By agreement with the publisher, this book is accessible by the search feature, but cannot be browsed.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Alberts B, Bray D, Lewis J, et al. Molecular Biology of the Cell. 3rd edition. New York: Garland Science; 1994.

Molecular Biology of the Cell. 3rd edition.
Show detailsIntroduction
Actin is involved in a remarkably wide range of structures, from stiff and relatively permanent extensions of the cell surface to the dynamic three-dimensional networks at the leading edge of a migrating cell. Very different structures based on actin coexist in every living cell. In every case the fundamental structure of the actin filament is the same. It is the length of these filaments, their stability, and the number and geometry of their attachments (both to one another and to other components of the cell) that varies in different cytoskeletal assemblies. These properties in turn depend on a large retinue of actin-binding proteins, which bind to actin filaments and modulate their properties and functions.
In this section we describe some of the most important actin-binding proteins and the structures they form. Many of these are found at the perimeter of the cell in the actin-rich layer just beneath the plasma membrane called the cell cortex. This layer gives an animal cell mechanical strength and enables it to perform a variety of surface movements, such as phagocytosis, cytokinesis (cell division), and cell locomotion.
A Simple Membrane-attached Cytoskeleton Provides Mechanical Support to the Plasma Membrane of Erythrocytes 44
As noted in Chapter 10, the proteins spectrin and ankyrin were first discovered as prominent components of the membrane-associated cytoskeleton of mammalian red blood cells (erythrocytes). These unusual cells have lost their nucleus and internal membranes, and so the plasma membrane is the only membrane. It is supported by a two-dimensional network of spectrin tetramers that are connected at their ends by very short actin filaments. The spectrin is linked to the cytoplasmic tail of an abundant transmembrane carrier protein (band 3) by means of ankyrin bridges (see Figure 10-26). Close relatives of spectrin (also called fodrin) and of ankyrin are found in the cortex of many vertebrate cells. Thus the detailed arrangement of proteins in the erythrocyte cortex provides a simplified model for the actin-based cytoskeletal network that supports the plasma membrane in all other animal cells.
The actin filaments in the erythrocyte cortex are very short, acting only as cross-linking elements between spectrin tetramers. Those in a more typical cell cortex, by contrast, are much longer and thus project into the cytoplasm, where they form the basis of a three-dimensional actin filament network. It is uncertain whether ankyrinlike molecules anchor these more typical cortical arrays to the plasma membrane, although in some epithelial cells the transmembrane Na+/K+ ATPase (discussed in Chapter 11) is thought to link the plasma membrane to the cortical actin filament network through such molecules.
The cortical actin filament network generally determines the shape and mechanical properties of the plasma membrane. Many types of membrane attachments are needed for actin filaments to perform their various functions in the cortex; coupling to transmembrane proteins through ankyrin is only one. More dynamic attachments also exist, but the proteins that mediate them are just beginning to be characterized.
Cross-linking Proteins with Different Properties Organize Particular Actin Assemblies 43
The cortical actin filaments in animal cells are organized into three general types of arrays ( Figure 16-65). In parallel bundles, as found in microspikes and filopodia, the filaments are oriented with the same polarity and are often closely spaced (10-20 nm apart). In contractile bundles, as found in stress fibers and in the contractile ring that divides cells in two during mitosis, filaments are arranged with opposite polarities; they are more loosely spaced (30-60 nm apart) and contain the motor protein myosin-II (discussed later). In the gel-like networks of the cell cortex the filaments are arranged in a relatively loose, open array with many orthogonal interconnections. How are these different arrangements of the same actin filament generated and maintained within a single cell? While we do not know the complete answer, actin filament cross-linking proteins are clearly of central importance.

Figure 16-65
Three types of cortical arrays of actin filaments. A crawling cell is shown with three areas enlarged to show the arrangement of actin filaments drawn to scale. Arrowheads point toward the plus end of the filaments.
Actin filament cross-linking proteins can be divided into two classes - bundling proteins and gel-forming proteins - according to their effect on pure actin filaments in vitro. Bundling proteins cross-link actin filaments into a parallel array and are important for forming both the tight parallel arrays and the looser contractile bundles of actin filaments described above. Gel-forming proteins, by contrast, cross-link actin filaments at crosswise intersections, creating loose gels.
Fimbrin and α-actinin are widely distributed bundling proteins. Fimbrin is enriched in the parallel filament bundles at the leading edge of cells, particularly in microspikes and filopodia, and it is thought to be responsible for the tight association of actin filaments in these arrays. The second actin-bundling protein, α-actinin, is concentrated in stress fibers, where it is thought to be partly responsible for the relatively loose cross-linking of actin filaments in these contractile bundles; it also helps to form the anchorage for the ends of stress fibers where they terminate on the plasma membrane at focal contacts. As explained later, myosin is the motor protein in stress fibers and other contractile arrays that is responsible for their contractility. It seems likely that the very close packing of actin filaments caused by fimbrin excludes myosin, whereas the looser packing caused by α-actinin allows myosin molecules to enter; likewise, the very different spacing causes each of the two bundling proteins to exclude the other ( Figure 16-66).

Figure 16-66
The formation of two types of actin filament bundles. (A) α-actinin, which is a homodimer, cross-links actin filaments into loose bundles, which allow the motor protein myosin-II (not shown) to participate in the assembly. Fimbrin cross-links (more...)
Filamin is a widely distributed gel-forming protein. Although it is not present in stress fibers or the leading edge, it is enriched elsewhere in the cortex. Filamin is a homodimer that promotes the formation of a loose and highly viscous network by clamping together two actin filaments that cross each other ( Figure16-67). It is an abundant protein in many animal cells, reflecting the prevalence of the loose-network type of actin organization.
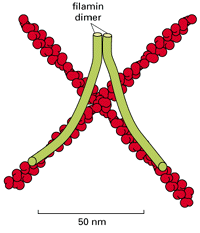
Figure 16-67
Filamin cross-links actin filaments into a three-dimensional network with the physical properties of a gel. Each filamin homo-dimer is about 160 nm long when fully extended and forms a flexible, high-angle link between two adjacent actin filaments. Filamin (more...)
Actin-binding Proteins with Different Properties Are Built Up from Similar Modules 45
Fimbrin, α-actinin, filamin, and spectrin each contain two actin-filament-binding domains, which is not surprising given that each needs to cross-link two filaments. Unexpectedly, however, in all of these proteins the actin-binding domains have a similar structure. The length and flexibility of the spacer sequences that separate the two actin-binding sites differ in the four proteins, and these differences determine the different properties of the four cross-linkers. Evidently, these proteins have diverged from a common ancestral actin-binding protein by adding different spacer sequences ( Figure 16-68).

Figure 16-68
The modular structures of four actin-binding proteins. Each of the proteins shown has two actin-binding sites ( red) that are related in sequence. Fimbrin has two directly adjacent actin-binding sites, so that it holds its two actin filaments very close (more...)
Gelsolin Fragments Actin Filaments in Response to Ca2+ Activation 46
Extracts prepared from many types of animal cells form a gel in the presence of ATP when they are warmed to 37°C. Although this gelation depends on both actin filaments and a cross-linking protein such as filamin, the gels exhibit more complex behavior than simple mixtures of actin filaments and filamin. If the Ca2+ concentration is raised above 10-7M, for example, the semisolid actin gel begins to liquefy - a process known as solation - and regions of the solating gel show vigorous local streaming when examined under a microscope. Clearly, there must be components besides actin and filamin in the extracts to account for this behavior. These components are likely to be involved in the cytoplasmic streaming observed in some large cells, where vigorous flowing movements are required to maintain an even distribution of metabolites and other cytoplasmic components. These movements seem to be associated with sudden local changes in the cytoplasm from a solid gel-like consistency to a more fluid state.
A number of proteins have been isolated from cell extracts that, when added to a gel formed from purified actin filaments and filamin, cause it to change to a more fluid state in the presence of Ca2+. The best characterized of these is gelsolin, which, when activated by the binding of Ca2+, severs an actin filament and forms a cap on the newly exposed plus end of the filament, thus breaking up the cross-linked network of actin filaments. Similar proteins are found in the cortex of many types of vertebrate cells; these severing proteins are activated by concentrations of Ca2+ (about 10-6 M) that occur only transiently in the cytosol.
One of the postulated functions of severing proteins is to help loosen or liquefy the cell cortex locally to allow membrane fusion events. When a phagocytic white blood cell engulfs a microorganism, for example, the resulting phagosome is initially coated on its cytoplasmic side with a thick network of actin filaments originating from the cortex. In order for this phagosome to fuse with lysosomes, these actin filaments must be depolymerized to allow intimate contact between the phagosome and lysosome membranes. This removal of actin can be prevented by artificially reducing the Ca2+ ion concentration, and it is thought that removal may depend on a local rise in Ca2+ through the action of gelsolin (or a similar protein). Gelsolin is also thought to be required for a cell to crawl along a substratum, although its exact role in this process is not clear.
While a mixture of purified actin filaments, filamin, and gelsolin is capable of undergoing Ca2+-dependent gel-to-sol transitions, it will not contract or show the streaming movements displayed by the cruder actin-rich gels obtained from cells. These activities require another type of actin-binding protein - the motor protein myosin. If myosin is selectively removed from the crude actin-rich gels, contractions and streaming no longer occur, suggesting that an interaction between actin and myosin generates the force for cytoplasmic streaming.
Multiple Types of Myosin Are Found in Eucaryotic Cells 47
Time-lapse cinematography reveals the cortex of cells to be continually moving. In the previous section we emphasized the importance of actin filament polymerization and depolymerization in these movements, but, as with microtubules, motor proteins are also important. All of the actin filament motor proteins identified to date belong to the myosin family. Myosins were originally isolated on the basis of their ability to hydrolyze ATP to ADP and Pi when stimulated by binding to actin filaments, and this remains a useful biochemical criterion for their identification. It is also possible to observe the motor activity of myosins directly by adsorbing them onto a glass coverslip: when fluorescent actin filaments are added together with ATP, the filaments can be observed with a fluorescence microscope to glide over the myosin-coated glass surface. Novel myosins have also been identified by DNA sequencing even before being characterized biochemically or functionally.
Myosin, along with actin, was first discovered in skeletal muscle, and much of what we know about the interaction of these two proteins was learned there. Muscle myosin belongs to the myosin-II subfamily of myosins, all of which have two heads and a long, rodlike tail: each head has both ATPase and motor activity. A myosin-II protein is composed of two identical heavy chains, each of which is complexed to a pair of light chains. The amino-terminal portion of the heavy chain forms the motor-domain head, while the carboxyl-terminal half of the heavy chain forms an extended α helix. Two heavy chains associate by twisting their α-helical tail domains together into a coiled-coil to form a stable dimer that has two heads and a single rodlike tail ( Figure16-69).
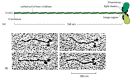
Figure 16-69
Myosin-II. (A) A myosin-II molecule is composed of two heavy chains (each about 2000 amino acids long) and four light chains. The light chains are of two types (one containing about 190 and the other about 170 amino acids), and one molecule of each type (more...)
A major role of the rodlike tail of myosin-II is to allow the molecules to polymerize into bipolar filaments. This polymerization is crucial for the function of myosin-II, which is to move groups of oppositely oriented actin filaments past each other, as seen most clearly in muscle contraction. Myosin-II is relatively abundant in the cell cortex; in fibroblasts, for example, there is roughly one myosin-II molecule per 100 actin molecules. Myosin-II filaments in the contractile ring are responsible for driving membrane furrowing during cell division, as discussed in Chapter 18, and they are thought to generate tension in stress fibers as well as much of the cortical tension that keeps the cell surface taut. Their role in muscle contraction is described at the end of the chapter.
In addition to myosin-II, which is generally the most abundant myosin in the cell, nonmuscle cells contain various smaller myosins, the best characterized of which is called myosin-I ( Figure 16-70). Myosin-I is thought to be more like the original, more primitive myosin from which myosin-II evolved. A single cell can contain multiple smaller myosins, each encoded by a different gene and performing a distinct function; the cellular slime mold Dictyostelium, for example, has at least nine. The common feature of all myosins is a conserved motor domain (motor head); the other domains vary from myosin to myosin and determine the specific role of the molecule in the cell. Thus myosin tails may have a membrane-binding site and/or a site that binds to a second actin filament independently of the head domain. Depending on its tail, a myosin molecule can move a vesicle along an actin filament, attach an actin filament to the plasma membrane, or cause two actin filaments to align closely and then slide past each other ( Figure 16-71).

Figure 16-70
Two myosin family members. On the left, myosin-I and myosin-II are drawn to scale and aligned with respect to their conserved ATP-binding and actin-binding sites. The relative shapes of the folded proteins are shown on the right.

Figure 16-71
Possible roles of myosin-I and myosin-II in a typical eucaryotic cell. The short tail of a myosin-I molecule contains sites that bind either to other actin filaments or to membranes. This allows the head domain to move one actin filament relative to another (more...)
All known myosins hydrolyze ATP to move along actin filaments from the minus end toward the plus end. Given the importance of oppositely directed motor proteins that move along microtubules (see Figure16-37), it would not be surprising to discover an additional class of motor proteins that move toward the minus end of an actin filament.
There Are Transient Musclelike Assemblies in Nonmuscle Cells 48
In higher eucaryotic cells, organized contractile bundles of actin filaments and myosin-II filaments often form transiently to perform a specific function and then disassemble. Most notably, cell division in animal cells is made possible by a beltlike bundle of actin filaments and myosin-II filaments known as the contractile ring. This ring appears beneath the plasma membrane during the M phase of the cell-division cycle; forces generated by it pull inward on the plasma membrane and thereby constrict the middle of the cell, leading to the eventual separation of the two daughter cells by a process known as cytokinesis ( Figure16-72). The contractile ring must be assembled from actin, myosin, and other proteins at the start of cell division, a process that can be monitored by staining dividing cells with fluorescent anti-myosin antibodies. In sea urchin eggs that are about to divide, for example, myosin-II molecules are at first distributed evenly beneath the plasma membrane and then move to the equatorial region as the contractile ring forms. Once cell division is complete, the myosin-II molecules disperse. It is not known how this process is controlled, but it seems likely that Ca2+ is involved, as Ca2+-dependent phosphorylation of myosin-II both increases its interaction with actin and promotes its assembly into short bipolar filaments ( Figure 16-73).

Figure 16-72
Musclelike contractile assemblies in nonmuscle cells. Each assembly contains myosin-II filaments in addition to actin filaments.

Figure 16-73
The controlled assembly of myosin-II into filaments. (A) The controlled phosphorylation of one of the two light chains has at least two effects in vitro:it causes a change in the conformation of the myosin head, exposing its actin-binding site, and it (more...)
Stress fibers, which are prominent components of the cytoskeleton of fibroblast cells in culture (see Figure 16-72), are a second example of a temporary contractile bundle of actin filaments and myosin-II. Although smaller and less highly organized, they resemble the tiny myofibrils in muscle (discussed later) in their structure and function. At one end they insert into the plasma membrane at special sites called focal contacts, where the external face of the cell is closely attached to the extracellular matrix ( Figure16-74); at the other end they insert into a second focal contact or into a meshwork of intermediate filaments that surrounds the cell nucleus. Stress fibers form in response to tension generated across a cell and are disassembled at mitosis when the cell rounds up and loses its attachments to the substratum. They also disappear rapidly if tension is released by suddenly detaching one end of the stress fiber from the focal contact by means of a laser beam. Stress fibers within fibroblasts in tissues are thought to allow the cells to exert tension on the matrix of collagen surrounding them - an essential process in both wound healing and morphogenesis (see Figure19-48). In epithelia, actin filament bundles spanning the cytoplasm from one cell-cell junction to another can appear and disappear in a similar way; such filament bundles, linked end to end via the cell-cell junctions, can form cables that transmit and generate tension along lines of particular stress in the multicellular sheet.
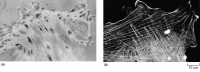
Figure 16-74
The relation between focal contacts and stress fibers in cultured fibroblasts. Focal contacts are best seen in living cells by reflection-interference microscopy (A). In this technique, light is reflected from the lower surface of a cell attached to a (more...)
Not all contractile assemblies of actin filaments and myosin in nonmuscle cells are transitory. Those associated with the intercellular anchoring junctions called adhesion belts, for example, are often more lasting. Adhesion belts (discussed in Chapter 19) are found near the apical surface of epithelial cells (see Figure 16-72). Among other functions, they are thought to play an important part in the folding of epithelial cell sheets during embryogenesis.
The mechanism of contraction of all of these cytoskeletal bundles is based on the ATP-driven sliding of interdigitated actin and myosin filaments, and it is thought to require a particular type of ordered assembly, which will be explained later when we discuss muscle.
Focal Contacts Allow Actin Filaments to Pull Against the Substratum 49
To pull on the extracellular matrix or on another cell, a stress fiber must be strongly anchored in the plasma membrane at the appropriate site. Attachments between actin filaments inside the cell and extracellular matrix on the outside of the cell are mediated by transmembrane linker glycoproteins in the plasma membrane. Those formed by cultured fibroblasts with the extracellular matrix are the best characterized. When fibroblasts grow on a culture dish, most of their cell surface is separated from the substratum by a gap of more than 50 nm; but at focal contacts ( adhesion plaques), this gap is reduced to 10 to 15 nm. Here the plasma membrane is attached to components of the extracellular matrix that have become adsorbed to the culture dish. Staining with anti-actin antibodies clearly shows these regions to be the sites where the ends of stress fibers attach to the plasma membrane (see Figure 16-74).
The main transmembrane linker proteins of focal contacts are members of the integrin family, whose external domain binds to an extracellular matrix component while the cytoplasmic domain is linked to actin filaments in stress fibers. The linkage is indirect and is mediated by multiple attachment proteins ( Figure 16-75). The cytoplasmic domain of the integrin binds to the protein talin, which in turn binds to vinculin, a protein found also in other actin-containing cell junctions, such as adherens junctions (discussed in Chapter 19). Vinculin associates with α-actinin and is thereby linked to an actin filament. Although the exact topology of protein interactions in the focal contact has not been established, a possible arrangement is shown in Figure 16-75B.

Figure 16-75
A model for how integrins in the plasma membrane connect intracellular actin filaments to the extracellular matrix at a focal contact. The formation of a focal contact occurs when the binding of matrix glycoproteins (such as fibronectin) on the outside (more...)
Besides their role as anchors for the cell, focal contacts can also relay signals from the extracellular matrix to the cytoskeleton. Several protein kinases, including the tyrosine kinase encoded by the src gene, are localized to focal contacts, and there are indications that their activity changes with the type of substratum on which the cell rests. These kinases can phosphorylate various target proteins, including components of the cytoskeleton, and hence regulate the survival, growth, morphology, movement, and differentiation of cells in response to the extracellular matrix in their environment.
Microvilli Illustrate How Bundles of Cross-linked Actin Filaments Can Stabilize Local Extensions of the Plasma Membrane 50
Microvilli are fingerlike extensions found on the surface of many animal cells. They are especially abundant on those epithelial cells that require a very large surface area to function efficiently. A single absorptive epithelial cell in the human small intestine, for example, has several thousand microvilli on its apical surface. Each is about 0.08 µm wide and 1 µm long, making the cell's absorptive surface area 20 times greater than it would be without them. The plasma membrane that covers these microvilli is highly specialized, bearing a thick extracellular coat of polysaccharide and digestive enzymes. The cytoskeleton of the microvillus has been studied in detail - a task that is made easier by its highly ordered structure, compared with the less specialized regions of cell cortex.
At the core of each intestinal microvillus is a rigid bundle of 20 to 30 parallel actin filaments that extend from the tip of the microvillus down into the cell cortex. The actin filaments in the bundle are all oriented with their plus ends pointing away from the cell body and are held together at regular intervals by actin-bundling proteins. Although fimbrin, the bundling protein in microspikes and filopodia, helps to bundle actin filaments into microvilli, the most important bundling protein is villin, which is found only in microvilli ( Figure 16-76). Villin, like fimbrin, cross-links actin filaments into tight parallel bundles, but it has a different actin-binding sequence. When villin is introduced into cultured fibroblasts, which do not normally contain villin and have only a few small microvilli, the existing microvilli become greatly elongated and stabilized, and new ones may also be induced, suggesting that villin is mainly responsible for the formation of the long microvilli in epithelial cells.

Figure 16-76
A microvillus. A bundle of parallel actin filaments held together by the actin-bundling proteins villin and fimbrin forms the core of a microvillus. Lateral arms (composed of myosin-I and the Ca2+-binding protein calmodulin) connect the sides of the actin (more...)
At the base of the microvillus the actin filament bundle is anchored into a specialized region of cortex at the apical end of the intestinal epithelial cell. This cortex, known as the terminal web, contains a dense network of spectrin molecules overlying a layer of intermediate filaments ( Figure16-77). The spectrin is thought to provide rigidity and stability to the cortex in this region, and the anchoring of the actin filaments to the terminal web is thought to stiffen the microvilli, keeping their actin bundles projecting outward at a right angle to the apical cell surface.

Figure 16-77
Freeze-etch electron micrograph of an intestinal epithelial cell, showing the terminal web beneath the apical plasma membrane. Bundles of actin filaments forming the core of microvilli extend into the terminal web, where they are linked together by a (more...)
The actin filament bundle is attached to the overlying plasma membrane of the microvillus by lateral bridges that can be seen in electron micrographs. The bridges are composed of a form of myosin-I that has several molecules of calmodulin (discussed in Chapter 15) bound to its tail region. The myosin is oriented with this tail region embedded in the membrane and its active ATP-binding head contacting the actin filaments. It is a mystery why a motor protein is used to link actin filaments to the membrane in microvilli. If the myosin-I in microvilli is motile, it should move toward the plus end of the actin filaments at the microvillus tip. This has lead to speculation that the myosin-I helps to pull the membrane up over the microvillus core, forming vesicles at its tip that are then released into the lumen of the intestine, where the digestive enzymes they carry continue their action.
The Behavior of the Cell Cortex Depends on a Balance of Cooperative and Competitive Interactions Among a Large Set of Actin-binding Proteins
The preceding examples show that the same actin filament can interact with different sets of actin-binding proteins at different locations in the cortex and that the actin-binding proteins can be segregated to different parts of the cell. What prevents the various sets of actin-binding proteins from mixing in the cytoplasm? It seems likely that both cooperative and competitive interactions among these proteins are important. One class of actin-binding proteins not yet discussed, for example, binds along the length of the actin filaments. The most widespread members of this class are the tropomyosins, which are rigid rod-shaped proteins named for similarities in their x-ray diffraction pattern to myosin-II. Like the tail of myosin-II, tropomyosin is a dimer of two identical α-helical chains that wind around each other in a coiled-coil. By binding along the length of an actin filament, the tropomyosin stabilizes and stiffens the filament. It also inhibits the binding of filamin to actin filaments, which probably explains why tropomyosin and filamin tend to be differentially distributed in cells. By contrast, tropomyosin binding to an actin filament increases the binding of myosin-II to the filament - an example of a cooperative interaction ( Figure16-78).

Figure 16-78
Some examples of competitive and cooperative interactions between actin-binding proteins. The arrowhead at the end of each actin filament indicates the minus end. Tropomyosin and filamin both bind strongly to actin filaments, but their binding is competitive. (more...)
We can now begin to see how stress fibers and cortical networks of actin filaments can coexist in a common cytoplasm. At one site in a cell - perhaps nucleated at a forming focal adhesion under the influence of activated Rho protein - tropomyosin, myosin-II, and α-actinin associate with actin filaments and exclude filamin; the contractile activity of myosin-II then promotes further organizational changes to produce a stress fiber. At another site in the cell tropomyosin-deficient actin filaments bind filamin, producing a loose network that provides few sites where α-actinin can bind to two filaments at once, so that it is excluded; bending of the filaments in the loose meshwork may also discourage tropomyosin-binding, since this molecule prefers a straight filament. While this picture is partly speculative, it illustrates the basic pathway by which a combination of cooperative and competitive interactions can give rise to spatially differentiated actin filament arrays in a common cytoplasm. It is not known how the postulated local differences that initiate the formation of these assemblages are established; nor is it known how many distinct types of actin filament arrays can coexist in the same cell - there are certainly more than the two we have just mentioned.
Some of the actin-binding proteins discussed in this section are summarized in Figure 16-79.

Figure 16-79
Some of the major classes of actin-binding proteins found in most vertebrate cells. Actin is shown in red,while the actin-binding proteins are shown in green. The molecular mass of each protein is given in kilodaltons (kD).
The Migration of Animal Cells Can Be Divided into Three Distinct Actin-dependent Subprocesses 51
The crawling movements of animal cells are among the most difficult to explain at the molecular level. Different parts of the cell change at the same time, and there is not a single, easily identifiable locomotory organelle (analogous to a flagellum, for example). Although actin forms the basis of animal cell migration, it undergoes many different transformations as the cell moves forward, assembling into lamellipodia and microspikes, associating with focal contacts, forming stress fibers, and so on. A complete account would have to give a molecular explanation for these transformations, explain how they are coordinated in time and space, and also account for important biophysical parameters such as the development of tension in the cortex and the formation of strong adhesions between the cell and its substratum.
In broad terms, three distinct processes can be identified in the crawling movements of animal cells: protrusion, in which lamellipodia and microspikes (or filopodia) are extended from the front of the cell; attachment, where the actin cytoskeleton makes a connection with the substratum; and traction, where the body of the cell moves forward.
Protrusion is a function of the leading edge of the cell. Actin-rich lamellipodia and microspikes (or filopodia) extend forward over the substratum, a process that is accompanied by actin polymerization, as described previously. It seems likely that the protrusion is driven by actin polymerization at the leading edge (see Figure 16-58), although this is still debated. Myosin-I motors attached to the plasma membrane could also drive the cell forward by actively walking along actin filaments. Yet another possibility, which has been suggested to apply in particular to the locomotion of giant amoebae, is that protrusions are squeezed out of the front of the cell by hydrostatic pressure generated by the contraction of the cortex elsewhere in the cell.
The attachment of cortical actin filaments to the substratum was discussed earlier when we described focal contacts, although these are specialized attachment structures present in fibroblasts in culture and associated with the ends of stress fibers. Rapidly motile cells - such as Dictyostelium amoebae and white blood cells - make more diffuse contacts with the substratum. It is thought, however, that similar principles apply to these contacts: transmembrane receptors for extracellular matrix proteins link the plasma membrane to the substratum, and actin filaments in the cytoplasm interact with the cytoplasmic domains of these receptors through actin-binding proteins. The details of these important interactions are uncertain, but it is clear that the cell contacts with the substratum must be continually made and broken as the cell moves forward.
Traction is perhaps the most mysterious part of cell locomotion. In many cases it is thought that the force for cell locomotion is generated near the front of the cell and that the nucleus and bulk cytoplasm are dragged forward passively. The force generation can be viewed in different ways. The leading part of the cell might actively contract like a muscle fiber and thus pull on the back of the cell. In another view polymerization of actin filaments at the front of the cell extends the actin cortex forward, and the rear of the cell is then carried forward by the contractile force of the resulting cortical tension ( Figure16-80).

Figure 16-80
One model of how forces generated in the actin-rich cortex might move a cell forward. The actin-dependent extension and firm attachment of a lamellipodium at the leading edge stretches the actin cortex. The cortical tension then draws the body of the (more...)
The Mechanism of Cell Locomotion Can Be Dissected Genetically 52
One of the most powerful ways to analyze the mechanism of a complex cellular process is to examine the effect of mutations that result in the deletion, overexpression, or modification of specific proteins. In the case of eucaryotic cell locomotion, the amoeboid cells of the slime mold Dictyostelium are particularly suitable for genetic analysis. These cells have a shape and a manner of moving that closely resemble those of the cells of higher organisms. But because they are haploid, they are readily manipulated by reverse genetic methods. Thus it has been possible to delete a number of actin-binding proteins from these cells and examine the consequences for cell locomotion.
The role of myosin-II, for example, has been tested genetically by two methods. In one, a defective form of the myosin-II gene is substituted for the existing gene by homologous recombination (see Figure7-47), leading to a mutant strain with no myosin-II. The second strategy is to use anti-sense RNA (see Figure7-43) to inactivate myosin-II mRNA, which has essentially the same effect. In a normal crawling Dictyostelium myosin-II is concentrated near the rear of the cell and myosin-I is concentrated in the leading edge ( Figure16-81). In the mutants the myosin-I is unchanged but myosin-II is gone.

Figure 16-81
The locations of myosin-I and myosin-II in a normal crawling Dictyostelium amoeba. The two forms of myosin were stained with specific antibodies, each coupled to a different fluorescent dye, and examined in a fluorescence microscope. Myosin-II ( orange (more...)
Remarkably, Dictyosteliumcells without myosin-II can still move over the substratum and respond chemotactically to a source of cyclic AMP, although both processes are somewhat impaired. Thus myosin-II is not absolutely essential for cell locomotion. Although protrusive activity at the leading edge of such mutant cells is quite normal, movement of the cell body forward is somewhat impaired, suggesting that myosin-II plays a role in generating traction. Nevertheless, myosin-I and/or actin polymerization must be able to drive the cell forward at a reasonable rate without the help of myosin-II.
Not surprisingly, the mutant cells are unable to form a contractile ring following mitosis and therefore develop into multinucleated giant cells. These cells eventually divide by using cell locomotion to tear themselves in two. It is interesting to speculate that such locomotion-dependent cytokinesis may represent a primitive cell division mechanism and that myosin-II might have evolved from myosin-I through natural selection for a more efficient cytokinetic apparatus.
Summary
The varied forms and functions of actin in eucaryotic cells depend on a versatile repertoire of actin-binding proteins that cross-link actin filaments into loose gels, bind them into stiff bundles, attach them to the plasma membrane, or forcibly move them relative to one another. Tropomyosin, for example, binds along the length of actin filaments, making them more rigid and altering their affinity for other proteins. Filamin cross-links actin filaments into a loose gel. Fimbrin and a-actinin form bundles of parallel actin filaments. Gelsolin mediates Ca2+-dependent fragmentation of actin filaments, thereby causing a rapid solation of actin gels. Various forms of myosin use the energy of ATP hydrolysis to move along actin filaments, either carrying membrane-bounded organelles from one location in the cell to another or moving adjacent actin filaments against each other. Sets of actin-binding proteins are thought to act cooperatively in generating the movements of the cell surface, including cytokinesis, phagocytosis, and cell locomotion. These movements are difficult to analyze because of the many components involved, but genetic approaches, in which genes encoding specific actin-binding proteins are mutated, can show the function of individual proteins in each process.
- Introduction
- A Simple Membrane-attached Cytoskeleton Provides Mechanical Support to the Plasma Membrane of Erythrocytes
- Cross-linking Proteins with Different Properties Organize Particular Actin Assemblies
- Actin-binding Proteins with Different Properties Are Built Up from Similar Modules
- Gelsolin Fragments Actin Filaments in Response to Ca2+ Activation
- Multiple Types of Myosin Are Found in Eucaryotic Cells
- There Are Transient Musclelike Assemblies in Nonmuscle Cells
- Focal Contacts Allow Actin Filaments to Pull Against the Substratum
- Microvilli Illustrate How Bundles of Cross-linked Actin Filaments Can Stabilize Local Extensions of the Plasma Membrane
- The Behavior of the Cell Cortex Depends on a Balance of Cooperative and Competitive Interactions Among a Large Set of Actin-binding Proteins
- The Migration of Animal Cells Can Be Divided into Three Distinct Actin-dependent Subprocesses
- The Mechanism of Cell Locomotion Can Be Dissected Genetically
- Summary
- Actin-binding Proteins - Molecular Biology of the CellActin-binding Proteins - Molecular Biology of the Cell
Your browsing activity is empty.
Activity recording is turned off.
See more...
