All rights reserved. No part of this book may be reprinted or reproduced or utilised in any form or by any electronic, mechanical, or other means, now known or hereafter invented, including photocopying and recording, or in any information storage or retrieval system, without permission in writing from the publishers. Enquiries in this regard should be directed to the British Psychological Society.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Collaborating Centre for Mental Health (UK). Generalised Anxiety Disorder in Adults: Management in Primary, Secondary and Community Care. Leicester (UK): British Psychological Society; 2011. (NICE Clinical Guidelines, No. 113.)
July 2019: Because of a risk of abuse and dependence, pregabalin is controlled under the Misuse of Drugs Act 1971 as a class C substance and scheduled under the Misuse of Drugs Regulations 2001 as schedule 3 (as of 1 April 2019). A footnote has been added by NICE to this guideline to reflect this change. Minor updates since publication July 2019: Recommendation 1.4.39 was amended to remove structured problem solving as a care and management option as this had originally been included in error. June 2018: Recommendation 1.2.13 was amended with advice on CBT. Recommendation 1.2.26 was updated with a link to the newest evidence on use of antipsychotics for treatment of GAD. Recommendation 1.4.7 was added to link to existing guidance on identifying treatment options for common mental health problems. Recommendation 1.4.10 on low-intensity interventions for mild to moderate panic disorder and 1.4.13 on treatment for moderate to severe panic disorder were added from the NICE guideline on common mental health disorders. Section 1.4 on stepped care for people with panic disorder was reordered.

Generalised Anxiety Disorder in Adults: Management in Primary, Secondary and Community Care.
Show details8.1. INTRODUCTION
The use of pharmacological interventions to manage anxiety is a far from recent phenomenon; for example, the consumption of alcohol and opiates for this purpose dates back centuries. In the 19th and early 20th century, medicines containing bromides were often prescribed by clinicians to treat what would then have been called ‘anxiety neurosis’ (Schwartz et al., 2005). The mid-20th century saw the introduction of barbiturates followed by the benzodiazepines, which were widely used for the medical treatment of anxiety between the 1960s and the 1980s. Towards the end of this period the limitations of benzodiazepines in terms of tolerance and dependence became apparent and at the same time the therapeutic benefits of anti-depressants in treating various kinds of anxiety disorders were more widely recognised (Davidson et al., 2010b).
Antidepressants, particularly SSRIs, are now commonly used in the management of anxiety disorders, including GAD. A number of other agents are also licensed for the treatment of GAD, some of which have a long history of use in this area, for example hydroxyzine (an antihistamine) and buspirone (a 5-hydroxytryptamine1A [5-HT1A] receptor agonist), while others, such as pregabalin (an anticonvulsant), have been introduced more recently (Baldwin et al., 2005).
The majority of research on pharmacological and physical interventions has concerned the use of interventions such as antidepressants and benzodiazepines. However there are a number of other interventions that are in relatively wide use or of interest in the treatment of GAD and include herbal interventions, acupuncture and hypnotherapy. These are reviewed at the end of this chapter.
8.1.1. Effectiveness of pharmacological interventions
There are currently several different kinds of pharmacological treatment available for the treatment of GAD. Placebo-controlled trials provide the best evidence of efficacy but such studies are not always easy to interpret because of the extent of the placebo response (Baldwin et al., 2005). In addition, in the general population, GAD is commonly comorbid with other anxiety disorders and depression, whereas participants recruited to placebo-controlled trials are more likely to have GAD as a sole diagnosis (Tyrer & Baldwin, 2006). This introduces uncertainty about the generalisability of findings from controlled trials to real-world clinical populations. There is also uncertainty about the length of time for which drug treatment should be continued once an initial response has been obtained. Related to this is the issue of the discontinuation symptomatology that often accompanies medication withdrawal (MHRA, 2004) and how patients may fare subsequently.
8.1.2. Current practice
Current clinical practice, as reflected in previous published guidelines (Baldwin et al., 2005; Davidson et al., 2010a), suggests that pharmacological treatment should be considered only at a certain level of clinical severity when there is evidence of persistent symptomatology that results in occupational and social disability. The presence of a comorbid mental disorder or physical illness may also influence the decision to offer medication (Davidson et al., 2010a).
When medication is recommended, current advice is to consider an antidepressant (either an SSRI or SNRI) as first-line treatment. Benzodiazepines are not advised because of the potential for the development of tolerance and dependence in a condition where treatment may need to be given for several months but they are still in relatively wide use.
8.2. PHARMACOLOGICAL INTERVENTIONS COMPARED WITH PLACEBO
8.2.1. Review question
In the treatment of GAD, which drugs improve outcomes compared with other drugs and with placebo?
8.2.2. Databases searched and inclusion/exclusion criteria
Information about the databases searched and the inclusion/exclusion criteria used for this section of the guideline can be found in Table 31 (further information about the search for health economic evidence can be found in Section 3.6). It should be noted that evidence on quetiapine was searched in order to inform a network meta-analysis of pharmacological treatments for people with GAD. Data on quetiapine were utilised in this meta-analysis to increase inference on other drugs. The results of the network meta-analysis supported the guideline economic analysis on pharmacological treatments for people with GAD. Methods and results of both the network meta-analysis and the guideline economic analysis of pharmacological treatments are reported in Section 8.8.3. The available evidence on quetiapine in the treatment of GAD was not assessed in this guideline as it is the subject of a forthcoming NICE Technology Appraisal.
Table 31
Databases searched and inclusion/exclusion criteria for clinical evidence.
8.2.3. Studies considered15
The review team conducted a new systematic search for RCTs that assessed the benefits and harms of pharmacological interventions for the treatment of people with GAD as defined in DSM-III-R or DSM-IV.
A total of 13,356 references were identified by the electronic search relating to clinical evidence; a further seven unpublished trials were identified through pharmaceutical company websites. Of these references, 13,220 were excluded at the screening stage on the basis of reading the title and/or abstract. The remaining 139 references were assessed for eligibility on the basis of the full text. Sixty-two trials met the eligibility criteria set by the GDG, providing data on 20,834 participants. Of these, seven were unpublished and 55 were published in peer-reviewed journals between 1992 and 2009. In addition, 77 studies were excluded from the analysis. Fifty studies did not provide an acceptable diagnosis of GAD; 19 were not RCTs; seven had less than ten participants per group, one was not double blind; and one did not use a relevant intervention. Further information about both included and excluded studies can be found in Appendix 15d.
8.2.4. Antidepressants versus placebo
Studies considered
There were a total of 29 trials comparing various antidepressants with placebo. Most trials were on venlafaxine (all studies used extended release [XL] preparations), duloxetine, escitalopram, sertraline and paroxetine. These trials were all large, high-quality studies funded almost exclusively by drug company sponsorship. There was no evidence of publication bias at the study level for any of the antidepressant comparisons as assessed by visual inspection of funnel plots and formally by the Egger’s test.
A summary of study characteristics can be found in Table 32 with full details in Appendix 15d, which also includes details of excluded studies.
Table 32
Study information table for trials comparing antidepressants with placebo.
Clinical evidence for antidepressants versus placebo
Evidence from the important outcomes and overall quality of evidence are presented in Table 33. The full GRADE profiles and associated forest plots can be found in Appendix 18c and Appendix 16c, respectively.
Table 33
Evidence summary table for trials of antidepressants versus placebo.
Clinical evidence summary
There was limited or no data for a number of interventions: there was only one study assessing imipramine; one study assessing citalopram; no data on mirtazapine, bupropion, trazodone, fluvoxamine, fluoxetine and amitriptyline; and no data on most TCAs (for example, clomipramine, doxepin, dosulepin, lofepramine, nortriptyline and trimipramine). A further limitation of the data was the lack of long-term studies (only two studies, one for venlafaxine and one for escitalopram, provided data on use beyond 6 months) and no available follow-up data beyond end of treatment.
The benefits in terms of reducing the risk of non-response, non-remission and the mean anxiety rating score was similar for most antidepressants suggesting a small-to-moderate improvement in anxiety relative to placebo.
The harms were also relatively consistent across drugs. Discontinuation due to adverse events was greater than placebo for most antidepressants but particularly high for paroxetine, duloxetine and venlafaxine. Specific side effects such as nausea and insomnia were more common in people receiving antidepressants compared with placebo. Sexual problems were relatively rare but there was an increased risk associated with antidepressants.
8.2.5. Pregabalin versus placebo
Studies considered
A total of eight trials compared pregabalin with placebo. A summary of study characteristics can be found in Table 34 with full details in Appendix 15d, which also includes details of excluded studies.
Table 34
Study information table for trials comparing pregabalin with placebo.
Clinical evidence for pregabalin versus placebo
Evidence from the important outcomes and overall quality of evidence are presented in Table 35. The full GRADE profiles and associated forest plots can be found in Appendix 18c and Appendix 16c, respectively.
Table 35
Evidence summary table for trials of pregabalin versus placebo.
Clinical evidence summary
Pregabalin was associated with a moderate benefit in terms of mean anxiety rating scores and non-response. However, although there was statistically significant evidence of benefit in relation to non-remission, the effect size was small.
In terms of harms, there was a small borderline statistically significant increase in the risk of discontinuation due to adverse events. For specific side effects, there was a different pattern from that found for antidepressants. There was no statistically significant increase in risk of experiencing nausea or insomnia. In addition, sexual problems were not reported as frequent side effects in any of the studies. However, there were large increases in risk of dizziness and fatigue (although for the latter this was not statistically significant).
8.2.6. Benzodiazepines versus placebo
Study characteristics
A total of four trials compared benzodiazepines with placebo. A summary of study characteristics can be found in Table 36 with full details in Appendix 15d, which also includes details of excluded studies.
Table 36
Study information table for trials comparing benzodiazepines with placebo.
Clinical evidence for benzodiazepines versus placebo
Evidence from the important outcomes and overall quality of evidence are presented in Table 37. The full GRADE profiles and associated forest plots can be found in Appendix 18c and Appendix 16c, respectively.
Table 37
Evidence summary table for trials of benzodiazepines versus placebo.
Clinical evidence summary
The evidence base for benzodiazepines was much smaller than for antidepressants and pregabalin reported above. There were inconsistent effects for most outcomes. On the mean anxiety rating score there were small-to-moderate benefits found but the effect for diazepam was not statistically significant. On non-response there was a moderate reduction for diazepam but no statistically significant effects were identified for lorazepam and alprazolam. For non-remission, no data was found for diazepam and there were no statistically significant effects for lorazepam or alprazolam.
There was inconsistent reporting of harms, therefore the data on side effects is relatively limited. There was no statistically significant increase in risk of discontinuation for diazepam and alprazolam but there was a higher risk in lorazepam. Increased risk of experiencing sexual problems was found for diazepam but this was not reported for the other drugs. There was an increased risk of dizziness for diazepam, lorazepam and alprazolam.
8.2.7. Buspirone versus placebo
Studies considered
There were a total of five trials comparing buspirone with placebo. A summary of study characteristics can be found in Table 38 with full details in Appendix 15d which also includes details of excluded studies.
Table 38
Study information table for trials comparing buspirone with placebo.
Clinical evidence for buspirone versus placebo
Evidence from the important outcomes and overall quality of evidence are presented in Table 39. The full GRADE profiles and associated forest plots can be found in Appendix 18c and Appendix 16c, respectively.
Table 39
Evidence summary table for trials of buspirone versus placebo.
Clinical evidence summary
There was a small benefit associated with buspirone on both the mean anxiety rating score and non-response. However, no data was reported on non-remission therefore it is not possible to draw conclusions on this outcome.
There was greater risk of discontinuation due to adverse events associated with buspirone. There was a higher risk of experiencing nausea and dizziness compared with placebo.
8.2.8. Hydroxyzine versus placebo
Studies considered
A total of three trials compared hydroxyzine with placebo. A summary of study characteristics can be found in Table 40 with full details in Appendix 15d, which also includes details of excluded studies.
Table 40
Study information table for trials comparing hydroxyzine with placebo.
Clinical evidence for hydroxyzine versus placebo
Evidence from the important outcomes and overall quality of evidence are presented in Table 41. The full GRADE profiles and associated forest plots can be found in Appendix 18c and Appendix 16c, respectively.
Table 41
Evidence summary table for trials of hydroxyzine versus placebo.
Clinical evidence summary
There was inconsistent reporting of data on hydroxyzine therefore it is difficult to draw conclusions concerning the harms and benefits of this drug. The mean anxiety rating score suggested a moderate reduction in anxiety. However, most studies did not report data in sufficient detail on non-response and non-remission. There were also very little data on discontinuation or reporting of specific side effects.
8.2.9. Quetiapine versus placebo
Studies considered
A total of four trials compared quetiapine with placebo. A summary of study characteristics can be found in Table 42 with full details in Appendix 15d, which also includes details of excluded studies.
Table 42
Study information table for trials comparing quetiapine with placebo.
Clinical evidence for quetiapine versus placebo
Evidence from the important outcomes and overall quality of evidence are presented in Table 43. The full GRADE profiles and associated forest plots can be found in Appendix 18c and Appendix 16c respectively.
Table 43
Evidence summary table for trials of quetiapine versus placebo.
Clinical evidence summary
A review of the clinical efficacy of quetiapine is included in a forthcoming NICE Technology Appraisal and therefore is not assessed in this guideline. The data is to inform the network-analysis only (see section 8.8.3).
8.3. HEAD-TO-HEAD TRIALS OF PHARMACOLOGICAL INTERVENTIONS
8.3.1. Antidepressants versus other antidepressants
Studies considered
There were a total of six trials comparing antidepressants with other antidepressants. A summary of study characteristics can be found in Table 44 with full details in Appendix 15d, which also includes details of excluded studies.
Table 44
Study information table for trials comparing antidepressants with other antidepressants.
Clinical evidence for antidepressants versus other antidepressants
Evidence from the important outcomes and overall quality of evidence are presented in Table 45. The full GRADE profiles and associated forest plots can be found in Appendix 18c and Appendix 16c, respectively.
Table 45
Evidence summary table for trials of antidepressants versus other antidepressants.
Clinical evidence summary
There was a small statistically significant effect in favour of escitalopram compared with paroxetine based on a reduction in HAM-A scores. In addition, there was a 40% reduction in risk of non-response for escitalopram compared with paroxetine. Moreover, there was greater risk (although not statistically significant) of discontinuation of treatment due to adverse events associated with paroxetine.
There were no differences found on reduction of anxiety symptoms between escitalopram and venlafaxine. However, venlafaxine was associated with a greater risk of discontinuation (although this was not statistically significant).
No difference was found between duloxetine and venlafaxine for reduction in anxiety but there was a greater risk of discontinuation for venlafaxine (although again this was not statistically significant)
There were no statistically significant differences found between paroxetine and sertraline on any outcomes. However, this was based on a small trial that was unlikely to have sufficient power to identify any differences.
8.3.2. Antidepressants versus other pharmacological interventions
Studies considered
There were a total of six trials comparing antidepressants with other pharmacological interventions. A summary of study characteristics can be found in Table 46 with full details in Appendix 15d, which also includes details of excluded studies.
Table 46
Study information table for trials comparing antidepressants with other pharmacological interventions.
Clinical evidence for antidepressants versus other pharmacological interventions
Evidence from the important outcomes and overall quality of evidence are presented in Table 47. Data for quetiapine, which is considered in the network meta-analysis, are reported in the same table. The full GRADE profiles and associated forest plots can be found in Appendix 18c and Appendix 16c, respectively.
Table 47
Evidence summary table for trials of antidepressants versus other pharmacological interventions.
Clinical evidence summary
Similar to the data in Section 8.3.1, there was limited data concerning comparisons between active interventions. There were no statistically significant differences in reduction in anxiety for venlafaxine in comparison with pregabalin, buspirone or diazepam. However there was an increased risk of discontinuation due to adverse events for venlafaxine compared with these drugs.
8.3.3. Head-to-head comparisons of pharmacological interventions other than antidepressants
Studies considered
There were a total of six head-to-head trials of pharmacological interventions other than antidepressants. A summary of study characteristics can be found in Table 48 with full details in Appendix 15d, which also includes details of excluded studies.
Table 48
Study information table for head-to-head comparisons of pharmacological interventions other than antidepressants.
Clinical evidence for head-to-head trials of pharmacological interventions other than antidepressants
Evidence from the important outcomes and overall quality of evidence are presented in Table 49. The full GRADE profiles and associated forest plots can be found in Appendix 18c and Appendix 16c, respectively.
Table 49
Evidence summary table for head-to-head comparisons of pharmacological interventions other than antidepressants.
Clinical evidence summary
As above, there was a lack of head-to-head comparisons. There were borderline statistically significant effects favouring pregabalin over lorazepam and alprazolam in reduction of anxiety. In addition, pregabalin was associated with a reduced risk of discontinuation due to adverse events compared with lorazepam. However, both lorazepam and alprazolam were less likely to be associated with reporting dizziness as a side effect.
There was a small but not statistically significant difference in favour of hydroxyzine compared with buspirone based on a reduction in HAM-A scores. In addition, no statistically significant differences were found between buspirone and lorazepam.
8.4. EFFECTS OF DOSE
8.4.1. Venlafaxine
Studies considered
There were four trials on venlafaxine comparing different doses. A summary of study characteristics can be found in Table 50 with full details in Appendix 15d, which also includes details of excluded studies.
Table 50
Study information table for trials of venlafaxine comparing different doses.
Doses used in studies of venlafaxine ranged from a mean of 37.5 mg to 225 mg but there was limited data for most comparisons. The most common comparison was of 75 mg versus 150 mg.
Clinical evidence for venlafaxine comparing different doses
Evidence from the important outcomes and overall quality of evidence are presented in Table 51. The full GRADE profiles and associated forest plots can be found in Appendix 18c and Appendix 16c, respectively.
Table 51
Evidence summary table for trials of venlafaxine comparing different doses.
Clinical evidence summary
There were no statistically significant differences between 37.5 mg and 75 mg of venlafaxine for discontinuation due to adverse events and dizziness. However, with 37.5 mg compared with 75 mg, there was a 35% reduction in the risk of nausea. There was a borderline statistically significant difference on mean HAM-A score in favour of 75 mg in comparison with 150 mg of venlafaxine based on a reduction in HAM-A scores (SMD −0.27; CI −0.57 to 0.03) and a reduction in the risk of side effects such as nausea (RR = 0.82; CI 0.68 to 0.98) and insomnia (RR = 0.59; CI 0.34 to 1.01). There were no statistically significant differences in regards to a reduction in the risk of non-response, in discontinuation for any reason and side effects such as nervousness, dizziness and asthenia.
There were no statistically significant differences between 150 mg and 255 mg for risk of side effects such as insomnia, nervousness, asthenia and dizziness.
8.4.2. Selective serotonin reuptake inhibitors
Studies considered
There were limited studies (only two trials) comparing doses for SSRIs. Comparisons could only be made for escitalopram and paroxetine, with just one study found for each drug. A summary of study characteristics can be found in Table 52 with full details in Appendix 15d, which also includes details of excluded studies.
Table 52
Study information table for trials of SSRIs comparing different doses.
Clinical evidence for selective serotonin reuptake inhibitors comparing different doses
Evidence from the important outcomes and overall quality of evidence are presented in Table 53. The full GRADE profiles and associated forest plots can be found in Appendix 18c and Appendix 16c, respectively.
Table 53
Evidence summary table for trials of SSRIs comparing different doses.
Clinical evidence summary
There were borderline statistically significant effects in the reduction of anxiety in favour of 10 mg of escitalopram compared with 5 mg based on mean HAM-A scores. There was no significant difference between the two groups regarding side effects with the exception of a reduction in the risk of reported headache with 5 mg compared with 10 mg of escitalopram. There was a reduced risk of reported headaches in the 20 mg group compared with the 10 mg escitalopram group.
There were no clear differences on outcomes between 20 mg and 40 mg of paroxetine.
8.4.3. Duloxetine
Studies considered
There were two trials on duloxetine comparing different doses. A summary of study characteristics can be found in Table 54 with full details in Appendix 15d, which also includes details of excluded studies.
Table 54
Study information table for trials of duloxetine comparing different doses.
Doses used in the studies ranged from a mean of 20 mg to a mean of 120 mg. Results were similar as those reported above – there was limited evidence of differences between doses.
Clinical evidence for duloxetine comparing different doses
Evidence from the important outcomes and overall quality of evidence are presented in Table 55. The full GRADE profiles and associated forest plots can be found in Appendix 18c and Appendix 16c, respectively.
Table 55
Evidence summary table for trials of duloxetine comparing different doses.
Clinical evidence summary
There was a reduction in anxiety in favour of 60 to 120 mg of duloxetine compared with 20 mg based on HADS-A scores. However, this did not reach statistical significance. There were no clear differences between 60 mg and 120 mg found on any outcomes.
8.4.4. Pregabalin
Studies considered
There were five trials on pregabalin comparing different doses. A summary of study characteristics can be found in Table 56 with full details in Appendix 15d, which also includes details of excluded studies. Dosages used in the studies ranged from a mean of 150 mg to a mean of 600 mg.
Table 56
Study information table for trials of pregabalin comparing different dosages.
Clinical evidence for pregabalin comparing different doses
Evidence from the important outcomes and overall quality of evidence are presented in Table 57. The full GRADE profiles and associated forest plots can be found in Appendix 18c and Appendix 16c, respectively.
Table 57
Evidence summary table for trials of pregabalin comparing different dosages.
Clinical evidence summary
There were few differences found between doses of pregabalin. However, there was some evidence that a mean of 600 mg was associated with greater reduction in anxiety compared with 150 mg. But 150 mg was associated with fewer reported side effects (particularly somnolence and dizziness). In addition, 400 mg was associated with greater benefits in reduction of anxiety compared with 600 mg.
8.4.5. Overall clinical evidence summary
The evidence from controlled trials indicates that SSRIs (sertraline, escitalopram and paroxetine) and SNRIs (duloxetine and venlafaxine) are efficacious in the treatment of GAD in that relative to placebo they produce greater reductions in HAM-A ratings and increase the probability of response to treatment. Generally, effect sizes are in the low to moderate range and do not seem to differ between the different antidepressants to a clinically significant extent, although there are much more data available for some drugs than others. There is no clear indication of a dose-response relationship where this has been specifically assessed. Nausea and insomnia are commonly experienced side effects. Discontinuation due to adverse events was more common in people receiving antidepressant treatment. There were few direct comparisons between antidepressants but there were indications that escitalopram may be slightly more effective than paroxetine.
Other drugs (particularly pregabalin) were also efficacious in GAD with effect sizes generally in the range of those seen with antidepressants. Again, comparative data did not yield evidence of consistent differences in efficacy, although the side-effect profile of the non-antidepressant agents differed from that of the SSRIs and SNRIs, consisting mainly of somnolence and dizziness.
8.5. MAINTENANCE TREATMENT
In many people GAD runs a chronic course and even where patients improve with treatment, relapse is common, particularly in those who remain symptomatic to some extent (Yonkers et al., 1996). Stopping treatment after a few weeks can lead to relapses in 60 to 80% of patients over the next year (Rickels & Schweizer, 1990). For this reason current guidelines (Baldwin et al., 2005; Davidson et al., 2010) suggest that where drug treatment is helpful it should be continued over the next 6 to 12 months if tolerance and efficacy are satisfactory. Establishing the efficacy of this practice is therefore important. How long treatment should be continued subsequently is unclear and guidelines suggest adapting an individualised approach depending on the needs and preferences of the patient (Davidson et al., 2010).
8.5.1. Databases searched and inclusion/exclusion criteria
Information about the databases searched and the inclusion/exclusion criteria used for this section of the guideline can be found in Table 58 (further information about the search for health economic evidence can be found in Section 3.6).
Table 58
Databases searched and inclusion/exclusion criteria for clinical evidence.
8.5.2. Studies considered
The review team conducted a new systematic search for RCTs that assessed the benefits and downsides of pharmacological interventions for the maintenance treatment of people with GAD. Maintenance treatment was defined as interventions for participants who had already responded to treatment in order to maintain reductions in anxiety. While all other antipsychotics were reviewed, quetiapine was not examined in this review as it will be formally evaluated in a forthcoming NICE Technology Appraisal.
A total of four trials met the eligibility criteria of the review, with one trial each comparing pregabalin, paroxetine, escitalopram and duloxetine with placebo.
A summary of study characteristics can be found in Table 59 with full details in Appendix 15d, which also includes details of excluded studies.
Table 59
Study information table for trials of maintenance treatment.
8.5.3. Clinical evidence for maintenance treatment
Evidence from the important outcomes and overall quality of evidence are presented in Table 60. The full GRADE profiles and associated forest plots can be found in Appendix 18c and Appendix 16c, respectively.
Table 60
Evidence summary table for trials of maintenance treatment.
8.5.4. Clinical evidence summary
There was only one trial each examining pregabalin, duloxetine, escitalopram, and paroxetine. The findings suggest that where people have responded to pharmacological treatment in the short-term, continuing treatment over the next 6 months resulted in fewer relapses than switching to placebo. These findings support current guidelines that drug treatment should be continued for at least 6 months in people who respond in the short-term (Baldwin, et al., 2005; Davidson et al., 2010). In addition, there was no difference between the drugs and placebo for reported side effects.
However, the main limitation of this review is the very high dropout reported in most studies particularly in the placebo groups. For example, 49% dropped out of the placebo group in the paroxetine trial and 45.5% dropped out in the placebo group in the duloxetine trial. In addition, there was some variability in the length of follow-up.
The high dropout raises questions concerning whether differences between groups is due to the benefit of continuing to receive pharmacological treatment or due to the effects of withdrawing the medication. In addition, there is a lack of controlled data to guide management of pharmacological treatment in the longer-term.
8.6. MANAGEMENT OF NON-RESPONSE TO PHARMACOLOGICAL INTERVENTIONS
8.6.1. Introduction
For many people, symptomatic remission is not achieved during pharmacological treatment for GAD. Guidelines emphasise the importance of giving initial drug treatment sufficient time to exert its effect because clinical improvement in GAD may be slow with both response and remission rates increasing beyond 2 months of drug treatment (Bielski & Bose, 2005; Davidson et al., 2010). Where clinician and service user agree that pharmacological treatment should be modified, there are three possible strategies: (i) increase the dose of the current treatment (if the maximum dose has not been reached); (ii) augment with another agent from a different pharmacological class; (iii) switch to an alternative agent. In general (i) and (ii) are favoured when there has been a partial response to initial treatment.
Conventional antipsychotic drugs such as trifluoperazine were previously used to treat anxiety where clinicians wished to avoid the use of benzodiazepines. There is currently interest in the possible role of a typical antipsychotic drugs in GAD because relative to conventional agents these drugs have a reduced propensity to cause serious movement disorders such as tardive dyskinesia (Correll et al., 2004). Some guidelines have advocated the use of atypical antipsychotic drugs such as olanzapine, risperidone and quetiapine to augment antidepressants in people who do not have a satisfactory response to antidepressant treatment alone (Davidson et al., 2010).
8.6.2. Databases searched and inclusion/exclusion criteria
Information about the databases searched and the inclusion/exclusion criteria used for this section of the guideline can be found in Table 61 (further information about the search for health economic evidence can be found in section 3.6).
Table 61
Databases searched and inclusion/exclusion criteria for clinical evidence.
8.6.3. Studies considered
The review team conducted a new systematic search for RCTs that assessed the benefits and downsides of pharmacological interventions for the treatment of people with GAD.
A total of four trials met the eligibility criteria for the review. Two trials compared risperidone with placebo, one trial compared olanzapine with placebo and one trial compared ziprasidone with placebo, as augmentation strategies in combination with pharmacological interventions for GAD.
No trials were identified on switching or sequencing pharmacological interventions.
8.6.4. Augmentation strategies
Studies considered
There were four trials on augmentation strategies. A summary of study characteristics can be found in Table 62 with full details in Appendix 15d, which also includes details of excluded studies.
Table 62
Study information table for trials of augmentation strategies.
Clinical evidence for augmentation strategies
Evidence from the important outcomes and overall quality of evidence are presented in Table 63. The full GRADE profiles and associated forest plots can be found in Appendix 18c and Appendix 16c, respectively.
Table 63
Evidence summary table for trials of augmentation strategies.
Clinical evidence summary
There was limited evidence because three of the four trials were small and there was high heterogeneity in HAM-A scores (I2 = 73%) for risperidone. There was no statistically significant evidence of benefit for any of the antipsychotic drugs assessed individually. When combining the antipsychotic data there was still limited evidence of benefit.
8.6.5. Overall clinical summary of non-response to pharmacological interventions
There was no data identified on increasing the dose or switching pharmacological treatments. There was only data available on atypical antipsychotics (olanzapine, risperidone and ziprasidone) for augmentation treatment. It appears such interventions were associated with limited benefit and greater risk of discontinuation due to adverse events.
8.7. SIDE EFFECTS OF PHARMACOLOGICAL INTERVENTIONS
8.7.1. Introduction
The purpose of this review is to assess the side effects and adverse events of pharmacological interventions for the treatment of GAD. However given the lack of data specifically focused on this disorder, data were examined for common mental health problems (that is, depression and anxiety disorders). Pharmacological interventions were limited to those most commonly used in clinical practice including antidepressants, pregabalin, benzodiazepines, hydroxyzine and buspirone.
8.7.2. Databases searched and inclusion/exclusion criteria
Information about the databases searched and the inclusion/exclusion criteria used for this section of the guideline can be found in Table 64 (further information about the search for health economic evidence can be found in section 3.6).
Table 64
Databases searched and inclusion/exclusion criteria for clinical evidence.
8.7.3. Studies considered
The review team conducted a new systematic search for systematic reviews that assessed the efficacy and safety of antidepressants.
Twenty systematic reviews relating to clinical evidence met the eligibility criteria set by the GDG. All were published in peer-reviewed journals between 1999 and 2009.
8.7.4. Clinical evidence for side effects and adverse events of antidepressants
The side effects and adverse events of antidepressants have already been reviewed in detail in the NICE guideline for depression in people with a chronic physical health problem (NCCMH, 2010b). The key characteristics of the included systematic reviews discussed in that guideline and relevant to the present guideline are summarised in Table 65.
Table 65
Study information table of included systematic reviews on side effects and adverse events.
The main adverse events associated with antidepressants are cardiovascular symptoms, bleeding, gastrointestinal symptoms, sexual dysfunction, weight change, and suicidal ideation and behaviour.
Cardiovascular symptoms
SSRIs do not appear to be associated with an increase risk in cardiovascular adverse events (for example, Swenson et al., 2006; Taylor, 2008) and are associated with a relatively low fatal toxicity index (number of poisoning deaths per million prescriptions). However, TCAs are associated with a higher risk of cardiovascular adverse events and have found to be cardiotoxic in overdose (Taylor, 2008).
Duloxetine was associated with small increases in diastolic blood pressure, tachycardia and cholesterol compared with placebo (Duggan & Fuller, 2004; Wernicke et al., 2007). In addition, there is evidence of moderate acute toxicity associated with venlafaxine (Taylor, 2008).
Bleeding
Several observational studies utilising data from national prescribing databases have found a relatively strong association (approximately a three-fold increase) between SSRIs and increased risk of gastrointestinal bleeding (Weinrieb et al., 2003; Yuan et al., 2006). However, it should be noted that the outcome was relatively rare with approximately four to five events per 1000 person years. This effect was particularly strong (approximately a 15-fold increase of bleeding) in people concurrently using non-steroidal anti-inflammatory drugs (NSAIDs) and SSRIs.
Gastrointestinal symptoms
There is consistent evidence both in depression and anxiety populations of the increased risk of gastrointestinal (GI) symptoms such as nausea, vomiting and diarrhoea associated with SSRI use (Brambilla et al., 2005; Beasley et al., 2000). This has been confirmed in the current systematic review of SSRIs for GAD (see section 8.2.3). TCAs also appear to be associated with higher risk of constipation when compared with fluoxetine (Beasley et al., 2000).
Sexual dysfunction
There was consistent evidence of sexual adverse events associated with SSRIs, duloxetine and venlafaxine in people with depression (Werneke et al., 2006; Gregorian et al., 2002; Beasley et al., 2000; Keller, 2000). These results have been replicated in people with GAD in the current systematic review (see section 8.2.3).
Weight change
Fluoxetine appears to be associated with greater loss in weight compared with placebo (Beasley et al., 2000), TCAs and other SSRIs (Brambilla et al., 2005). However, as noted by Demyttenaere and Jaspers (2008), these effects are reported early on in treatment. When assessing continuation studies there is a possibility that paroxetine and fluoxetine may actually be associated with weight gain but this needs further research to establish this finding.
In addition, there is some evidence that duloxetine was associated with weight loss with a mean reduction of 2.2 kg compared with 1 kg for placebo (Duggan & Fuller, 2004).
Suicidal ideation and behaviour
One systematic review was identified on the association between antidepressant use and suicidal ideation and/behaviour (Stone et al., 2009). For those aged under 25 years there was an increased odds of suicidal behaviour (OR 2.30; 95% CI 1.04, 5.09) for people taking antidepressants compared with placebo. There was a borderline statistically significant increase in odds of suicidal ideation and suicidal behaviour (OR 1.62; 95% CI 0.97, 2.71).
8.7.5. Clinical evidence for side effects for pregabalin
The included reviews are summarised in Table 66. Three reviews were included; however there are a number of limitations to their quality. The methods of identifying the included studies, data extraction and so on were not reported. In addition, the results were almost exclusively concerned with the results of short-term RCTs therefore no long-term evidence of the safety and side effects of pregabalin was examined.
Table 66
Study information table of included systematic reviews of pregabalin.
Pregabalin appeared to be well tolerated by most participants but was associated with greater risk of headaches, dizziness and somnolence.
8.7.6. Clinical evidence for side effects for buspirone
No systematic reviews were identified that specifically assessed the side effects of buspirone.
8.7.7. Clinical evidence for side effects for hydroxyzine
No systematic reviews were identified that specifically assessed the side effects of hydroxyzine.
8.7.8. Clinical evidence for side effects for benzodiazepines
The three included reviews are summarised in Table 67. As above there were no high-quality systematic reviews available. Very few, if any, details are reported on inclusion criteria, search strategies, data extraction, and so on. The most common reported problem with benzodiazepine use was risk of dependence. This suggests only short-term use of this treatment is appropriate and that particular caution should be exercised for people with comorbid alcohol or drug misuse.
Table 67
Study information table of included systematic reviews of benzodiazepines.
There were a number of cognitive side effects reported, including impairment of speech and memory. In addition, sedation, fatigue and ataxia were commonly associated with benzodiazepine use.
8.7.9. Overall clinical summary for side effects of pharmacological interventions
The systematic review confirms the characteristic side-effect profile of the various drugs used in pharmacological interventions in GAD. Many of the studies of antidepressants concern the use of these agents in conditions other than GAD; however, there do not seem to be important differences in the nature and frequency of the side effects experienced across diagnoses. SSRIs are well known to be associated with nausea, insomnia and sexual dysfunction and a similar profile of effect is seen with SNRIs. Discontinuation symptoms are common after antidepressant drug withdrawal and appear to be more frequent after withdrawal of agents with relatively short half-lives such as paroxetine and venlafaxine. SSRIs can also be associated with serious bleeding problems such as gastrointestinal haemorrhage, a risk that is significantly increased by co-administration of NSAIDs. Although it should be acknowledged that these events are relatively rare. SSRIs are generally safe in patients with cardiovascular problems though SNRIs carry a risk of increasing blood pressure. Venlafaxine appears more toxic in overdose than SSRIs.
In contrast to the SSRIs and SNRIs, pregabalin and benzodiazepines cause more sedation and dizziness but are less likely to be associated with nausea and sexual problems. Benzodiazepines are well known to be associated with tolerance and dependence and cause a withdrawal syndrome upon discontinuation. Withdrawal effects after pregabalin have not yet been well characterised. In keeping with its action at central 5-HT receptors, buspirone causes nausea and dizziness while the antihistamine, hydroxyzine, is associated with sedation.
8.8. HEALTH ECONOMIC EVIDENCE
8.8.1. Research question
What is the cost effectiveness of pharmacological treatments compared with other interventions in the treatment of GAD?
8.8.2. Systematic literature review
The systematic search of the economic literature undertaken for the guideline identified five eligible studies on pharmacological treatments for people with GAD (Guest et al., 2005; Heuzenroeder et al., 2004; Iskedjian et al., 2008; Jørgensen et al., 2006; Vera-Llonch et al., 2010). Two studies were conducted in the UK (Guest et al., 2005; Jørgensen et al., 2006), one in Spain (Vera-Llonch et al., 2010), one in Canada (Iskedjian et al., 2008) and one in Australia (Heuzenroeder et al., 2004). Details on the methods used for the systematic review of the economic literature are described in Chapter 3; references to included studies and evidence tables for all economic evaluations included in the systematic literature review are provided in Appendix 15f. Completed methodology checklists of the studies are provided in Appendix 17. Economic evidence profiles of studies considered during guideline development (that is, studies that fully or partly met the applicability and quality criteria) are presented in Appendix 18c, accompanying the respective GRADE clinical evidence profiles.
Jørgensen and colleagues (2006) evaluated the cost effectiveness of escitalopram versus paroxetine in the treatment of people with GAD in the UK. A decision-analytic model was constructed for this purpose. The study population consisted of newly diagnosed people with GAD with a HAM-A score of 18 or more, who were treated in a primary care setting. The primary measure of outcome in the analysis was the rate of initial response as well as the rate of maintained response (that is, initial response and no relapse until the end of the time horizon). Initial response was defined by a Clinical Global Impressions (CGI) Improvement score of 1 or 2. Relapse was defined as a HAM-A total score of 15 or more, a CGI-S score of 4 or more, or discontinuation due to lack of efficacy. Response and discontinuation rates were taken from Bielski and Bose (2005); relapse data and other clinical input parameters were based on published literature and further assumptions. The study adopted a societal perspective but an analysis using NHS costs only was also provided. Estimates of resource use (medication, GP and/or psychiatrist visits as well as productivity losses) were based on recommendations from the previous NICE guideline on anxiety (NICE, 2004a) and the expert opinion of the GDG; UK national unit costs were used. The time horizon of the analysis was 9 months.
According to the results of the analysis, escitalopram dominated paroxetine in both the NHS and societal perspectives considered. Escitalopram demonstrated a higher rate of initial response (14.4% more responders) and a higher rate of maintained response (7.7% more responders) than paroxetine. The mean total costs of escitalopram and paroxetine over 9 months, estimated from an NHS perspective, were £447 and £486 per person treated, respectively (2005 prices). Results were robust to changes in response rates, tolerance and acquisition costs.
The study is directly applicable to the review question and the NHS setting. The methods appear to be rigorous overall; however, the study has been funded by the pharmaceutical industry, which raises issues about potential conflicts of interest.
Guest and colleagues (2005) examined the cost effectiveness of venlafaxine XL compared with diazepam in the treatment of people with GAD in primary care in the UK, from the perspective of the NHS. The study was based on decision-analytic modelling. The primary outcome measure was the percentage of successful treatment, defined as the percentage of people in remission at 6 months, with remission defined as a CGI score of 1. The source of clinical effectiveness data was Hackett and colleagues (2003). Resource use estimates were based on expert opinion; national prices were used. The time horizon of the analysis was 6 months.
Venlafaxine XL was shown to be more effective and more costly than diazepam. The percentage of successful treatment was 27.6% with venlafaxine XL, versus 16.8% with diazepam. The mean total costs of venlafaxine XL and diazepam were £352 and £310 per person treated, respectively (2001 prices). Venlafaxine XL incurred an extra £381 per successfully treated person compared with diazepam. Results were sensitive to changes in rates of response, remission, relapse and discontinuation, as well as to changes in resource use estimates. Probabilistic analysis revealed that venlafaxine XL dominated diazepam in at least 25% of iterations. The authors concluded that venlafaxine XL was more cost effective than diazepam for the treatment of people with GAD. However, the results are difficult to interpret due to lack of use of QALYs as the measure of outcome. In addition, the study is at risk of bias as it was funded by the pharmaceutical industry.
Vera-Llonch and colleagues (2010) examined the cost effectiveness of pregabalin compared with venlafaxine XL in the treatment of people with GAD in Spain, from the perspective of a third-party payer. The study was based on decision-analytic modelling. The measure of outcome was the number of QALYs gained. Clinical data were taken from Kasper and colleagues (2009). Resource use estimates were based on published and unpublished data; national unit costs were used. The time horizon of the analysis was 12 months.
Pregabalin was found to be more effective and more costly than venlafaxine XL. The ICER of pregabalin versus venlafaxine was estimated at €23,909 per QALY, ranging from €19,829 to €35,993 per QALY in sensitivity analysis (2007 prices). Converted and uplifted to 2009 UK pounds, the ICER of pregabalin versus venlafaxine becomes £17,565 per QALY, ranging from £14,567 to £26,442 per QALY in sensitivity analysis. Results were sensitive to changes in utility values, the time horizon, and whether discontinuation was assumed. The probability of pregabalin being cost effective at a threshold of roughly €25,000/QALY (£20,000/QALY) was approximately 95% (as read from a graph). Based on these results, the authors concluded that paroxetine was likely to be more cost effective than venlafaxine XL for the treatment of people with GAD in a Spanish healthcare setting. However, the study was conducted in Spain and therefore is not directly applicable to the UK setting. In addition, a major limitation of the analysis is that it was assumed that the treatment effect lasted for 44 weeks following end of treatment (that is, from 8 weeks until 12 months). Over this period it was assumed that all people retained the level of clinical improvement achieved by the end of treatment and no relapse was observed. Finally, the study is at risk of bias as it was funded by the pharmaceutical industry.
Iskedjian and colleagues (2008) undertook a modelling study to compare the costs and benefits of escitalopram versus paroxetine over 24 weeks, for the treatment of people with GAD in Canada. The study used both a Ministry of Health and a societal perspective. The primary measure of outcome was the number of symptom-free days, defined by a score of 1 or 2 on the CGI-I. Response and discontinuation rates were taken from Bielski and Bose (2005); other clinical input parameters were based on published literature and expert opinion. Resource use estimates were also based on expert opinion; national unit prices were used.
From a Ministry of Health perspective, escitalopram was shown to be more effective than paroxetine at an extra cost of $6.56 per symptom-free day, or $2,362 per symptom-free year (2005 Canadian dollars); converted and uplifted to 2009 UK pounds, this makes £3.4 per symptom-free day or £1,240 per symptom-free year. When a societal perspective was considered, escitalopram dominated paroxetine, that is, escitalopram was more effective, and at the same time was associated with lower total costs, compared with paroxetine. These results were robust to changes in rates of response, tolerance and adherence. Based on their results, the authors concluded that escitalopram was more cost effective than paroxetine for the treatment of GAD. However, the results are difficult to interpret due to lack of use of QALYs as the outcome measure. In addition, the study was conducted in Canada and therefore is not directly applicable to the UK setting. Finally, the study is at risk of bias as it was funded by the pharmaceutical industry.
The fifth study included in the systematic economic literature review was a modelling study that compared venlafaxine XL versus standard care for the treatment of GAD from the perspective of the healthcare sector in Australia (Heuzenroeder et al., 2004). Standard care was defined as a mixture of care based on evidence-based medicine principles (27%), non-evidence-based medicine principles (28%) and no care (45%). The study population was the total estimated adult population with GAD in Australia, according to national surveys. The measure of outcome was the number of DALYs saved. The source of clinical effectiveness data was a meta-analysis of two RCTs (Allgulander et al., 2001; Davidson et al., 1999). Resource use estimates were based on assumptions; national unit prices were used. The study reported that use of venlafaxine XL for the treatment of the adult population in Australia incurred an extra Aus$77 million and saved 3,300 DALYs compared with standard care, resulting in an incremental cost of $30,000/DALY saved, which ranged between $20,000 and $51,000/DALY saved in sensitivity analysis. The study, although meeting the systematic review inclusion criteria, was considered to be non-applicable to the UK setting for the following reasons: it was conducted in Australia, the outcome measure was DALYs saved, which limited the interpretability of the study findings, and standard care, according to its definition, was likely to differ significantly from standard care in the NHS. For these reasons the study was not considered further during the guideline development process.
8.8.3. Economic modelling
Introduction – objective of economic modelling
The cost effectiveness of pharmacological interventions relative to other available treatments for people with GAD was considered by the GDG as an area with likely significant resource implications. The GDG was particularly interested in the cost effectiveness of pharmacological interventions compared with low- and high-intensity psychological interventions, as well as in the relative cost effectiveness between different pharmacological interventions, including no treatment (placebo).
The development of an economic model comparing pharmacological interventions with low- and high-intensity psychological interventions using clinical effectiveness data from the guideline systematic review was not possible: no RCTs directly comparing pharmacological with psychological interventions were identified in the systematic clinical literature review. Indirect (clinical) comparisons between pharmacological and psychological interventions using a common ‘baseline’ comparator were problematic because of important differences in study designs in terms of the following:
- Comparators: psychological studies used mainly waitlist or standard care as a comparator, while studies on pharmacological treatments used placebo as control (but never waitlist or standard care); therefore, it was not possible to make indirect comparisons between pharmacological and psychological interventions using a common ‘baseline’ comparator.
- Reported clinical outcomes: psychological studies tended to report mainly continuous outcomes. Few psychological studies reported rates of response or remission, which were often used as outcome measures in pharmacological studies; even then, the definitions of response and remission in psychological studies were not the same as the respective definitions in pharmacological studies.
- Study population: a number of studies on low-intensity psychological interventions were conducted on people with mixed anxiety rather than GAD only; in contrast, only studies on people with GAD were included in the systematic literature review of pharmacological interventions.
Due to the above limitations, which did not allow consideration of both psychological and pharmacological treatments in one economic analysis, an economic model was developed to assess the relative cost effectiveness between different pharmacological interventions for people with GAD in the UK. This analysis was considered as a priority by the GDG, because of the likely significant resource implications associated with the choice of drug in the treatment of people with GAD. Moreover, existing economic evidence in the area of pharmacological treatment for people with GAD is rather limited and not directly applicable to the UK setting, since only two of the five studies were conducted in the UK. The economic studies included in the systematic review were characterised by a number of limitations; besides, they did not assess the whole range of drugs available in the UK for the treatment of people with GAD.
Economic modelling methods
Interventions assessed
The choice of drugs assessed in the economic analysis was determined by the availability of respective clinical data included in the guideline systematic literature review. The economic analysis considered all drugs with an acceptable risk-to-benefit ratio, as demonstrated by the systematic review of clinical evidence, that were deemed appropriate as first-line pharmacological treatment options for people with GAD. Based on the findings of the clinical systematic review, the following drugs were assessed in the economic analysis: duloxetine, escitalopram, paroxetine, pregabalin, sertraline and venlafaxine XL. It must be noted that sertraline was included in the economic analysis, despite the fact that it is not licensed for the treatment of people with GAD, because available evidence suggested that this is an effective drug in the treatment of GAD, with an acceptable risk-to-benefit ratio. Sertraline is widely used in the UK for the treatment of depression and mixed depression and anxiety; the GDG acknowledged that it is likely to be less commonly used in the treatment of GAD, but that this is probably because people presenting with anxiety in primary care are not often diagnosed with GAD. The model also considered no pharmacological treatment (placebo), consisting of GP visits only, as one of the treatment options.
Model structure
A decision-analytic model in the form of a decision-tree was constructed using Microsoft Office Excel 2007. The structure of the model was determined by the availability of clinical data. According to the model structure, hypothetical cohorts of people with GAD were initiated on each of the six drugs assessed (first-line drug) or no pharmacological treatment. People initiated on the first-line drug could either continue treatment for 8 weeks, or discontinue due to intolerable side effects during this 8-week period. For modelling purposes, it was assumed that drug discontinuation because of intolerable side effects occurred at 2 weeks following initiation of treatment; this was based on the GDG’s estimate that the majority of people discontinuing treatment because of intolerable side effects do so within 2 weeks from starting treatment. People who continued on the first-line drug either responded to treatment or did not respond. Those who responded were given maintenance treatment (consisting of the same drug) for 6 months. During this period, they either experienced a relapse or did not relapse. In each cohort, people discontinuing the first-line drug due to intolerable side effects and those not responding to the first-line drug were switched to a second-line drug, which was a mixture of all drugs assessed in the economic analysis, except the first-line drug administered to this cohort. People taking the second-line drug were all assumed to continue treatment with this drug. From that point onwards they followed the same pathways as people who continued the first-line drug (that is, no response or response and maintenance treatment, during which they could relapse or not relapse). People receiving no pharmacological treatment were assumed to either discontinue treatment, in which case they did not clinically improve (‘no response’), or continue their treatment and follow a similar pathway to that experienced by people continuing pharmacological treatment (that is, no response or response followed by relapse or no relapse). The time horizon of the analysis was 42 weeks, based on the optimal duration of initial pharmacological treatment (8 weeks) and maintenance treatment (26 weeks), and in order to allow for switching to second-line treatment in case the 8-week first-line treatment did not lead to response. A schematic diagram of the decision-tree is presented in Figure 6.
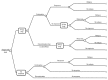
Figure 6
Schematic diagram of the decision-tree constructed for the assessment of the relative cost effectiveness of pharmacological interventions for people with GAD.
Costs and outcomes considered in the analysis
The economic analysis adopted the perspective of the NHS and personal social services, as recommended by NICE (2009a). Costs consisted of intervention costs (drug acquisition and GP visit costs) and other health and social care costs incurred by people with GAD not responding to treatment or experiencing a relapse following response (including contacts with healthcare professionals such as GPs, psychiatrists, psychologists, mental health nurses and social workers, community care, inpatient and outpatient secondary care). The measure of outcome was the QALY.
Clinical input parameters and overview of methods employed for evidence synthesis
Clinical input parameters consisted of the probability of drug discontinuation due to intolerable side effects, the probability of response for those not discontinuing treatment due to side effects (conditional response), and the probability of relapse following response to treatment.
The guideline systematic review of the clinical literature on pharmacological treatments identified two dichotomous outcomes that could be utilised in economic modelling: response (defined in the vast majority of studies as 50% reduction in HAM-A scores) and remission (defined in the vast majority of studies as a HAM-A score below 7). Utilisation of both types of data was not possible because not all studies provided data on both outcomes; therefore, it was not possible to estimate the numbers of people with GAD who responded to treatment but did not meet criteria for remission, and of those who responded to treatment and remitted for the whole dataset. For the economic model, it was decided to utilise response (rather than remission) data for the following reasons:
- response data were available from a larger number of studies including a higher number of participants, compared with data on remission
- available relapse data referred to people who had responded to treatment; no relapse data following remission were available in the guideline systematic review
- utility data were available for the health state of ‘response’ but not for the health state of ‘remission’; in addition, there were utility data available for ‘relapse following response’ but not for ‘relapse following remission’.
The availability of clinical and utility data, and the subsequent selection of response data in order to populate the model, determined the economic model structure.
To take all trial information into consideration, network (mixed treatment comparison) meta-analytic techniques were employed to synthesise evidence on discontinuation due to intolerable side effects, as well as evidence on conditional response (the methods used can be found in Appendix 13). Network meta-analysis is a generalisation of standard pair-wise meta-analysis for A versus B trials to data structures that include, for example, A versus B, B versus C and A versus C trials (Lu & Ades, 2004). A basic assumption of network meta-analysis is that direct and indirect evidence estimate the same parameter; in other words, the relative effect between A and B measured directly from an A versus B trial is the same with the relative effect between A and B estimated indirectly from A versus C and B versus C trials. Network meta-analytic techniques strengthen inference concerning the relative effect of two treatments by including both direct and indirect comparisons between treatments and, at the same time, allow simultaneous inference on all treatments examined in the pair-wise trial comparisons while respecting randomisation (Lu & Ades, 2004; Caldwell et al., 2005). Simultaneous inference on the relative effect of a number of treatments is possible provided that treatments participate in a single ‘network of evidence’, that is, every treatment is linked to at least one of the other treatments under assessment through direct or indirect comparisons.
Details on the methods and clinical data utilised in the two network meta-analyses that were undertaken to estimate the probability of discontinuation due to intolerable side effects and the probability of conditional response for each treatment option considered in the economic analysis (that is, each first-line drug or no pharmacological treatment) are presented in Appendix 13. The findings of the two network meta-analyses are discussed in the next sub-section. The probability of response for the second-line drug in each decision node of the model was calculated as the average probability of conditional response of all drugs except the one that was used as a first-line treatment in this particular node of the model.
The probability of relapse following response to treatment was estimated based on relevant data included in the guideline systematic review. Four placebo-controlled trials assessed the efficacy of pharmacological treatments in preventing relapse in people with GAD: two of them assessed an SSRI (ALLGULANDER2006 – escitalopram; STOCCHI2003 – paroxetine), one assessed an SNRI (DAVIDSON2008 –duloxetine) and one assessed pregabalin (FELTNER2008). According to the expert opinion of the GDG, pregabalin is not a drug routinely used in the maintenance treatment of people with GAD. Moreover, the relative risk of relapse of pharmacological treatment versus placebo was higher in FELTNER2008 compared with the respective relative risk estimated for each of the other three studies, indicating that pregabalin may be potentially less effective than the other three drugs in preventing relapse in people with GAD that has responded to initial treatment. Inclusion of data from FELTNER2008 in a meta-analysis of the above four trials increased the heterogeneity of the analysis considerably (87% when data from all four studies were pooled together versus 0% when data from FELTNER2008 were excluded). For the above reasons, the estimation of the relative risk of relapse of pharmacological treatment versus placebo was based on meta-analysis of the remaining three studies (ALLGULANDER2006, DAVIDSON2008, STOCCHI2003). This estimate was utilised in all decision nodes of the model that involved pharmacological treatment, including the pregabalin node. Nevertheless, the probability of relapse following response in the ‘no treatment’ node of the model was estimated by pooling data from the placebo arms of all four studies.
Table 71 provides all the clinical input parameters utilised in the economic model.
Table 71
Input parameters utilised in the economic model of pharmacological treatments for people with GAD.
Findings of the network meta-analyses undertaken to inform the economic analysis
The summary statistics of a number of parameters of the two network meta-analyses undertaken to inform the economic analysis, including the log hazard ratios of all drugs considered in the economic analysis versus placebo and the between-trial variation, are reported in Appendix 13.
Table 68 provides the results of the network meta-analysis of data on drug discontinuation due to side effects, as well as the findings of classical pair-wise comparisons of each drug versus placebo on the same outcome. Results of the network meta-analysis are reported as mean values with 95% credible intervals, which are analogous to confidence intervals in frequentist statistics. Only results on options considered in the economic analysis are presented. The table shows the probability of each option leading to discontinuation side effects over 8 weeks of treatment, the probability of each option being the ‘best’ among available options in averting discontinuation due to side effects, as well as the hazard ratio of each drug versus placebo in this outcome. In addition to these three parameters examined in the network meta-analysis, the table shows the relative risk of each drug versus placebo for discontinuation due to side effects (mean and 95% confidence intervals), as estimated in the guideline classical, pair-wise meta-analysis. Treatment options have been ranked from ‘best’ to ‘worst’ in terms of their ability to minimise discontinuation due to side effects, according to the results of the network meta-analysis.
Table 68
Pharmacological treatment discontinuation due to side effects: findings of the network meta-analysis and of classical pair-wise comparisons versus placebo.
The results of the network meta-analysis indicated that placebo had the lowest probability of discontinuation due to side effects (mean 5.8% over 8 weeks). Among drugs, sertraline had the lowest probability of leading to discontinuation due to side effects (mean 7.2% over 8 weeks), followed by pregabalin, escitalopram, paroxetine, venlafaxine XL and, finally, duloxetine (mean 17.5% over 8 weeks). The probability of sertraline being the best drug in limiting discontinuation due to side effects reached 61%. All drugs showed a significantly higher hazard of discontinuation compared with placebo, except sertraline. The results of the guideline classical meta-analysis of placebo-controlled trials were consistent overall with the findings of the network meta-analysis: it can be seen that the ranking of relative risks of each drug versus placebo with respect to discontinuation due to side effects (classical, pair-wise meta-analysis) was the same with the ranking of the hazard ratios (network meta-analysis), with the exception of venlafaxine XL. The relative effect of sertraline versus placebo was found to be non-significant in the classical pair-wise meta-analysis, in accordance with the finding in the network meta-analysis. However, it must be noted that data on sertraline were taken from a small number of studies relative to other drugs (two placebo-controlled studies with 706 participants). On the other hand, in contrast to pair-wise comparisons, which failed to demonstrate a significant effect of pregabalin compared with placebo in terms of discontinuation due to side effects, data combined in the network meta-analysis had sufficient strength to demonstrate that pregabalin significantly increases the risk of discontinuation due to side effects compared with placebo.
Table 69 provides the results of the network meta-analysis of data on conditional response (that is, response in people who have not discontinued the drug due to side effects), as well as the findings of classical pair-wise comparisons of each drug versus placebo on non-response. It must be noted that the classical meta-analysis was based on an intention-to-treat (ITT) approach, and therefore considered all trial participants, without excluding those who discontinued due to side effects (exclusion of people discontinuing due to side effects in the network meta-analysis was dictated by the economic model structure). The table shows the probability of conditional response of each option considered in the economic analysis over 8 weeks of treatment, the probability of each option being the ‘best’ in leading to conditional response among available options, as well as the hazard ratio of each drug versus placebo with respect to conditional response. In addition to these three parameters examined in the network meta-analysis, the table shows the relative risk of each drug versus placebo regarding non-response (mean and 95% confidence intervals), as estimated in the guideline classical, pair-wise meta-analysis. Treatment options have been ranked from ‘best’ to ‘worst’ in terms of their ability to achieve conditional response, according to the results of the network meta-analysis.
Table 69
Response to pharmacological treatment: findings of the network meta-analysis and of classical pair-wise comparisons versus placebo.
The results of the network meta-analysis indicated that duloxetine had the highest probability of conditional response (mean 65.1% over 8 weeks), followed by sertraline, venlafaxine XL, pregabalin, escitalopram and paroxetine (mean 51.9% over 8 weeks). Placebo had the lowest probability of conditional response among options assessed (mean 42.8% over 8 weeks). The probability of duloxetine being the best drug in terms of response in people who have not discontinued their drug treatment was approximately 37%. Hazard ratios demonstrated that all drugs were significantly better than placebo in the outcome of conditional response. On the other hand, the relative risks derived from the guideline classical meta-analysis of placebo-controlled trials showed that all drugs significantly reduced the risk of non-response compared with placebo, with the exception of paroxetine; the latter demonstrated a positive effect which, nevertheless, did not reach statistical significance. However, it must be noted that since the data in the classical meta-analyses were based on ITT, cases of discontinuation due to side effects were counted as non-responders. This means that the classical meta-analysis considered both people who discontinued due to side effects, as well as people who did not discontinue due to side effects but did not respond to treatment either, as non-responders. This difference between network and classical meta-analysis may explain the discrepancies observed between the results: for example, duloxetine was shown to have the highest probability of conditional response (that is, the highest probability of response in those not discontinuing due to side effects) in the network meta-analysis, but not the lowest relative risk versus placebo in terms of non-response in the classical meta-analysis. The latter finding may be attributed to the fact that duloxetine is characterised by a high rate of discontinuation due to side effects, which reduces the response rate measured using an ITT approach. The lowest relative risk versus placebo in terms of non-response in the classical meta-analysis was that of sertraline. This is consistent with the fact that sertraline had the lowest probability of discontinuation due to side effects among the drugs considered, and, at the same time, the second highest probability of response in people who did not discontinue treatment due to side effects (that is, of conditional response).
The probability of discontinuation due to intolerable side effects and the probability of conditional response of each treatment option comprised the outcomes of the network meta-analyses that were utilised in the economic model. These data are also provided in Table 71.
Utility data and estimation of quality-adjusted life years
In order to express outcomes in the form of QALYs, the health states of the economic model needed to be linked to appropriate utility scores. Utility scores represent the HRQoL associated with specific health states on a scale from 0 (death) to 1 (perfect health); they are estimated using preference-based measures that capture people’s preferences on the HRQoL experienced in the health states under consideration. The systematic search of the literature identified two studies that reported utility scores for specific health states associated with GAD (Allgulander et al., 2007; Revicki et al., 2008). Details on the studies, their methods and reported utility data are provided in the respective section of the economic model described in Section 6.6.4.
According to NICE guidance regarding the selection of utility scores for use in cost-utility analysis, the measurement of changes in HRQoL should be reported directly from people with the condition examined, and the valuation of health states should be based on public preferences elicited using a choice-based method, such as TTO or SG, in a representative sample of the UK population. NICE recommends the EQ-5D (Brooks, 1996) as the preferred measure of HRQoL in adults for use in cost-utility analysis. When EQ-5D scores are not available or are inappropriate for the condition or effects of treatment, NICE recommends that the valuation methods be fully described and comparable to those used for the EQ-5D (NICE, 2008a).
Available utility data for people with GAD were not generated using EQ-5D. However, both studies included in the respective review used SF-6D for the estimation of utility scores in this population. SF-36 (and its shorter form SF-12) is a validated generic measure of HRQoL. The SF-6D algorithm can generate utility scores for all health states described from SF-36 (Brazier et al., 2002) and SF-12 (Brazier & Roberts, 2004), which have been elicited from a representative sample of the UK general population using SG; thus the valuation method meets NICE criteria.
The utility data reported in Allgulander and colleagues (2006) corresponded to the health states described in the economic model (that is, response, non-response, relapse following response, and no relapse following response); moreover, the definition of response in Allgulander and colleagues (2006) was the same as that used in all RCTs considered in the network meta-analysis that provided data on conditional response for the economic model. In contrast, the utility data reported in Revicki and colleagues (2008) corresponded to the health states of mild, moderate and severe anxiety, which could not be directly linked to the model health states of response, no response, relapse following response and no relapse following response. Therefore, it was decided to use the utility data reported in Allgulander and colleagues (2006) in the economic analysis.
It was assumed that the improvement in utility for people with GAD responding to treatment occurred linearly over the 8 weeks of treatment, starting from the utility value of non-response and reaching the utility value of response. People responding and not relapsing were assumed to experience a linear increase in their utility during the 6 months of maintenance treatment, starting from the utility value of response and reaching the utility value of response and no relapse. In contrast, people relapsing following response were assumed to experience a linear reduction in their utility during maintenance treatment, starting from the utility value of response and reaching the utility value of relapse following response.
Side effects of medication are expected to result in a reduction in utility scores corresponding to GAD-related health states. However, no studies on people with GAD reporting such ‘disutility’ due to side effects were identified in the literature. On the other hand, Revicki and Wood (1998) examined the effect of the presence of side effects associated with antidepressants in the HRQoL of people with depression. According to the study, people with a side effect reported lower utility scores compared with those not experiencing side effects. The observed mean disutility ranged from 0.01 for dry mouth and nausea to 0.12 for nervousness and light-headedness. However, except for light-headedness and dizziness, the reduction in utility caused by side effects did not reach statistical significance.
Clinical evidence on people with GAD suggests that side effects from drugs considered in the economic analysis consist mainly of nausea, insomnia and sexual problems (SSRIs and SNRIs), as well as dizziness, fatigue and headaches (pregabalin). Less common side effects include palpitations, tachycardia and orthostatic hypotension associated with duloxetine; SNRIs may increase blood pressure. Both SSRIs and SNRIs may result in suicidal thinking and self-harming behaviour in a minority of young people. Finally, SSRIs can cause gastrointestinal bleeding, especially if they are administered alongside NSAIDs.
Data on the risk for common, tolerable side effects have not been consistently collected and reported across RCTs included in the guideline systematic review, but available evidence indicates that all drugs are associated with such side effects in a similar degree. Data on less common but more severe side effects were sparser. On the other hand, discontinuation due to intolerable side effects was consistently reported in clinical trials. Development of intolerable side effects is expected to reduce more significantly the HRQoL of people with GAD compared with tolerable side effects.
Based on the above facts, data availability and limitations of available evidence, the economic analysis did consider the reduction in utility caused by intolerable side effects, given that data on discontinuation due to side effects were consistently reported for all drugs and analysed using network meta-analysis. The disutility caused by intolerable side effects was assumed to equal 0.12, which was the highest reduction in utility caused by the presence of side effects reported by people with depression taking antidepressants (Revicki & Wood, 1998). This reduction in utility due to intolerable side effects was assumed to last only 2 weeks, as discontinuation of drug treatment due to intolerable side effects was estimated to occur usually within 2 weeks from initiation of the particular drug. However, the reduction in utility caused by tolerable side effects was not considered in the economic analysis for two reasons: (i) inconsistent reporting of clinical data on tolerable side effects in RCTs might introduce bias in the economic analysis, should such data be included in the economic model, and (ii) available evidence on people with depression indicated that the majority of common side effects of antidepressants do not significantly reduce the HRQoL. Regarding less common and more severe side effects, such as gastrointestinal bleeding and suicidal thinking, these are likely to have a stronger negative impact on the HRQoL, but, given their low frequency, the implications of their omission (in terms of utility losses) are deemed to be less substantial at a study population level. Nevertheless, the lack of full consideration of the impact of side effects on the HRQoL of people with GAD treated with medication is acknowledged as a limitation of the economic analysis.
Cost data
Costs associated with the pharmacological treatment of people with GAD were calculated by combining resource-use estimates with respective national unit costs. Costs consisted of intervention costs and other health and social care costs incurred by people with GAD not responding to treatment or relapsing following response. Intervention costs of pharmacological treatment consisted of drug acquisition costs and GP visit costs. Intervention costs of no pharmacological treatment related to GP visit costs only. All costs were expressed in 2009 prices, uplifted, where necessary, using the HCHS Pay and Prices Index (Curtis, 2009). Discounting of costs was not necessary since the time horizon of the analysis was shorter than 1 year.
Drug acquisition costs were taken from the British National Formulary (BNF) 59 (British Medical Association and the Royal Pharmaceutical Society of Great Britain, March 2010). For each drug the lowest reported price was selected and used in the analysis; where available, costs of generic forms were considered. The average daily dosage of each drug was determined according to optimal clinical practice (the expert opinion of the GDG) and was consistent with the respective average daily dosage reported in the RCTs considered in the economic model. People discontinuing treatment due to intolerable side effects were assumed to have been already prescribed 1 month’s drug supply for their initiated drug, and therefore incurred the initiated drug cost over 4 weeks before switching to second-line treatment. The average daily dosages and acquisition costs as well as the total ingredient costs over 8 weeks of initial treatment and 6 months of maintenance treatment for all drugs are presented in Table 70. The ingredient cost of the second-line drug in each arm of the model was assumed to equal the average ingredient cost of all drugs except the one that was used as first-line treatment in this particular arm.
Table 70
Average daily dosage, acquisition costs and estimated 8-week and 6-month ingredient costs of drugs used in the treatment of people with GAD included in the economic model.
Regarding GP visits, these included one visit at initiation, two visits over the first 8 weeks of treatment, and another visit during maintenance treatment. People who discontinued their first-line treatment due to intolerable side effects were assumed to pay one extra visit to their GP, and then were initiated on second-line drug treatment following the same pattern of GP visits as that estimated for the first-line drug treatment. This pattern of GP visits was also assumed to apply to the cohort of people under no pharmacological treatment.
Costs of managing tolerable side effects were not considered separately in the analysis, partly due to inconsistent reporting of side-effect data in the RCTs included in the guideline systematic review of clinical evidence. Nevertheless, the GDG estimated that the majority of common tolerable side effects, such as nausea, insomnia, sexual problems (associated with SSRIs and SNRIs), dizziness, fatigue and headaches (associated with pregabalin), as well as the less commonly observed suicidal thinking (associated with antidepressants administered to younger people), palpitations and tachycardia (associated with duloxetine), would be discussed during monitoring GP visits which were considered at the estimation of intervention costs relating to initial and maintenance pharmacological treatment. It was the GDG’s view that even if the presence of these common side effects led to extra GP visits and incurred additional costs, these were unlikely to be considerable compared with total intervention costs. Regarding less common side effects, such as hypertension (associated with SNRIs) and gastrointestinal bleeding (associated with SSRIs), these were thought to result in higher management costs at an individual level, but given their low frequency they were deemed to entail smaller economic implications at a study population level. Therefore, although omission of costs associated with management of tolerable side effects is acknowledged as a limitation of the analysis, it is not considered to have substantially affected the economic modelling results.
The extra health and social care costs incurred by people with GAD not responding to treatment or relapsing following response to treatment were estimated based on data reported in the Adult Psychiatric Morbidity in England survey (McManus et al., 2009), supported by the expert opinion of the GDG. Data on resources used by people with GAD (including inpatient care, outpatient services, contacts with GPs, psychiatrists, psychologists, community psychiatric nurses, social workers and services provided by community day care centres) were combined with appropriate national unit costs (Curtis, 2009; DH, 2010) in order to estimate a total weekly cost incurred by people with GAD. The average length of stay for people with GAD receiving inpa-tient care was taken from national hospital episode statistics (NHS, The Information Centre, 2009). Based on the above data, the health and social care cost incurred by people with GAD not responding to treatment or relapse following response was approximately £804 per year or £15 per week. Details on the methods of estimation of this cost are provided in the economic analysis described in Section 6.6.4. People who did not respond to second-line pharmacological treatment and those who did not respond to no pharmacological treatment were assumed to incur this weekly health and social care GAD-related cost for the remaining time horizon of the analysis following no response. People who relapsed following response to treatment were assumed to incur maintenance treatment costs over 3 months and this health and social care GAD-related cost over the remaining 3 months of the 6-month maintenance treatment period that led to relapse.
Costs of treating tolerable side effects were not considered in the economic analysis due to lack of consistency in reporting appropriate side-effect data across all drugs.
Table 71 reports the mean (deterministic) values of all input parameters utilised in the economic model and provides information on the distributions assigned to specific parameters in probabilistic sensitivity analysis.
Data analysis and presentation of the results
Two methods were employed to analyse the input parameter data and present the results of the economic analysis.
First, a deterministic analysis was undertaken, where data are analysed as point estimates; results are presented as mean total costs and QALYs associated with each treatment option are assessed. Relative cost effectiveness between alternative treatment options is estimated using incremental analysis: all options are initially ranked from most to least effective; options that are dominated (they are more expensive and less effective than other options) are excluded from further analysis. Subsequently, ICERs are calculated for all pairs of consecutive options. ICERs express the additional cost per additional unit of benefit associated with one treatment option relative to its comparator. Estimation of such a ratio allows consideration of whether the additional benefit is worth the additional cost when choosing one treatment option over another.
After excluding cases of extended dominance (which occur when an intervention is less effective and more costly than a linear combination of two other options), ICERs are recalculated. The treatment option with the highest ICER below the cost-effectiveness threshold is the most cost-effective option.
One-way sensitivity analyses explored the following:
- The impact of the uncertainty characterising the monthly health and social care cost incurred by people with GAD not responding to treatment or relapsing following response on the results of the deterministic analysis. Since the estimation of this cost was based on a number of assumptions and data extrapolations, a scenario of a 70% change in this cost was tested to investigate whether the conclusions of the analysis would change.
- The impact of an increase in the extra GP visits following discontinuation of the first-line treatment due to intolerable side effects. The impact of three extra GP visits on the results was tested (in base-case analysis one extra visit was assumed).
- The uncertainty around the probability of response achieved by second-line drug treatment. In the base-case analysis, this probability was calculated as the average probability of conditional response of all drugs considered in the analysis except the one that was used as first-line treatment in each particular decision node of the model. However, it is possible that responsiveness to a drug used as second-line is lower than that observed when the drug is used as first-line. Therefore a scenario in which the responsiveness of the second-line drug was reduced by 15% was tested.
In addition to deterministic analysis, a probabilistic analysis was also conducted. In this case, all model input parameters were assigned probability distributions (rather than being expressed as point estimates), to reflect the uncertainty characterising the available clinical and cost data. Subsequently, 10,000 iterations were performed, each drawing random values out of the distributions fitted onto the model input parameters. This exercise provided more accurate estimates of mean costs and benefits for each intervention assessed (averaging results from the 10,000 iterations), by capturing the non-linearity characterising the economic model structure (Briggs et al., 2006).
The distributions of the probability of discontinuation due to intolerable side effects and the probability of conditional response for each drug, which were obtained using mixed treatment comparison techniques, were defined directly from values recorded in each of the 10,000 iterations performed in WinBUGS, as described in Appendix 13.
The probability of relapse for no pharmacological treatment was given a beta distribution. Beta distributions were also assigned to utility values, using the method of moments. The relative risk of relapse of drug treatment versus no treatment was assigned a log-normal distribution. The estimation of distribution ranges was based on available data in the guideline meta-analysis (relapse data) and the published sources of evidence (utility data). Costs (with the exception of drug acquisition costs) were assigned a gamma distribution; in order to define the distribution, a 30% standard error around the mean costs was assumed.
Table 71 provides details on the types of distributions assigned to each input parameter and the methods employed to define their range.
Results of probabilistic analysis are presented in the form of CEACs, which demonstrate the probability of each treatment option being the most cost effective among the strategies assessed at different levels of willingness-to-pay per unit of effectiveness (that is, at different cost-effectiveness thresholds the decision maker may set). In addition, the cost-effectiveness acceptability frontier is provided alongside CEACs, showing which treatment option among those examined offers the highest average net monetary benefit (NMB) at each level of willingness-to-pay (Fenwick et al., 2001). The NMB of a treatment option at different levels of willingness-to-pay is defined by the following formula:
Where E is effectiveness (number of QALYs), C is the costs associated with the treatment, and λ is the level of the willingness-to-pay per unit of effectiveness.
8.8.4. Economic modelling results
Results of deterministic analysis
According to deterministic analysis, sertraline was the most cost-effective option among those assessed because it produced the highest number of QALYs and was associated with the lowest costs (dominant option). ‘No pharmacological treatment’ was dominated by all drugs except pregabalin; the latter was more effective than placebo at an extra cost of £3,768 per QALY.
Table 72 provides mean costs and QALYs for every treatment option assessed in the economic analysis. The seven options have been ranked from the most to the least effective in terms of number of QALYs gained. It can be seen that sertraline is associated with lowest costs and highest benefits (QALYs) and consequently dominates all other drugs as well as no treatment. Figure 7 provides the cost-effectiveness plane showing the incremental costs and QALYs of all drugs versus paroxetine. It can be seen that sertraline is in the southeast quadrant and has the highest number of QALYs and the lowest costs relative to all other drugs assessed (no treatment is not shown in this graph).
Table 72
Mean costs and QALYs for each pharmacological treatment option for people with GAD assessed in the economic analysis - results per 1,000 people.

Figure 7
Cost-effectiveness plane of all drugs assessed in the economic analysis plotted against paroxetine – incremental costs and QALYs per 1,000 people with GAD.
Results were robust under all scenarios examined in one-way sensitivity analyses: sertraline remained dominant when the health and social care costs incurred by people with GAD not responding to treatment or relapsing following response increased by 70%, when three extra GP visits (instead of one) were assumed in the case of discontinuation of first-line treatment, and when conditional response for the second-line drug was reduced by 15%. Sertraline dominated all options except no treatment when the health and social costs incurred by people with GAD not responding to treatment or relapsing following response decreased by 70%. In this case, the ICER of sertraline versus no treatment was £946 per QALY gained, which is well below the lower cost-effectiveness threshold of £20,000 per QALY set by NICE (NICE, 2008b).
Results of probabilistic analysis
Results of probabilistic analysis were very similar to those of deterministic analysis: sertraline dominated all other treatment options when mean costs and QALYs derived from 10,000 iterations were estimated. Sertraline had also the highest probability of being the most cost-effective treatment option, at any level of willingness-to-pay per additional QALY gained. At the lower NICE cost-effectiveness threshold of £20,000/QALY (NICE, 2008b) the probability of sertraline being cost effective was 0.70, whereas venlafaxine XL, which was the second most cost-effective option, had a probability of only 0.13. The cost-effectiveness acceptability frontier coincided with the CEAC for sertraline, because sertraline produced the highest average net benefit at any level of willingness to pay.
Figure 8 shows the CEACs generated for each pharmacological treatment option assessed in the economic model. Table 73 shows the probability of each treatment option being cost effective at various cost-effectiveness thresholds, that is, at various levels of willingness-to-pay per QALY gained.
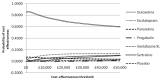
Figure 8
CEACs of all pharmacological treatment options for people with GAD assessed in the economic analysis.
Table 73
Probability of each pharmacological treatment option being cost effective at various levels of willingness-to-pay per QALY gained (WTP).
Discussion – limitations of the analysis
The results of the economic analysis suggest that sertraline is likely to be the most cost-effective pharmacological treatment for people with GAD. Sertraline dominated all other treatment options and had the highest probability of being the most cost-effective option at any level of willingness-to-pay per QALY gained, which reached 0.70 at the lower NICE cost-effectiveness threshold of £20,000 per QALY. The cost effectiveness of sertraline is attributed to a number of factors: sertraline had the lowest average probability of discontinuation due to intolerable side effects among all drugs assessed, and the second best probability of conditional response; in addition, sertra-line had the lowest acquisition cost among all drugs, as it is available in generic form. It must be noted that sertraline is currently not licensed for the treatment of people with GAD.
Clinical data on discontinuation due to intolerable side effects as well as response for those who did not discontinue due to intolerable side effects (conditional response) were synthesised using network meta-analytic techniques. Such methods enable evidence synthesis from both direct and indirect comparisons between treatments, and allow simultaneous inference on all treatments examined in pair-wise trial comparisons while respecting randomisation (Lu & Ades, 2004; Caldwell et al., 2005).
One limitation of the economic analysis was that data on conditional response for first-line drug treatment were also used to estimate the probability of response for second-line drug treatment, which, in every decision node of the model, was calculated as the average probability of conditional response of all drugs except the one that was used as first-line treatment in this particular node. This assumption was necessary in order to populate the model due to lack of response data on people with GAD switched to a second-line drug. However, it is possible that responsiveness to a drug used as second-line is lower than that observed when the drug is used as first-line. Nevertheless, one-way sensitivity analysis, in which the responsiveness of the second-line drug was assumed to be reduced by 15%, demonstrated that the results of the economic analysis were robust to this assumption.
Another limitation of the economic analysis is that it did not take into account the reduction in HRQoL and the costs associated with the management of tolerable side effects, which do not lead to treatment discontinuation. Consideration of these factors was not possible as there was no consistent reporting of side effects across trials included in the systematic review. Moreover, there is limited evidence on the reduction in HRQoL caused by the presence of side effects from drugs considered in the analysis and no such evidence in people with GAD. Regarding the reduction in HRQoL associated with the presence of side effects from antidepressants, available evidence has demonstrated that this is largely insignificant in people with depression (Revicki & Wood, 1998). Regarding costs associated with the management of tolerable side effects, these were considered to be non-substantial, as most side effects are expected to be managed during GP monitoring visits, which have already been considered at the estimation of intervention costs of pharmacological treatment. It should be noted that the economic analysis did consider the impact of the development of intolerable side effects, which lead to treatment discontinuation, on costs and HRQoL associated with pharmacological treatment of people with GAD.
The economic analysis revealed that drug acquisition costs may be an important factor in determining the relative cost effectiveness of pharmacological treatments for GAD: sertraline, which was found to be the most cost-effective option resulting also in lowest total costs, has currently the lowest acquisition cost, as it is available in generic form. Paroxetine, which is also available in generic form and has the second lowest acquisition cost among the drugs assessed, was ranked the second least costly drug and fourth most cost-effective option at a cost-effectiveness threshold of £20,000 per QALY (with probability of being cost effective about 0.05), despite the fact that it had one of the highest probabilities of discontinuation due to side effects and the lowest probability of conditional response among drugs. Venlafaxine XL, which has the lowest acquisition cost among patented drugs included in the analysis, was ranked the third least costly drug and second most cost-effective option at a cost-effectiveness threshold of £20,000 per QALY (with probability of being cost effective about 0.13). Based on these findings, it is expected that the relative cost effectiveness of drugs for the treatment of GAD is likely to change in the future, as eventually drugs will become available in generic form, resulting in a considerable reduction in their acquisition costs.
8.8.5. Overall conclusions from economic evidence
Existing economic evidence is limited in the area of pharmacological treatment for people with GAD. Of the five studies meeting the inclusion criteria, one was considered as non-applicable to the UK setting (Heuzenroeder et al., 2004) and was therefore not considered at formulation of recommendations. One study conducted in Canada (Iskedjian et al., 2008) concluded that escitalopram was more cost effective than paroxetine. Another modelling study conducted in Spain concluded that paroxe-tine might be more cost effective than venlafaxine XL. Both studies are partially applicable to the UK context. Two other modelling studies that were conducted in the UK and were thus directly applicable to the guideline development process concluded that escitalopram was more cost effective than paroxetine (Jørgensen et al., 2006) and that venlafaxine XL was more cost effective than diazepam (Guest et al., 2005). All four studies, considered at the development of guideline recommendations, were funded by the pharmaceutical industry, which may have introduced bias in the analyses. Overall, the choice of drugs evaluated in previously published economic literature is very limited. Therefore, it is difficult to draw conclusions on the cost effectiveness of particular pharmacological interventions for the treatment of people with GAD based on existing evidence.
The economic analysis undertaken for this guideline concluded that sertraline was the most cost-effective drug in the treatment of people with GAD, as it was associated with the highest number of QALYs and lowest total costs among all treatments assessed, including no treatment. Sertraline had the highest probability of being cost effective at any cost-effectiveness threshold, which reached 0.70 at the lower NICE cost-effectiveness threshold of £20,000/QALY.
8.9. FROM EVIDENCE TO RECOMMENDATIONS
Acute treatment
Short-term efficacy studies suggested that a range of pharmacological interventions were associated with a small to moderate benefit in reducing anxiety symptoms and reducing the risk of non-response and non-remission. Head-to-head studies were limited in number but suggested little difference between treatments. There was also consistent evidence of a higher probability of experiencing side effects for people receiving pharmacological treatment and a greater risk of discontinuation due to these side effects. In addition, it was noted that benzodiazepines appeared to be associated with risk of dependence therefore did not appear to be an appropriate medication for routine use for people with GAD who often require long-term treatment. Clinical data on quetiapine, although collected to increase inference in the network meta-analysis undertaken to support the guideline economic analysis, were not assessed, as this is the subject of a future NICE Technology Appraisal.
The GDG weighed up the evidence for benefit and harm for each of the acute treatments and identified interventions with sufficient clinical effectiveness data to be considered for further cost-effectiveness analysis. The following drugs were considered to have sufficient clinical effectiveness data and an acceptable harm-to-benefit ratio, and were thus considered as potentially suitable first-line pharmacological treatments: esci-talopram, duloxetine, paroxetine, pregabalin, sertraline and venlafaxine XL. It must be noted that sertraline was included in the economic analysis, despite the fact that it is not licensed for the treatment of people with GAD, because available evidence suggested that this is an effective drug in the treatment of GAD, with an acceptable risk-to-benefit ratio. Sertraline is widely used in the UK for the treatment of depression and mixed depression and anxiety; the GDG acknowledged that it is likely to be less commonly used in the treatment of GAD, but that this is probably because people presenting with anxiety in primary care are not often diagnosed with GAD.
Several drugs that were assessed in the guideline systematic review and meta-analysis were not considered in the economic analysis. Lorazepam, alprazolam and diazepam were excluded from further consideration because of the well documented withdrawal syndrome associated with them. The GDG considered it would not be appropriate to recommend these drugs as first-line pharmacological treatments for GAD as it is a chronic disorder often requiring long-term treatment. Hydroxyzine and buspirone did not have sufficient evidence of clinical effectiveness as both were found to have no statistically significant difference from placebo in terms of non-response. Finally, citalopram and imipramine were not included in the economic model because for both drugs there was only one small trial, which was not sufficient to draw conclusions on their clinical effectiveness.
The network meta-analysis that was undertaken to inform the guideline economic analysis demonstrated that sertraline had the lowest probability of discontinuation due to intolerable side effects, followed by pregabalin, escitalopram, paroxetine, venlafaxine XL and duloxetine. Duloxetine had the highest probability of conditional response (that is, response in people not discontinuing pharmacological treatment due to intolerable side effects), followed by sertraline, venlafaxine XL, pregabalin, esci-talopram and paroxetine. Network meta-analysis demonstrated that sertraline was the best drug in limiting discontinuation due to side effects, and the second best drug (following duloxetine) in achieving conditional response (that is, based on a completer analysis). Although duloxetine was the drug with the highest probability of conditional response, it was also associated with the highest risk of discontinuation due to side effects, indicating that its overall probability of response (in an ITT rather than a completer analysis) will be lower than the respective probability for sertraline. This is reinforced by a review of the relative risks of non-response of all drugs versus placebo in the classical meta-analysis, where an ITT approach was adopted, which shows that the relative risk of non-response versus placebo was lowest for sertraline, indicating that sertraline is likely to have the highest probability of overall response.
The guideline economic analysis demonstrated that sertraline dominated all other treatment options (that is, it was associated with lowest total costs and higher number of QALYs) and had the highest probability of being cost effective, which reached 0.70 at a willingness-to-pay of £20,000/QALY. All drugs were shown to be more effective and less costly than placebo, with the exception of pregabalin, which was more effective at an additional cost of roughly £3,800 per QALY, which is well below the NICE lower cost-effectiveness threshold of £20,000 per QALY (NICE, 2008b).
However, given the consistent evidence of a greater risk of side effects and discontinuation from treatment compared with placebo the GDG concluded that pharmacological interventions should only be routinely offered to people who have not benefited from low- or high-intensity psychological interventions.
Relapse prevention
There was a lack of data for most medications with only one trial each on paroxetine, escitalopram, pregabalin and duloxetine. In all of the four studies, continuing the treatment was more effective than being randomised to placebo and was not associated with greater risk of side effects.
Augmentation
There were limited data on the effectiveness of antipsychotics (olanzapine, risperidone and ziprasidone) as an augmentation treatment. There was no evidence to conclude that antipsychotics were effective as an augmentation treatment for reducing anxiety. In addition, there was evidence of an increase in discontinuation due to adverse events. The GDG therefore concluded, given the current evidence, that the benefits did not appear to justify the harms associated with antipsychotic augmentation. Therefore the GDG judged that such treatment should not be routinely used and should only be provided in specialist settings.
In addition, it was the judgment of the GDG that antipsychotics should not be offered in primary care as stand-alone or augmentation treatment, as this would require specialist expertise.
Side effects
There was consistent evidence that SSRIs were associated with an increased risk of gastrointestinal bleeding particularly in elderly people. Although such events were relatively rare, the GDG considered this was still important to take into account when considering prescribing an SSRI.
In addition, there was evidence that antidepressant use was associated with an increased probability of suicidal behaviour in participants under 25 years of age. The GDG also took into account related advice on risk of suicide from both the Medicines and Healthcare products Regulatory Agency (MHRA, 2004) and the NICE guideline on depression (NICE, 2009a) which suggested a cut-off of under 30 years of age. Therefore the GDG judged that it would be important for prescribers to inform this group of the potential risk and to monitor the risk early on in treatment.
8.10. RECOMMENDATIONS
Drug treatment
- 8.10.1.1.
For people with GAD and marked functional impairment, or those whose symptoms have not responded adequately to step 2 interventions:
- Offer either
- –
an individual high-intensity psychological intervention (see 7.11.1.2–7.11.1.6) or
- –
drug treatment (see 8.10.1.2–8.10.1.12).
- Provide verbal and written information on the likely benefits and disadvantages of each mode of treatment, including the tendency of drug treatments to be associated with side effects and withdrawal syndromes.
- Base the choice of treatment on the person’s preference as there is no evidence that either mode of treatment (individual high-intensity psychological intervention or drug treatment) is better. 16
- 8.10.1.2.
If a person with GAD chooses drug treatment, offer a selective serotonin reuptake inhibitor (SSRI). Consider offering sertraline first because it is the most cost-effective drug, but note that at the time of publication (January 2011)17 sertraline did not have UK marketing authorisation for this indication. Informed consent should be obtained and documented. Monitor the person carefully for adverse reactions.
- 8.10.1.3.
If sertraline is ineffective, offer an alternative SSRI or a serotonin–noradren-aline reuptake inhibitor (SNRI), taking into account the following factors:
- tendency to produce a withdrawal syndrome (especially with paroxe-tine and venlafaxine)
- the side-effect profile and the potential for drug interactions
- the risk of suicide and likelihood of toxicity in overdose (especially with venlafaxine)
- the person’s prior experience of treatment with individual drugs (particularly adherence, effectiveness, side effects, experience of withdrawal syndrome and the person’s preference).
- 8.10.1.4.
If the person cannot tolerate SSRIs or SNRIs, consider offering pregabalin.
- 8.10.1.5.
Do not offer a benzodiazepine for the treatment of GAD in primary or secondary care except as a short-term measure during crises. Follow the advice in the ‘British national formulary’ on the use of a benzodiazepine in this context.
- 8.10.1.6.
Do not offer an antipsychotic for the treatment of GAD in primary care.
- 8.10.1.7.
Before prescribing any medication, discuss the treatment options and any concerns the person with GAD has about taking medication. Explain fully the reasons for prescribing and provide written and verbal information on:
- the likely benefits of different treatments
- the different propensities of each drug for side effects, withdrawal syndromes and drug interactions
- the risk of activation with SSRIs and SNRIs, with symptoms such as increased anxiety, agitation and problems sleeping
- the gradual development, over 1 week or more, of the full anxiolytic effect
- the importance of taking medication as prescribed and the need to continue treatment after remission to avoid relapse.
- 8.10.1.8.
Take into account the increased risk of bleeding associated with SSRIs, particularly for older people or people taking other drugs that can damage the gastrointestinal mucosa or interfere with clotting (for example, NSAIDS or aspirin). Consider prescribing a gastroprotective drug in these circumstances.
- 8.10.1.9.
For people aged under 30 who are offered an SSRI or SNRI:
- warn them that these drugs are associated with an increased risk of suicidal thinking and self-harm in a minority of people under 30 and
- see them within 1 week of first prescribing and
- monitor the risk of suicidal thinking and self-harm weekly for the first month.
- 8.10.1.10.
For people who develop side effects soon after starting drug treatment, provide information and consider one of the following strategies:
- monitoring the person’s symptoms closely (if the side effects are mild and acceptable to the person) or
- reducing the dose of the drug or
- stopping the drug and, according to the person’s preference, offering either
- –
an alternative drug (see 8.10.1.13–8.10.1.4) or
- –
a high-intensity psychological intervention (see 7.11.1.2–7.11.1.6).
- 8.10.1.11.
Review the effectiveness and side effects of the drug every 2–4 weeks during the first 3 months of treatment and every 3 months thereafter.
- 8.10.1.12.
If the drug is effective, advise the person to continue taking it for at least a year as the likelihood of relapse is high.
Inadequate response
- 8.10.1.13.
If a person’s GAD has not responded to drug treatment, offer either a high-intensity psychological intervention (see 7.11.1.2–7.11.1.6) or an alternative drug treatment (see 8.10.1.13–8.10.1.4)).
- 8.10.1.14.
If a person’s GAD has partially responded to drug treatment, consider offering a high-intensity psychological intervention in addition to drug treatment.
8.10.2. Research recommendations
- 8.10.2.1.
A comparison of the clinical and cost effectiveness of sertraline and CBT in people with GAD that has not responded to guided self-help and psychoeducation
What is the relative effectiveness of sertraline compared with CBT in people with GAD that has not responded to guided self-help and psychoeducation in a stepped-care model?
This question should be addressed using a randomised controlled design in which people with GAD that has not responded to step 2 interventions are allocated openly to treatment with sertraline, CBT or waiting-list control for 12–16 weeks. The control group is important to demonstrate that the two active treatments produce effects greater than those of natural remission. The period of waiting-list control is the standard length of CBT treatment for GAD and is also commonly the length of time that it would take for specialist CBT to become available in routine practice. After 12–16 weeks all participants should receive further treatment chosen in collaboration with their treating clinicians.
The outcomes chosen at 12–16 weeks should include both observer- and participant-rated measures of clinical symptoms and functioning specific to GAD, and of quality of life. An economic analysis should also be carried out alongside the trial. The trial needs to be large enough to determine the presence or absence of clinically important effects and of any differences in costs between the treatment options using a non-inferiority design. Mediators and moderators of response should be investigated. Follow-up assessments should continue over the next 2 years to ascertain whether short-term benefits are maintained and, in particular, whether CBT produces a better long-term outcome.
Why is this important?
Both sertraline and CBT are efficacious in the treatment of GAD but their relative efficacy has not been compared. In a stepped-care model both CBT and sertraline are treatment options if step 2 interventions (guided self-help and/or psychoeducation) have not resulted in a satisfactory clinical response. At present, however, there are no randomised trial data to help prioritise next-step treatments and no information on how individuals with GAD may be matched to particular therapies. Clarification of the relative short- and longer-term benefits of sertraline and CBT would be helpful in guiding treatment.
8.11. OTHER INTERVENTIONS
8.11.1. Introduction
There are a variety of herbal interventions that have been considered as possible treatments for GAD; these include chamomile, ginkgo biloba, combined plant extracts, valerian extract, galphimia glauca, lavender and passion flower. Chamomile is a common name for several daisy-like plants, which are best known for their ability to be made into a tea. It is not licensed as a medicine in the UK but can be bought ‘over the counter’ from health food shops, herbalists, supermarkets and community pharmacies. Many different branded preparations are available (Mann & Staba, 1986). Ginkgo biloba is one of the oldest living tree species and has been used in the past to treat circulatory disorders and to enhance memory. Similarly, gingko biloba is not licensed in the UK but can be bought ‘over the counter’. Moreover, various preparations are available such as capsules, tablets, liquid extracts and dried leaves for teas (Johne & Roots, 2005). Combined plant extracts (that is, Sympathyl) consists of hawthorn berry extract, California poppy extract and magnesium. This particular combination of plant extracts is not licensed as a medicine in the UK but can be bought online (Hanus et al., 2004). Valerian is an extract of the roots of the Valeriana officinalis plant. Many different branded preparations are available and it is most commonly found in capsule form, but can also be consumed as a tea. Valerian is used for insomnia and other disorders as an alternative to benzodiazepines. Oral forms are available in both standardised and unstandardised forms. However, standardised products may be preferable considering the wide variation of chemicals in the dried root. Standardisation is a percentage of valerenic or valeric acid (Johne & Roots, 2005). Galphimia glauca is an extract from the Thryallis shrub and, again, is available in various preparations but is not licensed as a medicine in the UK. This herb is not widely available but can be bought online (Herrera-Arellano, 2007). There are a number of different species of lavender that are available in several forms, such as drops, capsules and oils among others. Similar to the other herbal remedies, it is not licensed in the UK but can be bought ‘over the counter’ (Woelk & Schalke, 2010). Passion flower is derived from the family of plants called Passifloraceae and has been used for medicinal purposes for many years including the treatment of anxiety-related disorders. It is not as widely available as other herbal remedies but may be bought online. Similar to the other herbal interventions, this remedy is not licensed in the UK (Akhondzadeh et al., 2001).
Acupuncture has received much public interest and has widely been applied in different medical conditions including GAD. Generally acupuncture is regarded as having a more acceptable safety profile than conventional medications for GAD and therefore the literature has been reviewed on the efficacy and safety of acupuncture as an alternative or combinational treatment for this indication. Guizhen and colleagues (1998) state that, according to traditional Chinese medicine, a causative factor for disease (such as anxiety disorders) is an excess or decline in yin or yang, which can lead to an imbalance and disorders of the ‘qi’ (energy flow) and blood, leading to dysfunction of internal organs. By correctly selecting and needling acupuncture points it is argued there can be a removal of obstructions of qi and blood, which normalises the yin-yang balance and effectively cures the disease. Zhang and colleagues (2003) suggest that because the characteristic symptoms of ‘anxiety neurosis’ include anxiety, restlessness and constant fear, people should receive treatment designed to regulate the heart qi, although in practice acupuncture points can vary according to different treatment approaches.
8.11.2. Databases searched and inclusion/exclusion criteria
Information about the databases searched and the inclusion/exclusion criteria used for this section of the guideline can be found in Table 74 (further information about the search for health economic evidence can be found in Section 3.6).
Table 74
Databases searched and inclusion/exclusion criteria for clinical evidence.
8.11.3. Studies considered
The review team conducted a new systematic search for RCTs that assessed the benefits and harms of herbal interventions for the treatment of people with GAD as defined in DSM-III-R or DSM-IV.
A total of 3,397 references were identified by the electronic search relating to clinical evidence. Of these references, 3,353 were excluded at the screening stage on the basis of reading the title and/or abstract. The remaining 44 references were assessed for eligibility on the basis of the full text and 30 studies were excluded from the analysis. Five studies did not meet the criteria for GAD, nine studies did not provide an acceptable diagnosis of GAD, nine studies did not use a relevant intervention, five studies did not have a suitable study design, one study had fewer than 10 participants, and one other was not written in the English language. Further information about both included and excluded studies can be found in Appendix 15d.
Fourteen trials met the eligibility criteria set by the GDG, providing data on 1,627 participants. All were published in peer-reviewed journals between 1998 and 2010.
8.11.4. Herbal interventions versus placebo
Studies considered
There were a total of four trials comparing various herbal interventions with placebo. These were all small- to medium-sized trials, all of which were high quality. For two of the studies funding was provided by drug company sponsorship and one other from a national grant. One study failed to declare any funding. These trials could not be meta-analysed and therefore they are narratively reviewed below.
A summary of study characteristics can be found in Table 75 with full details in Appendix 15d, which also includes details of excluded studies. An evidence summary is provided in Table 76.
Table 75
Study information table for trials comparing herbal interventions with placebo.
Table 76
Evidence summary table for trials of herbal interventions versus placebo.
Narrative review of herbal interventions versus placebo
AMSTERDAM2009 conducted a randomised, double-blind efficacy trial in an outpatient clinic in the US comparing chamomile (n = 28) with placebo (n = 29) in participants with GAD. Participants met the DSM-IV diagnostic criteria for GAD and had a HAM-A score of greater than nine. Participants in the treatment group received one to five 220 mg capsules daily depending on tolerability levels. The placebo group received up to five capsules containing lactose monohydrate per day depending on their tolerability levels. Both treatment courses lasted for 8 weeks. Based on the evidence of this study, there is a moderate effect for chamomile over placebo in the reduction of clinician-rated anxiety scores. However, as this result has wide confidence intervals that just include the line of non-significance it should be interpreted with caution. This study also examined the difference in response rates as measured by a 50% reduction in HAM-A scores between the two groups. No statistically significant differences between the two groups were found. However, there was a 29% reduction in the level of non-response in favour of chamomile. With regards to discontinuation due to adverse events, there was no difference between the groups, suggesting that neither group was more likely to discontinue due to adverse events. Due to the limited evidence, it is difficult to draw any clear conclusions regarding the relative efficacy of chamomile to placebo.
WOELK2007 conducted a double-blind RCT in multiple outpatient centres in Germany, evaluating the therapeutic efficacy of gingko biloba (n = 70) versus placebo (n = 37) in participants with GAD. Some participants met the DSM-III-R diagnostic criteria for GAD (n = 82) and others met the diagnostic criteria for adjustment disorder with anxious mood by DSM-III-R (n = 25). Participants in the active treatment group received either a mean dose of 240 mg (n = 36) or a mean dose of 480 mg (n = 34) over 4 weeks. The placebo group took two film-coated drugs per day that were of the same appearance as the gingko biloba pills. Based on this limited evidence, there was a statistically significant moderate effect in favour of gingko biloba in the reduction of clinician-rated anxiety scores. For non-response, which was measured by a 50% reduction in HAM-A scores, there was a 25% reduction in non-response, which was statistically significant suggesting that those in the active treatment group were more likely to respond than those in the placebo group. In contrast, there were no significant differences in relation to non-remission between the two conditions. Finally, there were no significant differences in relation to dropout due to any reason. However, these results should be interpreted with caution as they are based on one medium-scale study, and given the wide confidence intervals it is difficult to make any firm conclusions from this evidence about the relative efficacy of ginkgo biloba to placebo.
HANUS2004 conducted a double-blind RCT in multiple outpatient centres in Paris, evaluating the therapeutic efficacy of combined plant extracts (n = 130) in comparison with placebo (n = 134) in participants with GAD. Participants met the DSM-IV diagnostic criteria for GAD and had a HAM-A score of between 16 and 28. Participants in the active treatment group received a mean dose of 375 mg (two tablets per day) of combined plant extracts (that is, Crataegus oxyacantha, Eschscholzia californica and magnesium) over a period of 3 months. The placebo group were given an indistinguishable tablet that was made from the same ingredients as the study drug except for the active ingredients. Firstly, in relation to HAM-A scores, there was a statistically significant small effect between treatments in favour of the combined plant extracts. Secondly, in relation to non-response (again measured as a 50% reduction in HAM-A scores), there was a 20% reduction in non-response for those taking the active treatment, which was statistically significant. Finally, there was no statistically significant difference between treatments in relation to dropout due to adverse events. Once more, firm conclusions are subject to cautious interpretation due to the limited evidence available and the small sample size.
ANDREATINI2002 conducted a double-blind RCT in Brazil, evaluating the therapeutic efficacy of valerian extract (n = 12) in comparison with placebo (n = 12) in participants with GAD. All participants met the DSM-III-R diagnostic criteria for GAD. Participants in the active treatment group received a mean dosage of 81.3 mg per day of valerian extract over 4 weeks. The placebo group were given identical capsules, which were administered three times per day. Firstly, in relation to HAM-A scores, there was no statistically significant differences between valerian extract versus placebo. There was no data reported for either non-response or non-remission. Finally, there was no statistically significant difference between treatments in relation to dropout due to any reason. Again, conclusions are subject to cautious interpretation because of the limited evidence available.
8.11.5. Herbal interventions versus benzodiazepines
Studies considered
There were a total of four trials comparing various herbal interventions with benzodiazepines, including lorazepam, diazepam and oxazepam. These trials were all small to medium sized and of high quality. One study was funded by drug company sponsorship and the other three studies failed to declare any funding. These trials could not be meta-analysed therefore they are narratively reviewed below. A summary of study characteristics can be found in Table 77 with full details in Appendix 15d, which also includes details of excluded studies. An evidence summary is provided in Table 78.
Table 77
Study information table for trials comparing herbal interventions with benzodiazepines.
Table 78
Evidence summary table for trials of herbal interventions versus benzodiazepines.
Narrative review of herbal interventions versus benzodiazepines
Only one study (HERRERA-ARELLANO2007) examined the effectiveness of galphimia glauca (n = 72) in comparison with lorazepam (n = 80) for treating the symptoms of GAD. This was a medium-scale, high-quality RCT in an outpatient setting in Mexico. With regard to the comparative beneficial effects of these two treatments in reducing clinician-rated anxiety scores, there was no statistically significant difference at post-treatment. Also, there was no statistically significant difference in dropout for any reason between the two groups. However, there was a statistically significant difference in favour of the herbal intervention with regard to dropout due to adverse events, with only 7% dropping out due to adverse events in the herbal intervention compared with 20% in the lorazepam group. However, the results as a whole should be interpreted with caution due to the lack of placebo group, wide confidence intervals, and lack of statistical significance.
WOELK2010 conducted a double-blind RCT in multiple outpatient centres in Germany, comparing lavender capsules (n = 40) with lorazepam treatment (n = 37) in participants with GAD as diagnosed by DSM-IV criteria. Participants in the herbal treatment group received one capsule (80 mg) of silexan (an oil-produced form of lavender) and one capsule of lorazepam placebo. Participants in the drug condition received one capsule (0.5mg) of lorazepam and one capsule of silexan placebo. Both treatment courses lasted 6 weeks. In terms of reducing clinician-rated anxiety scores, there was a non-significant difference between the two treatments.
In addition, there was a 20% reduction in the risk of non-response in favour of lavender, however, this was not statistically significant. Moreover, there was an 18% reduction in the risk of non-remission in favour of lavender, which again was not statistically significant. Finally, there were no statistically significant differences between treatments in the risk of dropout for any reason. Due to the limited evidence, it is difficult to come to any firm conclusions about the relative efficacy of these two treatments.
ANDREATINI2002 is a double-blind RCT in Brazil, comparing diazepam (n = 12) with valerian extract (n = 12) in participants with GAD as diagnosed by DSM-III-R criteria. Participants in the active treatment group received a mean dosage of 81.3 mg per day of valerian extract over 4 weeks. Participants in the diazepam condition received a dosage of 6.5 mg per day in capsule form. The capsules were administered three times a day with the lowest dose consisting of two placebo pills and one active capsule based on response. In terms of reducing clinician-rated anxiety scores, there was a small but statistically insignificant effect in favour of diazepam treatment. There was no data available for either response or remission. Finally, there was no statistically significant difference between the two conditions on the outcome of dropout for any reason. It is difficult to come to any clear conclusions about the relative efficacy of these two treatments due to the small sample size, lack of statistical significance and large confidence intervals.
Only one study examined the effectiveness of passion flower extract (n = 18) versus oxazepam (n = 18) for the treatment of GAD (AKHONDZADEH 2001A). The study consisted of a double-blind RCT conducted in an outpatient setting in Iran. Both passion flower extract and oxazepam were found to be effective in reducing clinician-rated anxiety scores from baseline severity. In both groups, post-hoc comparisons of the baseline HAM-A scores at post-treatment revealed a significant reduction from baseline (p < .001). The differences between the two treatments were significant at day four (t = 2.84, df = 30, p = .008), however, after the fourth day the differences were no longer significant. Moreover, significantly more problems relating to impairment of job performance were encountered with people taking oxazepam (p = .049). However, there was no significant differences between the two treatments in terms of total side-effect profile (p = .83). These results are based on one small-scale study and thus it is difficult to make any firm conclusions from this evidence.
8.11.6. Acupuncture
Narrative review of acupuncture
There were no studies concerning people with GAD who had received a diagnosis that met the eligibility criteria of the GDG. However, this partly reflected the fact that all identified studies were conducted in China and used the Chinese Classification of Mental Disorders (CCMD) criteria.
Zhiling and colleagues (2006) conducted a RCT in China, comparing acupuncture treatment (n = 35) with a medication control group (n = 30) in participants with GAD. Participants met the CCMD-3 criteria and had Self-rating Anxiety Scale (SAS) scores of 50 or more. Acupuncture treatment (which consisted of six acupuncture points) was given once daily. The control group were administered 0.5 to 2 mg of lorazepam (two or three times a day) with 20 mg of oryzanol, which is a mixture of plant chemicals (three times a day), or 10 to 20 mg of propanolol. Both treatment courses lasted for 30 days. The therapeutic effects between the groups looked at a measure of remission (disappearance of symptoms with stable emotions). No statistically significant difference between groups was found (RR = 0.90; CI, 0.65 to 1.24). Of participants in the treatment group, 65.7% did not achieve remission compared with 73.3% in the control group. For response (apparent improvement of symptoms with occasional anxious state) no statistical difference was found (RR = 0.90; CI, 0.59 to 1.38). Of participants in the treatment group, 54.3% did not respond compared to 60% in the control group.
Yuan and colleagues (2007) conducted a quasi-randomised trial, also in China, comparing the therapeutic efficacy of needling therapy with Western medication and a combination treatment. Participants were diagnosed with GAD using the CCMD-3-R criteria and had a HAM-A score of 15 or more. Participants in the Western medication group (n = 29) were treated with 20 mg of fluoxetine or paroxetine. In addition, 0.4 to 1.6 mg of alprazolam was given according to the participant’s condition. All drugs were administered once daily for 6 weeks. There were nine to ten acupuncture points selected in the needling therapy group (n = 29) and the treatment was given once daily, 6 times a week for 6 weeks. The same method for both Western medication and needling therapy groups was used for participants in the combination treatment group (n = 28). Clinical efficacy was scored using the CGI which includes a general index subscale. A high general index score indicates an inferior therapeutic effect. There was no statistically significant difference between the Western medication and needling therapy groups (SMD = 0.09; 95% CI, −0.44 to 0.63) or between the needling therapy and combination treatment groups (SMD = −0.16; 95% CI, −0.70 to 0.38).
Ruan (2003) conducted an RCT in China, comparing combined treatment of Chinese medicine with acupuncture (COM) with Western medication. Participants were diagnosed with ‘anxiety neurosis’ using CCMD-2 and the self-rated Anxiety Neurosis Scale. Those scoring more than 50 were eligible to participate. They were randomised into the COM group (n = 86) or the Western medication group (n = 83). The COM group were treated with Chinese medicine, taken twice each day, and received acupuncture daily for 30 to 60 minutes. The Western medication group were given the TCA doxepin (an average of 150 mg per day). Treatment lasted for 30 days. Thirty-nine out of 86 in the COM group and 30 out of 83 in the Western medication group remitted. There was no statistically significant difference between the COM and Western medication group. Clinical efficacy was scored using SAS-CR and there was no statistically significant difference between the COM and Western medication group (SMD = −0.14; 95% CI, −0.45 to 0.16).
Zhou and colleagues (2003) conducted a randomised trial comparing the combined effect (n = 50) of acupuncture and flupentixol (an antipsychotic drug) with flupentixol only (n = 50). Participants were diagnosed with ‘anxiety neurosis’ using CCMD-2-R. Participants were given acupuncture once per day for 10 days. They took 5 days’ rest before the second wave of treatment. There were three waves of treatment in total. They also took 20 mg of flupentixol three times daily continuously for 40 days. According to remission rates, combined treatment was statistically significantly better than treatment with a single drug (RR = 0.71; CI, 0.57 to 0.89). Of participants in the treatment group, 64% did not achieve remission compared with 90% in the control group. Participants also reported side effects. One person experienced dry mouth in the combined treatment group, and one person experienced insomnia in the drug-only group. One from each group had dizziness.
Zhang and colleagues (2003) conducted a randomised trial comparing the clinical efficacy of acupuncture (n = 157) and doxepin (n = 139). They selected people with ‘anxiety neurosis’ according to CCMD-2 and an SAS score of more than 50. The acupuncture group had treatment once a day, with a 1-day interval after six consecutive treatments; there were 30 sessions in total. They used any two of four methods (varying in methodology), one of which included giving an injection at an acupuncture point. Participants in the comparison group were given 25 mg of doxepin three times a day for 4 weeks, which was modified according to therapeutic or adverse events. The therapeutic effects between the groups looked at a measure of remission (disappearance of symptoms with stable emotions). No statistical difference was found (RR = 0.86; CI, 0.71 to 1.03). Of participants in the treatment group, 56.1% did not achieve remission compared with 65.5% in the control group. For response (clinical symptoms relieved with occasional emotional fluctuation) no statistical difference was found (RR = 1.04; CI, 0.90 to 1.21). Of participants in the treatment group, 72.1% did not respond compared with 69.1% in the control group.
Guizhen and colleagues (1998) conducted a randomised trial comparing the clinical efficacy of acupuncture (n = 80) with behavioural desensitisation (n = 80) and a combination of both treatments (n = 80) on people with ‘anxiety neurosis’ (with SAS scores of 50 or more). The acupuncture-only group were treated once every other day for ten sessions (this comprised one course). Each participant received between one and three courses. Behavioural desensitisation involved self-relaxation techniques (twice daily) and psychotherapy that incorporated desensitisation therapy (twice weekly for ten sessions). For acupuncture combined with behavioural desensitisation, each participant received both treatments in the same day and between one and four courses of treatment with 3 to 7 day intervals between courses. Physical examination and SAS evaluation measured remission (disappearance of symptoms – SAS score of less than 45). The results for the combined treatment group were significantly better than the acupuncture-only (RR = 0.59; CI, 0.46 to 0.77) or the behavioural desensitisation-only (RR = 0.64; CI, 0.49 to 0.84) groups. Of participants in the acupuncture-only group, 80% did not remit; in the behavioural desensitisation-only group, 73.8% did not remit; 47.5% in the combined treatment group did not remit. For response (marked improvement in symptoms and significant decrease in SAS scores, that is, more than 20 points) combined treatment was significantly higher than the acupuncture-only (RR = 1.30; CI, 1.02 to 1.65) and behavioural desensitisation-only (RR = 1.24; CI, 0.98 to 1.57) groups. Of participants in the combined treatment group, 72.3% did not respond compared with 55% and 57.5% in the acupuncture-only and behavioural desensitisation-only groups, respectively.
8.11.7. Hypnotherapy
Zhao and colleagues (2005) conducted a randomised trial, comparing clinical efficacy of hypnotherapy and alprazolam. Participants were diagnosed with GAD using CCMD-3, with a HAM-A score of over 14. Participants were randomly assigned into the hypnotherapy group (n = 32) and a comparison group (n = 30). The hypnotherapy group received treatment twice each week for 30 to 40 minutes each session. The comparison group received 0.8 mg of alprazolam twice each day and met with a doctor twice a week. The total length of treatment was 4 weeks. When looking at response (defined as 50% or more reduction in HAM-A scores), there was no statistically significant difference between groups (SMD = 0.10; 95% CI, −0.40 to 0.60). Evidence appeared to suggest no difference in effect between hypnotherapy and alprazolam.
8.11.8. Overall clinical summary of other interventions
Most of the herbal interventions were more effective than placebo in reducing anxiety-related symptoms with the exception of valerian extract. Moreover, no significant differences were found between herbal interventions and benzodiazepines in relation to anxiety-related outcomes. This evidence must be interpreted with caution, however, due to the small evidence base and the quality of the studies.
The results indicate that acupuncture may be of equivalent effectiveness to medication in the treatment of GAD or ‘anxiety neurosis’. It is important to note, however, that these trials use a range of medications as comparison conditions, many of which have uncertain effectiveness in the treatment of GAD. In addition, there are differences between the CCMD diagnoses of GAD and ‘anxiety neuroses’ and the DSM or ICD classification systems, for example, in duration of symptoms required to meet diagnostic criteria. Therefore this is an important limitation of the review. Furthermore, the trials are only medium sized and also of low to moderate quality, which makes it difficult to arrive at a confident conclusion.
There was very limited evidence for hypnotherapy, which proved inconclusive.
8.11.9. From evidence to recommendations
Due to the limited evidence base for most interventions reviewed in this section, the GDG concluded that it was not yet possible to generate recommendations on the use of any of these interventions for the treatment of GAD.
Existing research shows initial evidence for herbal interventions to be effective when compared with placebo, however, due to the small number of studies and small sample sizes, larger RCTs examining the effectiveness of these herbal interventions, any possible side effects and potential herb-drug interactions are necessary to increase confidence in these initial findings.
8.11.10. Research recommendation
- 8.11.10.1.
The effectiveness of chamomile and ginkgo biloba in the treatment of GAD
Is chamomile/ginkgo biloba more effective than placebo in increasing response and remission rates and decreasing anxiety ratings for people with GAD?
This question should be addressed using a placebo-controlled, double-blind randomised design to compare the effects of a standardised dose of chamomile (220–1100 mg) or ginkgo biloba (30–500 mg) in a readily available form, for example a capsule, with placebo. This should assess outcomes at the end of the trial and at 12-month post-trial follow-up. The outcomes chosen should include both observer-and participant-rated measures of clinical symptoms and functioning specific to GAD, and of side effects. There should be a health economic evaluation included and an assessment of quality of life. The trial should be large enough to determine the presence or absence of clinically important effects using a non-inferiority design. Mediators and moderators of response should be investigated.
Why this is important
GAD is a common mental health disorder and the results of this study will be generalisable to a large number of people. There is evidence for the efficacy of chamomile and ginkgo biloba in reducing anxiety in people with GAD but the evidence base is small (one study). However, the scarce literature on the effectiveness of other herbal interventions for treating GAD points to chamomile and ginkgo biloba as two of the more effective herbal interventions. Moreover, both these herbal remedies are widely available and relatively inexpensive. Furthermore, at present there is no scientific evidence of side effects or drug–herbal interactions in relation to chamomile or ginkgo biloba. As both these herbal interventions are readily available and have no known side effects, they could be used at an early stage as a means of preventing progression to drug treatments, which are associated with a number of undesirable side effects and dependency.
Footnotes
- 15
Here and elsewhere in the guideline, each study considered for review is referred to by a study ID in capital letters (primary author and date of study publication, except where a study is in press or only submitted for publication, then a date is not used).
- 16
This recommendation also appears in section 7.7.1.1 where the psychological data is presented.
- 17
The date of publication of the NICE guideline containing the recommendations only on the NICE website: http://guidance
.nice.org.uk/CG113
- INTRODUCTION
- PHARMACOLOGICAL INTERVENTIONS COMPARED WITH PLACEBO
- HEAD-TO-HEAD TRIALS OF PHARMACOLOGICAL INTERVENTIONS
- EFFECTS OF DOSE
- MAINTENANCE TREATMENT
- MANAGEMENT OF NON-RESPONSE TO PHARMACOLOGICAL INTERVENTIONS
- SIDE EFFECTS OF PHARMACOLOGICAL INTERVENTIONS
- HEALTH ECONOMIC EVIDENCE
- FROM EVIDENCE TO RECOMMENDATIONS
- RECOMMENDATIONS
- OTHER INTERVENTIONS
- Generalised anxiety disorder in adults: Evidence Update September 2012: A summary of selected new evidence relevant to NICE clinical guideline 113 'Generalised anxiety disorder and panic disorder (with or without agoraphobia) in adults: management in primary, secondary and community care' (2011)
- Clinical Guidelines for the Management of Anxiety: Management of Anxiety (Panic Disorder, with or without Agoraphobia, and Generalised Anxiety Disorder) in Adults in Primary, Secondary and Community Care (NICE guideline CG22)
- PHARMACOLOGICAL AND PHYSICAL INTERVENTIONS - Generalised Anxiety Disorder in Adu...PHARMACOLOGICAL AND PHYSICAL INTERVENTIONS - Generalised Anxiety Disorder in Adults
Your browsing activity is empty.
Activity recording is turned off.
See more...
