo et al. 1987; Leyton and Saling 1989). This crosslinking opens calcium channels to increase the concentration of calcium in the sperm (Leyton and Saling 1992; Florman et al. 1998). The mechanism by which ZP3 induces the opening of the calcium channels and the subsequent exocytosis of the acrosome remains controversial, but it may involve the receptor’s activating a cation channel (for sodium, potassium, or calcium), which would change the resting potential of the sperm plasma membrane. The calcium channels in the membrane would be sensitive to this change in membrane potential, allowing calcium to enter the sperm.
The difference between the acrosomal reaction in sea urchins and mammals may be due to the thickness of the extracellular envelopes surrounding the egg. In the sea urchin, the vitelline envelope is very thin and porous. Once a sperm has bound there, it is very close to the egg plasma membrane, and, indeed, the bindin receptor may extend through the vitelline envelope. In mammals, however, the zona pellucida is a very thick matrix, so the sperm is far removed from the egg. By undergoing the acrosomal reaction directly on the zona, the sperm is able to concentrate its proteolytic enzymes to lyse a hole in this envelope. Indeed, sperm that undergo the acrosomal reaction before they reach the zona pellucida are unable to penetrate it (Florman et al. 1998).
Secondary binding of sperm to the zona pellucida
During the acrosomal reaction, the anterior portion of the sperm plasma membrane is shed from the sperm (see Figure 7.11). This region is where the ZP3-binding proteins are located, and yet the sperm must still remain bound to the zona in order to lyse a path through it. In mice, it appears that secondary binding to the zona is accomplished by proteins in the inner acrosomal membrane that bind specifically to ZP2 (Bleil et al. 1988). Whereas acrosome-intact sperm will not bind to ZP2, acrosome-reacted sperm will. Moreover, antibodies against the ZP2 glycoprotein will not prevent the binding of acrosome-intact sperm to the zona, but will inhibit the attachment of acrosome-reacted sperm. The structure of the zona consists of repeating units of ZP3 and ZP2, occasionally crosslinked by ZP1 (Figure 7.18). It appears that the acrosome-reacted sperm transfer their binding from ZP3 to the adjacent ZP2 molecules. After a mouse sperm has entered the egg, the egg cortical granules release their contents. One of the proteins released by these granules is a protease that specifically alters ZP2 (Moller and Wassarman 1989). This inhibits other acrosome-reacted sperm from moving closer toward the egg.
In guinea pigs, secondary binding to the zona is thought to be mediated by the protein PH-20. Moreover, when this inner acrosomal membrane protein was injected into adult male or female guinea pigs, 100% of them became sterile for several months (Primakoff et al. 1988). The blood sera of these sterile guinea pigs had extremely high concentrations of antibodies to PH-20. The antiserum from guinea pigs sterilized in this manner not only bound specifically to PH-20, but also blocked sperm-zona adhesion in vitro. The contraceptive effect lasted several months, after which fertility was restored. These experiments show that the principle of immunological contraception is well founded.
Box
Action at a Distance: Mammalian Gametes.
Footnotes
- *
Such exocytotic reactions are seen in the release of insulin from pancreatic cells and in the release of neurotransmitters from synaptic terminals. In all cases, there is a calcium-mediated fusion between the secretory vesicle and the cell membrane. Indeed, the similarity of acrosomal vesicle exocytosis and synaptic vesicle exocytosis may actually be quite deep. Studies of acrosomal reactions in sea urchins and mammals (Florman et al. 1992; González-Martínez et al. 1992) suggest that when the receptors for the sperm-activating ligands bind these molecules, they cause a depolarization of the membrane that would open voltage-dependent calcium ion channels in a manner reminiscent of synaptic transmission. The proteins that dock the cortical granules of the egg to the plasma membrane also appear to be homologous to those used in the axon tip (Bi et al. 1995).
- †
Bindin is probably the fastest evolving protein known. Closely related species may have near-identity of every other protein, but their bindins may have diverged significantly. For more information on bindin evolution, see Chapter 22.
- Recognition of Egg and Sperm - Developmental BiologyRecognition of Egg and Sperm - Developmental Biology
Your browsing activity is empty.
Activity recording is turned off.
See more...