NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Jamison DT, Feachem RG, Makgoba MW, et al., editors. Disease and Mortality in Sub-Saharan Africa. 2nd edition. Washington (DC): The International Bank for Reconstruction and Development / The World Bank; 2006.
The global burden of disease study of the World Health Organization (WHO) estimated that about 177 million people in the world had diabetes in the year 2000 (WHO 2003). In the second edition of the International Diabetes Federation's Diabetes Atlas it is estimated that 194 million people had diabetes in the year 2003, and about two-thirds of these people lived in developing countries (IDF 2003). In 1901 Albert Cook, a medical missionary in Uganda, reported that "diabetes is rather uncommon and very fatal" (Cook 1901). Over the next 50 to 60 years diabetes continued to be regarded as rare in Sub-Saharan Africa. Communicable diseases still make up the greatest disease burden, but by 2020, noncommunicable diseases, including hypertension and diabetes, will outstrip communicable diseases as a cause of death (Murray and Lopez 1997). Even allowing for the uncertainties of predicting future disease patterns posed by the unfolding of the human immunodeficiency virus (HIV) epidemic in Sub-Saharan Africa, it is clear that the relative importance of noncommunicable diseases will increase (Panz and Joffe 1999). This situation is a result of demographic change (populations with older age structures), increasing urbanization (WHO 1998), and associated changes in risk-factor levels, such as tobacco smoking, obesity, and physical inactivity (Hunter et al. 2000; Kaufman et al. 1999; Pavan et al. 1997). Countries of Sub-Saharan Africa are in various stages of the epidemiological transition with a multiple burden of diseases.
The available evidence suggests that noncommunicable diseases currently contribute substantially to the burden of mortality and morbidity in adults. Age-specific levels of diabetes and hypertension in many urban areas of Sub-Saharan Africa are as high as, or higher than, those in most Western European countries (Aspray et al. 2000; Edwards et al. 2000; Mollentze et al. 1995). In a demographic surveillance system in Tanzania they account for between one in six and one in three adult deaths (Kitange et al. 1996; Setel et al. 2000; Walker et al. 2000), with age-specific death rates from non-specific, noncommunicable diseases being as high or higher than in developed countries (Unwin et al. 1999).
Diabetes mellitus can be classified into four principal types (WHO 1999). This includes type 1 diabetes, type 2 diabetes, other specific types of diabetes, and gestational diabetes mellitus. The most common types of diabetes seen in Sub-Saharan Africa are type 2 and type 1 diabetes mellitus. This chapter focuses on the published data on the burden of type 1 and type 2 diabetes in Sub-Saharan Africa. Although type 1 diabetes is not caused by the adverse effects of lifestyle, as type 2 can be, the chronic complications of both type 1 and type 2 diabetes on the eyes, cardiovascular system, nerves, and kidneys are similar.
Sources of Data
The data search was limited to studies published after 1979, because data collected before 1980 may no longer reflect the current prevalence of diabetes. The Medline database and the Internet were used for the literature search, but also diabetes researchers and clinicians were asked to provide information on the burden of diabetes for their Sub-Saharan Africa country or subregion. The Medline search was undertaken for diabetes prevalence and for each complication: retinopathy, neuropathy, nephropathy, and so on. In the absence of data from any country, data were extrapolated from the socioeconomically, ethnically, and geographically most similar country. The data obtained were from prevalence studies, hospital-based studies, registry reports, hospital statistics, government estimates, and the like. Some of the specific data sources are mentioned in table 19.1. The sources of data for the 2003 estimates of diabetes mellitus and IGT are listed in table 19.2.
Table 19.1
Data Sources for the Prevalence of Type 2 Diabetes and IGT, by Year of Study.
Table 19.2
Data Sources for Prevalence Estimates of Diabetes Mellitus and IGT, by Country, 2003.
There is still a dearth of published studies describing the burden of diabetes in Sub-Saharan Africa. The prevalence rates of diabetes and its complications (table 19.3) have been drawn from the few country data available as applied to the population distribution of that country or a similar country. Clinic-based studies have serious limitations, so their generalizability is limited. Therefore the data presented here are only general indicators of diabetes frequency and should be interpreted with caution. As new and better epidemiological data become available, it will be possible to have actual rates of diabetes in Sub-Saharan Africa.
Table 19.3
Data Sources for the Prevalence of Diabetes Complications, by Disease and Year.
Epidemiology of Diabetes
Diabetes mellitus is a chronic metabolic disease characterized by hyperglycemia resulting from defects in insulin secretion, insulin action, or both. Uncontrolled chronic hyperglycemia results in long-term damage, particular dysfunction, and failure of the eyes, heart, blood vessels, nerves, and kidneys.
Type 1 diabetes results from autoimmune destruction of the pancreatic beta cells, causing the loss of insulin production. Children are usually affected by this type of diabetes, although it occurs at all ages and the clinical presentation can vary with age. Patients with this type of diabetes require insulin for survival.
Type 2 diabetes is characterized by insulin resistance and abnormal insulin secretion, either of which may predominate but both of which are usually present. The specific reasons for the development of these abnormalities are largely unknown. Type 2 is the most common type of diabetes. Type 2 diabetes can remain asymptomatic for many years, and the diagnosis is often made from associated complications or incidentally through an abnormal blood or urine glucose test.
Other specific types of diabetes include those due to genetic disorders, infections, diseases of the exocrine pancreas, endocrinopathies, and drugs. This last type of diabetes is relatively uncommon.
Gestational diabetes mellitus (GDM) is defined as any degree of glucose intolerance with onset or first recognition during pregnancy. The definition applies whether insulin or only diet modification is used for treatment and whether the condition persists after pregnancy. It does not exclude the possibility that unrecognized glucose intolerance may have antedated or begun concomitantly with the pregnancy. Approximately 7 percent of all pregnancies are complicated by GDM. The prevalence may range from 1 to 14 percent of all pregnancies, depending on the population studied and the diagnostic tests employed.
Impaired glucose tolerance (IGT) is asymptomatic, and its diagnosis is confirmed by an elevated nondiabetic level of blood glucose two hours after a 75 gram oral glucose tolerance test. Impaired fasting glycemia (IFG) is an elevated nondiabetic fasting blood glucose level. Both IGT and IFG are transitional stages in the development of type 2 diabetes.
Prevalence and Incidence of Type 1 Diabetes
There is a dearth of published studies describing the incidence and prevalence of type 1 diabetes in Sub-Saharan Africa. Type 1 diabetes is considerably rarer than type 2 disease, and large populations need to be surveyed. Also, to assess incidence, the population surveyed should be accurately known, and this is in itself difficult, as complete censuses in Africa are rare and migration in and out of study areas common. Elamin and colleagues in the Sudan in 1992 reported a survey of nearly 43,000 schoolchildren (age 7 to 11 years) and found a prevalence rate of 0.95 per 1,000 (Elamin et al. 1992). This rate is comparable to a reported prevalence rate of 0.3 per 1,000 in Nigeria (Afoke et al. 1992). The reported incidence is 10.1 per 100,000 children per year in Sudan (Elamin et al. 1992) and 1.5 per 100,000 per year in Tanzania (Swai, Lutale, and McLarty 1993). The discrepancy between the Sudanese and Tanzanian studies may be explained by ethnic differences, and perhaps problems related to the design of the studies.
The question of whether type 1 diabetes is truly rarer in Africa than elsewhere remains unsettled, and more detailed surveys are needed. Nonetheless, it emerges from careful clinic studies that the behavior of type 1 diabetes is different in Sub-Saharan Africa from that in the rest of the world. Studies indicate that the age of onset in South Africa and Ethiopia is later than elsewhere (Kalk, Huddle, and Raal 1993; Lester 1984), and the peak age of onset of type 1 diabetes in Sub-Saharan Africa is a decade later than in the West (Afoke et al. 1992; Kalk, Huddle, and Raal 1993). In addition it afflicts more females than males. In South Africa it has been reported that the peak age of onset was about 13 years in the white South Africans (similar to Europeans) but about 23 years in the black South Africans (Kalk, Huddle, and Raal 1993). The reasons for this difference are obscure, although it has been suggested that prolonged breastfeeding, which is common in Africa, may be reducing the incidence and delaying the onset of type 1 diabetes. Early introduction of cow's milk protein does seem to be a risk factor for the later development of type 1 diabetes, possibly because, in neonates, bovine albumin can raise antibodies that mimic islet cell antibodies and attack pancreatic beta cells. This is, of course, speculative, and much still remains unknown about the pathogenesis and epidemiology of type 1 diabetes in Africa.
Genetic Factors
More than 90 percent of type 1 diabetes subjects in Sub-Saharan Africa, as in the rest of the world, have one or both human leukocyte antigens (HLA) DR3 and DR4. However, there appear to be specificities in the HLA susceptibility found in certain African populations. Recent studies using allele-specific (oligonucleotide) probes from Zimbabwe, Senegal, and Cameroon show positive and negative associations with some alleles (Garcia-Pacheco et al. 1992; Chauffert et al. 1995).
Immunological Factors
The main markers of immune islet cell attack are islet cell antibodies (ICA) and glutamic acid decarboxylase antibodies (anti-GAD). These substances are found in most Caucasian type 1 diabetic patients at diagnosis, but levels gradually decline with time. Interpretation of ICA and anti-GAD levels in type 1 diabetes is dependent on duration of disease, and this may explain the variable results found in the limited African studies so far carried out. McLarty, Kinabo, and Swai (1990) found that the prevalence of ICA antibodies was only 8 to 11 percent in newly diagnosed Tanzanian patients. In South Africa, Motala, Omar, and Pirie (2000) found that 44 percent of blacks with newly diagnosed type 1 diabetes were positive for GAD antibody. It appears from these preliminary results that the genetic susceptibility and risk factors for type 1 diabetes in Sub-Saharan Africa may be different from those in the Western world. It can be speculated that non-autoimmune factors are the major determinants of type 1 diabetes in Sub-Saharan Africa.
Environmental Factors
An environmental "trigger" factor for the onset of type 1 diabetes has long been sought. Its existence is supported by the well-known seasonality of presentation in Europe, and viral infection (perhaps of the coxsackievirus group) is considered a likely candidate. A seasonality of type 1 diabetes has been reported in Tanzania (with most cases presenting between August and November) (McLarty, Yusafai, and Swai 1989). It would therefore seem likely that potential viral triggers operate also in the rest of Africa.
Prevalence of Type 2 Diabetes
Before the 1990s, diabetes was considered a rare medical condition in Africa. Epidemiological studies carried out in that decade, however, provided evidence of a trend toward increased incidence and prevalence of type 2 diabetes in African populations (Sobngwi et al. 2001). Indeed, Africa is experiencing the most rapid demographic and epidemiological transition in world history (Mosley, Bobadilla, and Jamison 1993). It is characterized by a tremendous rise in the burden of noncommunicable diseases (NCDs), underlined by the increasing life expectancy and lifestyle changes resulting from the reduction in infectious diseases and increased fertility, as well as Westernization.
Almost all the reports published between 1959 and 1985 showed a prevalence of diabetes below 1.4 percent, except those from South Africa, where higher prevalence was reported. Differences in diagnostic methods and criteria, however, made comparison between countries difficult. Since then, uniform diagnostic criteria has become more available, allowing comparison across countries. Epidemiological studies carried out during this period show the rising prevalence of diabetes all over Africa (table 19.2).
The prevalence of diabetes in Africa was approximately 3 million in 1994; but the region is due to experience a two-to threefold increase by the year 2010 (Amos, McCarty, and Zimmet 1997). The highest prevalence is found in populations of Indian origin, followed by black populations and Caucasians. Among the population of Indian origin in South Africa and Tanzania, the prevalence is between 12 and 13 percent (Ramaiya, Swai, McLarty, and Alberti 1991). The prevalence in blacks follows a Westernization gradient, with that of rural Africa generally below 1 percent but that of urban Africa between 1 and 6 percent. In general the prevalence of type 2 diabetes is low in both rural and urban communities of West Africa except in urban Ghana, where a high rate of 6.3 percent was recently reported (Amoah, Owusu, and Adjei 2002). Moderate rates have been reported from South Africa: 4.8 percent in a semi-urban community in the Orange Free State, 6.0 percent in an urban community of the Orange Free State, 5.5 percent in Durban (mostly occupied by the Zulu tribe), and 8 percent in Cape Town (mostly occupied by the Xhosa tribe). Also, moderate rates have been reported in studies from Tanzania (table 19.1).
Estimates of the Prevalence of Type 2 Diabetes and IGT in 2003
There are marked discrepancies between the prevalence of diabetes among different communities in Sub-Saharan Africa. The studies from Tanzania (Aspray et al. 2000; McLarty et al. 1989), showing an urban-to-rural ratio of five to one, and from Cameroon (Mbanya et al. 1997), with a ratio of two to one, both confirm the urban-rural discrepancy in diabetes prevalence and suggest the consequent likely increases because of urban migration. The data used for the extrapolation of current prevalence rates (table 19.2) in distant and probably dissimilar countries and populations indicate the great need for more epidemiological investigations in Sub-Saharan Africa. Such a need is dictated by the prevalence of undiagnosed diabetes, which accounted for 60 percent of those with diabetes in Cameroon (Mbanya et al. 1997), 70 percent in Ghana (Amoah, Owusu, and Adjei 2002), and over 80 percent in the recent study in Tanzania (Aspray et al. 2000). It would therefore appear that in Sub-Saharan Africa, for every diagnosed person with diabetes, there are one to three undiagnosed cases.
The International Diabetes Federation estimated that in 2003 the number of people age 20 to 79 years with diabetes in Sub-Saharan Africa was over 7 million for a population of more than 295 million, giving a prevalence rate of 2.4 percent. About 65 percent of those affected with diabetes lived in the urban areas, whereas 35 percent lived in the rural communities. More than 46 percent (3.3 million) of the diabetic population were 40 to 59 years old, whereas 28 percent and 26 percent, respectively, were 20 to 39 and 60 to 79 years old (table 19.4). This has serious implications for the productivity of the region, since diabetes affects the active members of the community. The top five countries with the highest number of people affected by diabetes in Sub-Saharan Africa are Nigeria (about 1.2 million people), South Africa (841,000), the Democratic Republic of Congo (552,000), Ethiopia (550,000), and Tanzania (380,000) (table 19.4).
Table 19.4
Prevalence Estimates of Diabetes Mellitus, by Country, 2003.
The impact of type 2 diabetes is bound to continue if nothing is done to curb the rising prevalence of IGT, which now varies between 2.2 percent and 16.2 percent. The estimated prevalence rate of IGT in 2003 for Sub-Saharan Africa is 7.3 percent with a total population of affected individuals of over 21 million (table 19.5). Some 70 percent of these individuals are expected to develop type 2 diabetes unless something is done to reduce the risk factors associated with the development of the disease.
Table 19.5
Prevalence Estimates of IGT, by Country, 2003.
The projections of type 2 diabetes and IGT from 2003 to 2025 are shown in table 19.6. It is estimated that the burden of diabetes and IGT will just about double in 2025 from their 2003 levels. The rate at which new cases of diabetes are emerging poses an additional burden on countries already stretched to the limit by common life-threatening infections, such as malaria, tuberculosis, and HIV and acquired immune deficiency syndrome (AIDS).
Table 19.6
Projections of Diabetes and IGT from 2003 to 2025 in the Age Group of 20 to 79 Years.
Risk Factors for Type 2 Diabetes
There are marked differences between diabetic and nondiabetic individuals in the prevalence of some risk factors for diabetes and its complications, notably anthropometric variables, such as obesity. Although it is true that these data are from cross-sectional studies that have limitations in establishing causality, they at least support the hypothesis that increasing prevalence of diabetes can be attributed largely to changes in lifestyle resulting in reduced physical activity and increased calorie intake and subsequent weight gain. Such changes have important implications for the provision of health care and for health education to promote behavioral change in order to control the emergence of diabetes in Sub-Saharan Africa.
Age and Ethnicity
Age and ethnicity are the two main nonmodifiable risk factors of diabetes in Africa. Glucose intolerance in Sub-Saharan Africa, as in other regions of the world, increases with age in both men and women (figure 19.1); however, published studies lack uniformity on the age range in which the prevalence of diabetes is observed. According to King, Aubert, and Herman (1998), in most developed communities the peak of occurrence falls in the age group of 65 years or older, whereas in developing countries it is in the age group 45 to 64, and in Sub-Saharan Africa it is in the age groups 20 to 44 and 45 to 64 years. Yet data from 12 other studies from Sub-Saharan Africa indicate two peak age ranges of 45 to 64 and older than 65 years (see table 19.1 for references).
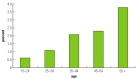
Figure 19.1
Prevalence of Diabetes with Increasing Age in Cameroon Source: Authors.
Two studies in Sub-Saharan Africa have examined ethnic differences in the prevalence of diabetes. A difference was found between Indians, blacks, and Caucasians in South Africa, where Indians had the highest predisposition and were followed by blacks and Caucasians (Levitt et al. 1999; Omar et al. 1994). In the Tanzanian study, the indigenous African population had lower diabetes prevalence than the migrant Asian group (1.1 percent as opposed to 9.1 to 7.1 percent) (McLarty et al. 1989; Ramaiya, Swai, McLarty, Bhopal, et al. 1991; Swai et al. 1990).
The prevalence of diabetes appears to be substantially higher in African-origin populations living abroad than in indigenous Africans. West Africans from Nigeria (Cooper et al. 1997) and central Africans from Cameroon (Mbanya et al. 1997) were compared with populations of West African origin in the Caribbean (Cooper et al. 1997; Mbanya et al. 1997), United Kingdom (Cooper et al. 1997; Mbanya et al. 1997), and the United States (Cooper et al. 1997). These studies suggest that environment determines diabetes prevalence in these populations of similar genetic origin.
Urban-Rural Differences
Residence seems to be a major determinant of diabetes in Sub-Saharan Africa, since urban residents have 1.5- to 4.0 times higher prevalence of diabetes than their rural counterparts. This is attributable to lifestyle changes associated with urbanization and Westernization. Urban lifestyle in Africa is characterized by changes in dietary habits involving an increase in the consumption of refined sugars and saturated fat and a reduction in fiber intake (Mennen et al. 2000). Sobngwi and colleagues (2002) have recently reported an increase in fasting plasma glucose in those whose lives have been spent in an urban environment, suggesting that both lifetime exposure to and recent migration to or current residence in an urban environment are potential risk factors for obesity and diabetes mellitus. The disease might represent the cumulative effects over years of dietary changes, decrease in physical activity, and psychological stress.
The population of Africa is predominantly rural, but the 1995–2000 urban growth rate was estimated at 4.3 percent (compared with 0.5 percent in Europe). Thus, more than 70 percent of the population of Africa will be urban residents by 2025 (UNFPA 2000). There will therefore be a tremendous increase in the prevalence of diabetes attributable to rapid urbanization. In addition, life expectancy at birth is rapidly increasing. For example, in Cameroon in 1960 it was about 35 years but in 1990 was raised to approximately 55 years. An increase in diabetes prevalence simply because of the change in the age structure of the population is therefore expected. However, the HIV pandemic may change these estimates and projections.
Family History of Diabetes
A significant proportion of the offspring of Cameroonians with type 2 diabetes have either type 2 diabetes (4 percent) or IGT (8 percent) (Mbanya et al. 2000). A positive family history seems to be an independent risk factor for diabetes, but this was not the case in the Cape Town study (Levitt et al. 1993), in which family history was not an independent risk factor.
Measure of Adiposity
Several studies from Sub-Saharan Africa have confirmed the association between the prevalence of diabetes and a surrogate of obesity, body mass index (BMI). Reports from Mali (Fisch et al. 1987), Nigeria (Cooper et al. 1997) and Tanzania (McLarty et al. 1989) have shown that the prevalence of diabetes increases with increasing BMI. BMI and obesity seem to be independent risk factors for diabetes (Levitt et al. 1993).
Physical Activity
There seems to be a significant relationship between physical inactivity and diabetes and obesity (Sobngwi et al. 2002). Physical activity is more common in rural than urban regions of Africa because rural populations rely on walking for transport and often have intense agricultural activities as their main occupation. In Sub-Saharan Africa, walking time and pace is drastically reduced (by factors of 2 to 4 for walking at a slow pace and 6 to more than 10 for walking at a brisk pace) in an urban community as compared with a rural community. The main difference in physical activity between the two types of community, however, is the use of walking in rural areas as a means of transportation.
The reduction in physical activity associated with life in a city partly explains the excess prevalence of obesity in urban areas. In a South African study, the prevalence of a sedentary lifestyle in Cape Town in subjects age 30 years and over was 39 percent for men and 44 percent for women (Omar et al. 1993). Low physical activity was normal for 22 percent of men and 52 percent of women in urban Tanzania, whereas it was usual for only 10 percent of men and 15 percent of women living in rural areas (Edwards et al. 2000). Cross-sectional data from 1,417 women age 15 to 83 years in a rural community and an urban community in Cameroon showed that in all age groups, fasting blood glucose levels were inversely associated with energy expenditure from walking (figure 19.2) (Sobngwi, Gautier, and Mbanya 2003). Rural dwellers' higher level of physical activity and related energy expenditure compared with urban subjects goes far to explain why obesity was found to be at least four times higher in urban areas than rural (Aspray et al. 2000). Thus, lack of physical activity appears to be a significant risk factor for diabetes in Sub-Saharan Africa.
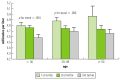
Figure 19.2
Mean Fasting Blood Glucose by Tertiles of Walking Energy Expenditure in Women: The Cameroon Study Source: Adapted from Sobngwi, Gautier, and Mbanya 2003.
Complications of Diabetes
The escalating prevalence of type 1 and type 2 diabetes and their complications in Sub-Saharan Africa are a major drain on health resources in financially difficult circumstances, in addition to having a considerable physical and social impact on the individual and community.
Acute Complications of Diabetes
The three main metabolic complications of diabetes in Sub-Saharan Africa are diabetic ketoacidosis, hyperosmolar nonketotic coma, and hypoglycemia. Diabetic ketoacidosis is a common diabetic emergency in developing countries and carries with it relatively high mortality, ranging from 25 percent in Tanzania to 33 percent in Kenya. The major contributing factors to such high mortality are the chronic lack of availability of insulin, delays in seeking medical assistance by newly diagnosed type 1 patients presenting in ketoacidosis, misdiagnosis of diabetes, and poor health care in general and diabetic care in particular (Rwiza, Swai, and McLarty 1986).
Hyperosmolar nonketotic coma is usually a complication of type 2 diabetes and is less common and accounts for about 10 percent of all hyperglycemic emergencies in developing countries (Zouvanis et al. 1997). Infection is the leading precipitating factor for both diabetic ketoacidosis and hyperosmolar nonketotic coma, followed by first presentation of diabetes at a health institution and noncompliance with a medical regimen (Zouvanis et al. 1997). It carries a high mortality of up to 44 percent according to studies from South Africa, which may be because the patients are usually elderly and have other major illnesses (Rolfe et al. 1995).
Hypoglycemia is also a serious complication of treatment in patients with diabetes. Of a total of 51 episodes in 43 patients admitted at the Baragwanath Hospital, Johannesburg, South Africa, 14 cases (33 percent) were associated with sulfonylurea treatment. The major cause precipitating the event was a missed meal (36 percent), although alcohol (22 percent), gastrointestinal upset (20 percent), and inappropriate treatment (18 percent) were also important contributory factors (Gill and Huddle 1993). No mortality was associated with hypoglycemia in this study.
Chronic Complications of Diabetes
The seriousness of diabetes is largely a result of its associated complications, which can be serious, disabling, and even fatal. Prevalence studies on complications reported up to the early 1990s gave widely variable figures. These have been reviewed in two studies and include figures ranging from 9 to 16 percent for cataract, 7 to 52 percent for retinopathy, 6 to 47 percent for neuropathy, 6 to 30 percent for nephropathy, and 1 to 5 percent for macroangiopathy (Mbanya and Sobngwi 2003; Rolfe 1997). The variations are due to diagnostic criteria problems, local and geographical factors, type of diabetes, and variation in duration of diabetes. Since 1995, however, many more vigorous and well-conducted studies have taken place, giving a much clearer picture of complication prevalence; these are summarized in table 19.3. It can be seen that there is generally less wide a range between these studies and also that the figures themselves are substantial.
The prevalence of diabetic retinopathy varies from 13 to 55 percent, depending on the duration of diabetes and glycemic control, with severe retinopathy representing 15 percent of all cases (table 19.3). At diagnosis, 21 to 25 percent of type 2 patients and 9.5 percent of type 1 patients have retinopathy. Ethnic differences in the prevalence of retinopathy have been observed in multiethnic communities. In South Africa, the highest prevalence of retinopathy is observed in Africans, rather than Indians or Caucasians (whites), at diagnosis and after a similar duration of follow-up (Kalk et al. 1997). Although genetic predisposition may not be ruled out, lack of blood glucose and blood pressure control because of difficult access to health care might account for most of these differences.
The prevalence of nephropathy varies between 32 and 57 percent after a mean duration of diabetes of 5 to 10 years and between 5 and 28 percent within the first year following the diagnosis of diabetes (table 19.3). Diabetic nephropathy also occurs early in the course of diabetes, because between 32 and 57 percent of diabetic patients with a mean duration of diabetes between 5 and 10 years have microalbuminuria (Kalk et al. 1997; Rahlenbeck and Gebre-Yohannes 1997; Sobngwi et al. 1999). The diagnosis of nephropathy may, however, be faulty because of the presence of proteinuria due to renal infections and sickle-cell anemia. In Africa, diabetes mellitus accounts for a third of all patients who are admitted to dialysis units (Diallo et al. 1997), and renal replacement is both expensive and not widely available. It appears, therefore, that diabetic end-stage renal failure is the first cause of hospital mortality in diabetic patients in Africa. In South Africa, for example, 50 percent of all causes of mortality in type 1 diabetic patients may be due to renal failure (Gill, Huddle, and Rolfe 1995).
The estimates of the prevalence of neuropathy vary widely, depending on the methodology used to assess them. Macrovascular complications of diabetes are considered rare in Africa despite a high prevalence of hypertension. Lower-extremity amputation varies from 1.5 to 7 percent, and about 12 percent of all hospitalized diabetic patients have foot ulceration. A high proportion of patients have lower-limb arterial disease that contributes to the development of diabetic foot lesions. It is common to see patients with diabetic foot ulcers as the presenting complaint of diabetes. Data from Tanzania have shown that the vast majority (over 80 percent) of ulcers are neuropathic in origin and not associated with peripheral vascular disease (Abbas, Lutale, and Morback 2000). Audits of diabetes care carried out in Cape Town, South Africa; Dar es Salaam, Tanzania; and Yaoundé, Cameroon, have demonstrated poor glycemic control and inadequate foot care as risk factors for diabetic foot. Fewer than 22 percent of patients had their feet examined during a year of attendance at primary health care clinics in these three cities, even though in Cape Town, 37 percent demonstrated either peripheral neuropathy or peripheral vascular disease (Abbas, Lutale, and Morback 2000; Boulton 1990). Limited patient knowledge of proper foot care, practices relating to foot care, and cultural beliefs, including the association of diabetes, leg ulcers, and lower extremity amputation with bewitchment, are also common problems encountered in Sub-Saharan Africa countries (Abbas, Lutale, and Morback 2000; Boulton 1990).
Data on cerebrovascular disease are scarce because of the mortality associated with this complication, the low proportion of patients seen in hospitals, and the lack of death certificates or proper records of the cause of death. Recent results from the general population of Tanzania, where a morbidity and mortality surveillance system has been set up, show that stroke mortality was three to six times that of England and Wales and that 4.4 percent of type 2 diabetic patients presented with stroke at the diagnosis of diabetes (Walker et al. 2000). Coronary heart disease may affect 5 to 8 percent of type 2 diabetic patients and cardiomyopathy up to 50 percent of all patients. Whereas microvascular complications of diabetes are highly preventable and occur early during the course of the disease, macrovascular disease is rare. Late diagnosis of diabetes, poor metabolic control, and nonstandardized diagnostic procedures rather than genetic predisposition may account for this difference with other populations around the world.
Mortality Associated with Diabetes
There have been relatively few structured mortality studies from Africa, making quantification of outcome difficult. However, a major study from Zimbabwe in 1980 (Castle and Wicks 1980) recorded follow-up of 107 newly diagnosed diabetic patients (both type 1 and type 2). In-patient mortality was 8 percent, and the survivors had a mortality rate of 41 percent within six years of follow-up. Most deaths were due to infection, hyperglycemic emergencies (ketoacidosis and nonketotic coma), or hypoglycemia. Particular risk factors for an adverse outcome were male gender, alcohol abuse, and insulin treatment.
Another outcome study was reported from Tanzania in 1990. A cohort of 1,250 newly diagnosed patients was followed from 1981 to 1987, and actuarial five-year survival rates were calculated (McLarty, Kinabo, and Swai 1990; Swai, Lutale, and McLarty 1990). Eighty-two percent of those not on insulin survived five years, but only 60 percent of the group on insulin treatment survived that long. Once again, the causes of death were predominantly metabolic and infective. The authors concluded that in Africa "diabetes was a serious disease with a poor prognosis." One reviewer also observed that the Tanzanian study indicated that five years from diagnosis, 40 percent of those on insulin would die, whereas in Europe 40 percent of similar patients would survive more than 40 years (Deckert, Poulsen, and Larsen 1978; Gill 1997).
There is some evidence, however, that at least in some parts of Africa the prognosis of diabetes is improving. Figures reported from Ethiopia (Lester 1991, 1996), for example, are considerably better than the Zimbabwean (1980) and Tanzanian (1990) data. Interestingly, although metabolic emergencies were still the major cause of death, the mortality from renal failure was substantial, presumably from diabetic nephropathy and large vessel disease. A cohort of type 1 diabetic patients who were followed in Soweto, South Africa, has also shown relatively prolonged survival (Gill, Huddle, and Rolfe 1995). At follow-up after 10 years, with a mean diabetes duration of 14 years, only 16 percent had died. This figure was still in excess of Western rates, although almost all these deaths were due to nephropathy, a complication mostly untreatable in Africa even now.
Obviously, many factors affect mortality patterns among diabetic patients in different parts of Africa. These include provision of medical care and supply of insulin and other treatment modalities, as well as a variety of social, cultural, and ethnic factors. As seen earlier, the gradual lengthening of the duration of diabetes in itself contributes to changing patterns of mortality, to which diabetic nephropathy and macroangiopathy are rapidly playing a larger part in many areas. Large vessel disease is likely to accelerate in prevalence also because of Western influences, such as smoking, obesity, reduced exercise, and high-fat diets.
Cost of Diabetes
Studies on the economics of diabetes care in Sub-Saharan Africa are limited. A Medline search of such studies over the past 20 years yielded only the Tanzanian study (Chale et al. 1992). In Tanzania about US$4 million would have been required to take care of all patients with diabetes in 1989/90, which translates to US$138 per patient per year. This sum is equivalent to 8.1 percent of the total budgeted health expenditure for that financial year and well above the allocated per capita health expenditure in Tanzania of US$2 for the year 1989/90 (Chale et al. 1992). In Cameroon the average direct medical cost of treating a patient with diabetes in 2001 was US$489, of which 56 percent was spent on hospital admissions, 33.5 percent on antidiabetic drugs, 5.5 percent on laboratory tests, and 4.5 percent on consultation fees. The direct medical costs for treating all diabetic patients in Cameroon represented about 3.5 percent of the national budget for the year 2001/2002 (Nkegoum 2002). Estimates of diabetes care management in Malawi, based on international prices for essential drugs and Malawi hospital cost data, suggest that a type 1 diabetic patient spends about US$100 per year for the purchase of insulin, and a type 2 patient spends US$25 annually on oral hypoglycemic agents (Vaughan, Gilson, and Mills 1989).
The average age at onset of diabetes in Tanzania was 44 years and the average age at death was 46 years; population life expectancy was 53 years. The calculated number of healthy life days (HLDs) lost because of diabetes was 4,100 days per patient, of which 69 percent was because of premature mortality. This calculation was based on an average case-fatality rate after five years of 29 percent and a severe chronic disablement rate of 14 percent. The estimated HLDs lost per capita because of diabetes were 820 person-days per 1,000 people per year (Chale et al. 1992). In Ghana the average age at onset of diabetes was 40 years, with 50 percent case fatality after 15 years and an average age at death of 55 years, with 30 percent disablement before death. The total days lost were calculated as 217 per 1,000 people per year, of which 52 percent were due to premature death (Vaughan, Gilson, and Mills 1989).
Table 19.7 reports the calculated estimates of the costs of diabetes care in Sub-Saharan Africa for persons age 20 to 79 years (IDF 2003). The table uses population estimates and diabetes prevalence estimates reported in table 19.4 for those age 20 to 79 years for 2003. The total health care budget for 20- to 79-year-olds can be derived by multiplying the population figures for that age group by the per capita health expenditures. Calculations are then presented for values of R of 2 and 3. R is the ratio of the cost of care for people with diabetes compared with the cost of care of people without diabetes. The data suggest that, at least for countries with high or moderate incomes, the value of R lies between 2 and 3 (IDF 2003). The top five countries with the highest costs of diabetes care in Sub-Saharan Africa are South Africa, Kenya, Zimbabwe, Nigeria, and Ghana (table 19.7).
Table 19.7
Calculated Estimates of the Costs of Diabetes Care, by Country.
Strategies for Control
During the last 10 years, health care spending in the developing countries has remained low. Of the 40 heavily indebted poor countries (HIPC) defined by the World Bank, 33 of them are in Sub-Saharan Africa. The average per capita income of HIPCs, is US$310 per year. The health care spending is approximately US$8 per person per year, and pharmaceutical spending is approximately US$2 to $3 per person per year (WHO 2003).
The economic cost of diabetes and its complications is unaffordable by most Sub-Saharan Africans. Their incomes are insufficient to purchase insulin, oral hypoglycemic agents, and other supplies for management of diabetes. The limited resources of the countries in Sub-Saharan Africa are divided between fighting poverty, implementing education strategies, providing housing and appropriate sanitation, and dealing with the socioeconomic and health burden of fighting the increasing incidence and prevalence of HIV/AIDS. Diabetes poses an additional burden on the limited health care delivery system.
The problems encountered in the management of diabetes in Sub-Saharan Africa include diagnosis; medical care; insulin and other drug supplies; monitoring; infections associated with diabetes, especially the diabetic foot; dietary advice; diabetes education; and the low priority placed on noncommunicable diseases.
The training of health care providers and organizations is not focused on effective and efficient treatment of people with diabetes. With modernization, economic well-being, and a Westernized lifestyle, the burden of diabetes and its complications also increases significantly. The resource-limited countries are unable to provide even minimum care in some instances, let alone secondary and tertiary care.
Over the last 10 years several assessments of health care services for diabetes have been done, particularly in South Africa (Whiting, Hayes, and Unwin 2003). The findings from these studies have shown the following:
- Patients' attendance is poor.
- Consultation times are short, resulting in little or no time for patient education.
- Staffing levels are inadequate, and staffs' knowledge is used inappropriately.
- Staff are poorly or inadequately trained, or both, and there exist hardly any continuous education programs.
- Monitoring and evaluation of complications of diabetes are lacking.
- The control of blood glucose and blood pressure is poor and inadequate.
- Referral systems are almost nonexistent.
- Education of people with diabetes is lacking.
- Overall organization of the clinics is not satisfactory.
- Record keeping is poor.
- Even if treatment guidelines are available, they are hardly used and are not up to date.
- Health care systems in Sub-Saharan Africa vary widely.
A structured, organized diabetes health care system is lacking. Many people with diabetes are managed by traditional health care providers and general practitioners who are inadequately integrated into the primary care system.
Other problems with or barriers to the quality of delivery and affordable care include the following:
- inadequate infrastructure
- irregular supply of medicines
- unaffordable insulin, oral hypoglycemic agents, and anti-hypertensives
- disproportionate distribution of health care facilities
- lack of information and clear roles for members of diabetes health care teams
- lack of appropriate and locally adapted diabetes education programs for people with diabetes and diabetes health care professionals
- lack of government support or subsidy, resulting in unaffordable costs.
Cost of medication, especially the high cost of insulin, is a major handicap to proper diabetes care in Sub-Saharan Africa. Indeed, in a recent International Diabetes Federation survey (IDF 2003), it was observed that 80 percent of the people with diabetes were unable to obtain insulin and insulin syringes because they could not afford them. The cost of insulin preparations was higher in Sub-Saharan Africa than elsewhere (figure 19.3). Insulin and insulin syringes were accessible to only 11 percent of all people with diabetes in Africa. In addition, only 25 percent of people with diabetes monitored their blood glucose. Self-monitoring of blood glucose was rarely used, mainly because of the cost of testing supplies in 90 percent and the unavailability of testing supplies in 70 percent of the countries in Africa (IDF 2003).
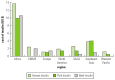
Figure 19.3
Cost of Different Types of Insulin in Relation to the Gross National Product Source: Adapted from IDF 2003.
It is clear that the organization of diabetes care in Sub-Saharan Africa has limitations at several levels of health delivery (box 19.1). There are solutions for each level of limitations and problems. Implementation strategies for effecting change will vary from country to country based on several factors. Care for people with diabetes needs to begin at the primary health care level, followed by secondary and tertiary health care. In Sub-Saharan Africa, the rural-to-urban migration results in a significant increase in the geographical spread of people with diabetes. The health care for people with diabetes tends to be concentrated in large urban hospitals, too far from most of the people who need to use the services. Outreach programs in which health care workers move out into the community are feasible, affordable, and achievable and will benefit the rural population. That will probably be the long-term solution to the problems of the management of diabetes.
Box 19.1
Organization of Diabetes Care.
Diabetes Health Care
More than 50 years ago people with diabetes were mostly treated in hospitals by specialists. With limited resources and shrinking health budgets, together with a sharp rise in the prevalence of type 2 diabetes, specialist care in a hospital is not possible for everyone. An increasing number of primary and community health care professionals are responsible for managing people with diabetes. The ongoing health sector reforms in most of the countries of Sub-Saharan Africa have promoted more responsive and appropriate planning through decentralized and demand-driven health care, in which district managers decide how to divide their budgets between prevention and control of different health problems (WHO 2000).
In order to plan for proper delivery of diabetes health care, Sub-Saharan Africa countries need epidemiological and health services information. There is also a need to know the estimates of the prevalence of diabetes, its risk factors, and its complications. Finally, adequate knowledge of the overall burden of diabetes in high-risk populations and countries is a prerequisite for effective diabetes health care delivery (King et al. 1998).
Prevention
Sufficient evidence exists from countries outside Africa that weight loss, diet, and exercise can prevent or delay diabetes in people with IGT (Pan et al. 1997; Tuomilehto et al. 2001; Vijan et al. 1997) and that physical activity may exert an independent effect on the prevention and control of diabetes (Wojtaszewski et al. 2000).
The strategies for primary prevention, mostly involving support for behavior change through different forms of focused education and mass-media campaigns, are highly cost-effective (Swai et al. 1990). Prevention strategies in Sub-Saharan Africa have their own limitations. Lack of awareness by the population of and facilities for detection and monitoring contributes to the high prevalence of diabetic complications, and poorly skilled or inadequate health care staff, delay in seeking medical attention, and lack of access to affordable drugs contribute to the high rate of diabetes-related mortality. Unless these factors are taken into account when planning effective preventive strategies, the objectives will not be attainable.
The United Kingdom Prospective Diabetes Study (UKPDS 1998a) and the Diabetes Control and Complication Trial (DCCT 1993) have shown that intensive control of glucose results in a 25 to 70 percent reduction in the number and severity of microvascular complications in people with diabetes. The UKPDS also demonstrated a 12 percent reduction in mortality related to type 2 diabetes. In the UKPDS, control of high blood pressure reduced the risk of microvascular complications by 37 percent and death from type 2 diabetes–related disease by 32 percent (UKPDS 1998b), better reductions than those from tight blood glucose control (UKPDS 1998a), although the combination of blood pressure and blood glucose control was the most effective.
Health beliefs are still deeply enshrined in the healing cultures of people in Sub-Saharan Africa, thereby predisposing most patients to alternate between modern and traditional clinics. There are places in Sub-Saharan Africa where chlorpropamide and tolbutamides are still drugs of choice (and the only ones available) together with alpha methyldopa (for blood pressure control) in people affected with diabetes. The newer classes of drugs—sulfonylurea group, glinides—are unaffordable for the majority of the population.
One of the major challenges facing insulin-treated patients in Sub-Saharan Africa is the lack of a constant supply of insulin at affordable cost (Yudkin 2000). The supply of insulin in Sub-Saharan Africa is erratic, even at large hospitals, and the prospects for people with type 1 diabetes are poor (Amoah et al. 1998; Dagogo-Jack 1995). The exact burden of poor insulin access in developing countries is still unknown, because no good scientific study has been carried out in these countries. However, 16 percent of the world's population in developed countries with about 35 percent of all diabetic patients use over 40 percent of the world's total insulin each year (Jervell 1996; King 1998). Moreover, a small percentage of type 2 diabetes patients in developing countries require insulin when they become severely wasted and hyperglycemic. Therefore, insulin is underutilized in Sub-Saharan Africa. In the second edition of Diabetes Atlas, the IDF's Task Force on Insulin Survey reports that no country in Africa had 100 percent accessibility to insulin. In fact two countries in Africa had the lowest accessibility in the world: the Democratic Republic of Congo, where people with type 1 diabetes had access to insulin for less than 25 percent of the time, and Zambia, where those with type 2 diabetes had access to insulin only 26 to 49 percent of the time. The high cost of insulin appears to be the most important cause of lack of access to insulin in people with type 1 diabetes in most countries of Africa (IDF 2003). There is therefore an urgent need for the initiation of international programs to alleviate the plight of insulin-treated patients in Africa. These programs may include (a) a selection of type of drug through the essential drug list; (b) improving affordability of the price charged by applying such measures as national price information, patent status, availability of generics, equity pricing schemes, review of general taxes and margins, and as a last resort, parallel import and compulsive licensing; and (c) sustainable financing of medical supplies through general tax levies, insurance schemes, copayment or full payment by the patient, loans, and donations (IDF 1998). One of the solutions to the problem being discussed is donation of insulin combined with a mechanism to support logistics, education, and monitoring. The limiting factor with this scheme is long-term sustainability.
Very few countries in Sub-Saharan Africa can afford to screen and treat the complications of diabetes (nephropathy, retinopathy, neuropathy, peripheral vascular disease) (Dagogo-Jack 1995). ACE inhibitors are a cost-effective way to reduce mortality and end-stage renal failure in people with type 1 diabetes (and type 2 diabetes) with microalbuminuria, but they are of limited use because most people cannot afford them (Hendry et al. 1997; Tooke, Thomas, and Viberti 2000). The potential for intervention and prevention of diabetic foot lesions is very high as a cost-effective strategy.
The Role of Diabetes Associations
The role of Diabetes Associations cannot be overemphasized. National Diabetes Associations should urge the government and nongovernmental organizations to ensure consistent and readily available insulin and other antidiabetes drugs and supplies at a subsidized cost. The national associations can also play a significant role in imparting education at different levels of health care and to the community at large.
The Role of the Patient
Patients must be empowered and motivated to join associations. They have to be informed about their rights. Together with the community, they would be able to remove misconceptions, mistrust, and the stigma of diabetes in Sub-Saharan Africa. The standard and quality of care being provided for people with diabetes has many limitations, which have to be overcome by a multisectoral approach.
Conclusion
Epidemiological data on diabetes mellitus in Sub-Saharan Africa are still limited. However, the prevalence and incidence of both type 1 and type 2 diabetes are increasing with the persistent rural-to-urban migration. Clearly, knowledge of the disease has increased since the 1990s; nevertheless, adoption of a Western lifestyle has greatly enhanced its development. The evidence shows that type 2 diabetes is a major cause of morbidity and mortality on the continent and that it is costly to manage diabetes and its complications. Given that the region still has a double and sometimes triple disease burden and that little priority is given to non-communicable diseases like diabetes, and in the absence of a health care system adapted to this new reality and able to use costly therapeutic interventions, well-planned cost-effective methods of prevention and treatment and refined tools to assess health services and monitor progress are therefore required.
References
- Abbas Z., Lutale J., Morback S. Outcome of Hospital Admissions for Diabetic Foot Lesions at Muhimbili Medical Centre, Dar es Salaam, Tanzania. Diabetologia. 2000;43(Suppl. 1):A246.
- Afoke A. O., Ejeh N. M., Nwonu E. N., Okafor C. O., Udeh N. J., Ludvigsson J. Prevalence and Clinical Picture of IDDM in Nigerian Ibo School Children. Diabetes Care. 1992;15:1310–12. [PubMed: 1425094]
- Ahren B., Corrigan C. B. Prevalence of Diabetes Mellitus in North-Western Tanzania. Diabetologia. 1984;26:333–36. [PubMed: 6734980]
- Amoah A. G., Owusu S. K., Adjei S. Diabetes in Ghana: A Community Based Prevalence Study in Greater Accra. Diabetes Research and Clinical Practice. 2002;56:197–205. [PubMed: 11947967]
- Amoah A. G., Owusu S. K., Saunders J. T., Fang W. L., Asare H. A., Pastors J. G., Sanborn C., Barrett E. J., Woode M. K., Osei K. Facilities and Resources for Diabetes Care at Regional Health Facilities in Southern Ghana. Diabetes Research and Clinical Practice. 1998;42:123–30. [PubMed: 9886749]
- Amos A. F., McCarty D. J., Zimmet P. The Rising Global Burden of Diabetes and Its Complications: Estimates and Projections to the Year 2010. Diabetic Medicine. 1997;14:S1–85. [PubMed: 9450510]
- Aspray T. J., Mugusi F., Rashid S., Whiting D., Edwards R., Alberti K. G., Unwin N. C. Rural and Urban Differences in Diabetes Prevalence in Tanzania: The Role of Obesity, Physical Inactivity and Urban Living. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2000;94:637–44. [PubMed: 11198647]
- Boulton A. Practical Diabetes Digest. 1990;1:35–37.
- Castle W. N., Wicks A. C. B. Follow-Up of 93 Newly Diagnosed African Diabetics for 6 Years. Diabetologia. 1980;18:121–23. [PubMed: 6988270]
- Chale S. S., Swai A. B. M., Mujinja P. G. M., McLarty D. G. Must Diabetes Be a Fatal Disease in Africa? Study of Costs of Treatment. British Medical Journal. 1992;304:1215–18. [PMC free article: PMC1881760] [PubMed: 1515790]
- Chauffert M., Cisse A., Cevenne D., Parfait B., Michel S., Trivin F. HLA-DR Beta 1 Typing and Non-Asp 57 Alleles in the Aborigine Population of Senegal. Diabetes Care. 1995;18:677–90. [PubMed: 8586006]
- CIA (Central Intelligence Agency). 2002. World Factbook 2002. Washington, DC: CIA. http://www
.cia.gov/publications /factbook/index.html. - Cook A. R. Notes on the Diseases Met with in Uganda, Central Africa. Journal of Tropical Medicine. 1901;4:175–78.
- Cooper R., Rotimi C., Kaufman J., Owoaje E., Fraser H., Forester T., Wilks R., Riste L. K., Cruikshank J. K. Prevalence of NIDDM among Populations of the African Diaspora. Diabetes Care. 1997;20:343–48. [PubMed: 9051385]
- Dagogo-Jack S. DCCT Results and Diabetes Care in Developing Countries. Diabetes Care. 1995;18:416–17. [PubMed: 7555491]
- DCCT Research Group. The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications of Insulin Dependent Diabetes Mellitus. New England Journal of Medicine. 1993;329:977–86. [PubMed: 8366922]
- Deckert T., Poulsen J. E., Larsen M. Prognosis of Diabetics with Diabetes Onset before the Age of Thirty-One. I. Survival, Causes of Death, and Complications. Diabetologia. 1978;14:363–70. [PubMed: 669100]
- Diallo A. D., Nochy D., Niamkey E., Yao Beda B. Etiologic Aspects of Nephrotic Syndrome in Black African Adults in a Hospital Setting in Abidjan. Bulletin Société Pathologie Exotique. 1997;90:342–45. [PubMed: 9507767]
- Dowse G. K., Gareeboo H., Zimmet P. Z., Alberti K. G., Tuomilehto J., Fareed D., Brissonnette L. G., Finch C. F. High Prevalence of NIDDM and Impaired Glucose Tolerance in Indian, Creole, and Chinese Mauritians. Mauritius Noncommunicable Disease Study Group. Diabetes. 1990;39:390–96. [PubMed: 2307296]
- Drabo P. P. Y., Kabore J., Lengani A. Complications of Diabetes Mellitus at the Hospital Center of Ouagadougou. Bulletin Société Pathologie Exotique. 1996;89:191–95. [PubMed: 8998413]
- Ducorps M., Baleynaud S., Mayaudon H., Castagne C., Bauduceau B. A Prevalence Survey of Diabetes in Mauritania. Diabetes Care. 1996;19:761–63. [PubMed: 8799635]
- Edwards R., Unwin N., Mugusi F., Whiting D., Rashid S., Kissima J., Aspray T. J., Alberti K. G. Hypertension Prevalence and Care in an Urban and Rural Area of Tanzania. Journal of Hypertension. 2000;18(2):145–52. [PubMed: 10694181]
- Elamin A., Omer M. I., Zein K., Tuvemo T. Epidemiology of Childhood Type I Diabetes in Sudan, 1987–1990. Diabetes Care. 1992;15:1556–59. [PubMed: 1468286]
- Elbagir M. N., Eltom M. A., Elmahadi E. M., Kadam I. M., Berne C. A Population-Based Study of the Prevalence of Diabetes and Impaired Glucose Tolerance in Adults in Northern Sudan. Diabetes Care. 1996;19:1126–1128. [PubMed: 8886561]
- Fisch A., Pichard E., Prazuck T., Leblanc H., Sidibe Y., Brucker G. Prevalence and Risk Factors of Diabetes Mellitus in the Rural Region of Mali West Africa: A Practical Approach. Diabetologia. 1987;30:859–62. [PubMed: 3446552]
- Garcia-Pacheco J. M., Herbut B., Culbush S., Hitman G. A., Zhonglin W., Magzoub M. et al. Distribution of HLA-DQA1 and DRBI Alleles in Black IDDM Controls from Zimbabwe. Tissue Antigens. 1992;40:145–49. [PubMed: 1440568]
- Gill, G. V. 1997. "Outcome of Diabetes in Africa." In Diabetes in Africa, ed. G. V. Gill, J.-C. Mbanya, and K. G. M. M. Alberti, 65–71. Cambridge: FSG Communications.
- Gill G. V., Huddle K. R. Hypoglycaemic Admissions among Diabetic Patients in Soweto, South Africa. Diabetic Medicine. 1993;10:181–83. [PubMed: 8458198]
- Gill G. V., Huddle K. R., Rolfe M. Mortality and Outcome of Insulin-Dependent Diabetes in Soweto, South Africa. Diabetic Medicine. 1995;12:546–50. [PubMed: 7648831]
- Hendry B. M., Viberti G. C., Hummel S., Bagust A., Piercy J. Modelling and Costing the Consequences of Using an ACE Inhibitor to Slow the Progression of Renal Failure in Type I Diabetic Patients. Quarterly Journal of Medicine. 1997;90(4):277–82. [PubMed: 9307762]
- Hunter J. M., Sparks B. T., Mufunda J., Musabayane C. T., Sparks H. V., Mahomed K. Economic Development and Women's Blood Pressure: Field Evidence from Rural Mashonaland, Zimbabwe. Social Science and Medicine. 2000;50:773–95. [PubMed: 10695977]
- IDF (International Diabetes Federation). 1998. Access to Insulin: A Report on the IDF Insulin Task Force on Insulin, 1994–1997. Brussels: IDF.
- ———. 2003. Diabetes Atlas. 2nd ed. Brussels: IDF.
- Jervell J. Variations in the Utilization and Cost of Insulin. International Diabetes Federation Bulletin. 1996;41:1–2.
- Kalk W. J., Huddle K. R. L., Raal F. J. The Age of Onset of Insulin-Dependent Diabetes Mellitus in Africans in South Africa. Postgraduate Medical Journal. 1993;69:552–56. [PMC free article: PMC2399890] [PubMed: 8415343]
- Kalk W. J., Joannou J., Ntsepo S., Mahomed I., Mahanlal P., Becker P. J. Ethnic Differences in the Clinical and Laboratory Associations with Retinopathy in Adult Onset Diabetes: Studies in Patients of African, European and Indian Origins. Journal of Internal Medicine. 1997;241:31–37. [PubMed: 9042091]
- Kaufman J. S., Owoaje E. E., Rotimi C. N., Cooper R. S. Blood Pressure Change in Africa: Case Study from Nigeria. Human Biology. 1999;71(4):641–57. [PubMed: 10453105]
- King H. Insulin: Availability, Affordability, and Harmonization. WHO Drug Information. 1998;12(4):230–34.
- King H., Aubert R. E., Herman W. H. Global Burden of Diabetes. Prevalence, Numerical Estimates and Projections. Diabetes Care. 1998;21:1414–31. [PubMed: 9727886]
- Kitange H. M., Machibya H., Black J., Mtasiwa D. M., Masuki G., Whiting D., Unwin N. et al. The Outlook for Survivors of Childhood in Sub-Saharan Africa: Adult Mortality in Tanzania. British Medical Journal. 1996;312:216–20. [PMC free article: PMC2349992] [PubMed: 8563587]
- Lester F. T. The Clinical Pattern of Diabetes in Ethiopians. Diabetes Care. 1984;7:6–11. [PubMed: 6705667]
- ———. 1991. Clinical Status of Ethiopian Diabetic Patients after 20 Years of Diabetes." Diabetic Medicine 8: 272–76. [PubMed: 1828744]
- ———1993Clinical Features, Complications and Mortality in Type 2 (Non-Insulin Dependent) Diabetic Patients in Addis Abeba, Ethiopia, 1976–1990 Ethiopian Medical Journal 31(2):109–26. [PubMed: 8513778]
- ———1996Mortality During Eight Years in a Cohort of Middle-Aged Ethiopian Diabetic Patients International Diabetes Digest 711–17.
- Levitt N. S., Bradshaw D., Zwarenstein M. F., Bawa A. A., Maphumolo S. Audit of Public Sector Primary Diabetes Care in Cape Town, South Africa: High Prevalence of Complications in Controlled Hyperglycaemia and Hypertension. Diabetic Medicine. 1997;14:1073–77. [PubMed: 9455936]
- Levitt N. S., Katzenellenbogen J. M., Bradshaw D., Hoffman M. N., Bonnici F. The Prevalence and Identification of Risk Factors for NIDDM in Urban Africans in Cape Town, South Africa. Diabetes Care. 1993;16:601–7. [PubMed: 8462387]
- Levitt N. S., Steyn K., Lambert E. V., Reagan G., Lombard C. J., Fourie J. M., Rossouw K., Hoffman M. Modifiable Risk Factors for Type 2 Diabetes Mellitus in a Peri-Urban Community in South Africa. Diabetic Medicine. 1999;16:946–50. [PubMed: 10588525]
- Mbanya J. C., Ngogang J., Salah J. N., Minkoulou E., Balkau B. Prevalence of NIDDM and Impaired Glucose Tolerance in a Rural and an Urban Population in Cameroon. Diabetologia. 1997;40:824–29. [PubMed: 9243104]
- Mbanya J. C., Pani L. N., Mbanya D. N., Sobngwi E., Ngogang J. Reduced Insulin Secretion in Offspring of African Type 2 Diabetic Parents. Diabetes Care. 2000;23:1761–65. [PubMed: 11128348]
- Mbanya J. C., Sobngwi E. Diabetes Microvascular and Macrovascular Disease in Africa. Journal of Cardiovascular Risk. 2003;10:97–102. [PubMed: 12668906]
- McLarty D. G., Kinabo L., Swai A. B. M. Diabetes in Tropical Africa: A Prospective Study, 1981–7. II. Course and Prognosis. British Medical Journal. 1990;300:1107–10. [PMC free article: PMC1662792] [PubMed: 2111723]
- McLarty D. G., Swai A. B. M., Kitange H. M., Masuki G., Mtinangi B. L., Kilima P. M., MaKene W. J., Chuwa L. M., Alberti K. G. Prevalence of Diabetes and Impaired Glucose Tolerance in Rural Tanzania. Lancet. 1989;(8643):871–75. [PubMed: 2564951]
- McLarty D. G., Yusafai A., Swai A. B. M. Seasonal Incidence of Diabetes Mellitus in Tropical Africa. Diabetic Medicine. 1989;6:762–65. [PubMed: 2533033]
- Mennen L. I., Mbanya J. C., Cade J., Balkau B., Sharma S., Chungong S., Kennedy J. The Habitual Diet in Rural and Urban Cameroon. European Journal of Clinical Nutrition. 2000;24:882–87. [PubMed: 10694786]
- Mollentze W. F., Moore A. J., Steyn A. F., Joubert G., Steyn K., Oosthuizen G. M., Weich D. J. V. Coronary Heart Disease Risk Factors in a Rural and Urban Orange Free State Black Population. South African Medical Journal. 1995;85:90–96. [PubMed: 7597541]
- Mosley, W. H., J. L. Bobadilla, and D. T. Jamison. 1993. "The Health Transition: Implications for Health Policy in Developing Countries." In Disease Control Priorities in Developing Countries, ed. D. T. Jamison, W. H. Mosley, A. R. Measham, and J. L. Bobadilla, 673–99. New York: Oxford University Press.
- Motala A. A., Omar M. A. K., Pirie F. J. Type 1 Diabetes Mellitus in Africa: Epidemiology and Pathogenesis. Diabetes International. 2000;10:44–47.
- Moukouri D. T., McMoli C., Nouedoui C., Mbanya J. C. Les aspects cliniques de la rétinopathie à Yaoundé Médecine d'Afrique Noire. 1995;42(89):423–28.
- Murray C. J. L., Lopez A. D. Mortality by Cause for Eight Regions of the World: Global Burden of Disease Study. Lancet. 1997;349:1269–76. [PubMed: 9142060]
- Nambuya A. P., Otim M. A., Whitehead H., Mulvaney D., Kennedy R., Hadden D. R. The Presentation of Newly-Diagnosed Diabetic Patients in Uganda. Quarterly Journal of Medicine. 1996;89:705–11. [PubMed: 8917747]
- Niang E. H., Diop S. N., Sidibe E. A., Badiane M., Lamouche J. R., Sow A. M. Echographic and Velocimetric Aspects of Arteriopathies in the Diabetic. Dakar Medical. 1994;39:37–42. [PubMed: 7493518]
- Nkegoum, A. V. 2002. "Coût direct et indirect du diabéte en l'absence de complications chroniques a Yaoundé, Cameroun." MD thesis, University of Yaoundé I, Cameroon.
- Ohwovoriole A. E., Kuti J. A., Kabiawu S. I. Casual Blood Glucose Levels and Prevalence of Undiscovered Diabetes Mellitus in Lagos Metropolis Nigerians. Diabetes Research and Clinical Practice. 1988;4(2):153–58. [PubMed: 3342734]
- Omar M. A., Seedat M. A., Dyer R. B., Motala A. A., Knight L. T., Becker P. J. South African Indians Show a High Prevalence of NIDDM and Bimodality in Plasma Glucose Distribution Patterns. Diabetes Care. 1994;17:70–74. [PubMed: 8112193]
- Omar M. A. K., Seedat M. A., Motala A. A., Dyer R. B., Becker P. The Prevalence of Diabetes Mellitus and Impaired Glucose Tolerance in a Group of South African Blacks. South African Medical Journal. 1993;83:641–43. [PubMed: 8310354]
- Pan X. R., Li G. W., Hu Y. H., Wang J. X., Yang W. Y., An Z. X., Hu Z. X. et al. Effects of Diet and Exercise in Preventing NIDDM in People with Impaired Glucose Tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20:537–44. [PubMed: 9096977]
- Panz V. R., Joffe B. I. Impact of HIV Infection and AIDS on Prevalence of Type 2 Diabetes in South Africa in 2010. British Medical Journal. 1999;318:1351. [PMC free article: PMC1115727] [PubMed: 10323831]
- Pavan L., Casiglia E., Pauletto P., Batista S. L., Ginocchio G., Kwankam M. M. Y. et al. Blood Pressure, Serum Cholesterol and Nutritional State in Tanzania and in the Amazon: Comparison with an Italian Population. Journal of Hypertension. 1997;15:1083–90. [PubMed: 9350582]
- Rahlenbeck S. I., Gebre-Yohannes A. Prevalence and Epidemiology of Micro- and Macroalbuminuria in Ethiopian Diabetic Patients. Journal of Diabetes Complications. 1997;11:343–49. [PubMed: 9365876]
- Ramaiya K. L., Swai A. B. M., McLarty D. G., Alberti K. G. M. M. Impaired Glucose Tolerance and Diabetes Mellitus in Hindu Indian Immigrants in Dar es Salaam. Diabetic Medicine. 1991;8:738–44. [PubMed: 1838065]
- Ramaiya K. L., Swai A. B. M., McLarty D. G., Bhopal R. S., Alberti K. G. M. M. Prevalences of Diabetes and Cardiovascular Disease Risk Factors in Hindu Indian Subcommunities in Tanzania. British Medical Journal. 1991;303:271–76. [PMC free article: PMC1670456] [PubMed: 1888926]
- Rolfe, M. 1997. "Chronic Complications of Diabetes in Africa." In Diabetes in Africa, ed. G. V. Gill, J.-C. Mbanya, and K. G. M. M. Alberti, 43–50. Cambridge: FSG Communications.
- ———1988The Neurology of Diabetes Mellitus in Central Africa Diabetic Medicine 5399–401. [PubMed: 2968895]
- Rolfe M., Ephraim G. G., Lincoln D. C., Huddle K. R. L. Hyperglycaemic Nonketotic Coma as a Cause of Emergency Hyperglycaemic Admission in Baragwanath Hospital. South African Medical Journal. 1995;85:173–76. [PubMed: 7777971]
- Rwiza H. T., Swai A. B. M., McLarty D. G. Failure to Diagnose Diabetic Ketoacidosis in Tanzania. Diabetic Medicine. 1986;3:181–83. [PubMed: 2951164]
- Setel P., Unwin N., Alberti K., Hemed Y. Cause-Specific Adult Mortality: Evidence from Community-Based Surveillance, Selected Sites, Tanzania, 1992–1998. Morbidity and Mortality Weekly Report. 2000;49:416–19. [PubMed: 10905820]
- Sobngwi E., Gautier J. F., Mbanya J. C. Exercise and the Prevention of Cardiovascular Events in Women. New England Journal of Medicine. 2003;348:77–79. [PubMed: 12510049]
- Sobngwi E., Mauvais-Jarvis E., Vexiau P., Mbanya J. C., Gautier J. F. Diabetes in Africans. Part 1: Epidemiology and Clinical Specificities. Diabetes and Metabolism. 2001;27:628–34. [PubMed: 11852370]
- Sobngwi E., Mbanya J-C., Moukouri E. N., Ngu K. B. Microalbuminuria and Retinopathy in a Diabetic Population of Cameroon. Diabetes Research and Clinical Practice. 1999;44:191–96. [PubMed: 10462142]
- Sobngwi E., Mbanya J. C., Unwin N. C., Kengne A. P., Fezeu L., Minkoulou E. M., Aspray T. J., Alberti K. G. Physical Activity and Its Relationship with Obesity, Hypertension and Diabetes in Urban and Rural Cameroon. International Journal of Obesity and Related Metabolic Disorders. 2002;26:1009–16. [PubMed: 12080456]
- Swai A. B. M., Lutale J., McLarty D. G. Diabetes in Tropical Africa: A Prospective Study 1981–7: Characteristics of Newly Presenting Patients in Dar es Salaam, Tanzania 1981–7. British Medical Journal. 1990;300:1103–7. [PMC free article: PMC1662826] [PubMed: 2344535]
- ———1993Prospective Study of Incidence of Juvenile Diabetes Mellitus over 10 Years in Dar es Salaam, Tanzania British Medical Journal 3061570–72. [PMC free article: PMC1678021] [PubMed: 8329915]
- Swai A. B. M., McLarty D. G., Sherrif F., Chuwa L. M., Maro E., Lukmanji Z., Kermali W., MaKene W., Alberti K. G. Diabetes and Impaired Glucose Tolerance in an Asian Community in Tanzania. Diabetes Research and Clinical Practice. 1990;8:227–34. [PubMed: 2340794]
- Teuscher T., Baillod P., Rosman J. B., Teuscher A. Absence of Diabetes in a Rural West African Population with a High Carbohydrate/Cassava Diet. Lancet. 1987;(8536):765–68. [PubMed: 2882181]
- Tooke J. E., Thomas S., Viberti G. C. Proteinuria in Diabetes. Journal of Royal College London. 2000;34:336–39. [PMC free article: PMC9665481] [PubMed: 11005067]
- Tuomilehto J., Lindstrom J., Eriksson J. G., Valle T. T., Hamalainen H., Ilanne-Parikka P., Keinanen-Kiukaanniemi S. et al. Prevention of Type 2 Diabetes Mellitus by Changes in Lifestyle among Subjects with Impaired Glucose Tolerance. New England Journal of Medicine. 2001;344:1343–50. [PubMed: 11333990]
- UKPDS Group. Intensive Blood-Glucose Control with Sulphonylureas or Insulin Compared with Conventional Treatment and Risk of Complications in Patients with Type 2 Diabetes: UKPDS 33. Lancet. 1998a;352:837–53. [PubMed: 9742976]
- ———1998bTight Blood Pressure Control and Risk of Macrovascular and Microvascular Complications in Type 2 Diabetes: UKPDS 38 British Medical Journal 317703–12. [PMC free article: PMC28659] [PubMed: 9732337]
- UNFPA (United Nations Population Fund). 2000. State of World Population. New York: United Nations.
- Unwin N., Mugusi F., Aspray T., Whiting D., Edwards R., Mbanya J. C. et al. Tackling the Emerging Pandemic of Non-Communicable Diseases in Sub-Saharan Africa: The Essential NCD Health Intervention Project. Public Health. 1999;113:141–46. [PubMed: 10910412]
- Vaughan P., Gilson L., Mills A. Diabetes in Developing Countries: Its Importance for Public Health. Health Policy and Planning. 1989;4:97–109.
- Vijan S., Stevens D., Hermann W., Funnell M., Standiford C. Screening, Prevention, Counseling, and Treatment for the Complications of Type II Diabetes Mellitus. Putting Evidence into Practice. Journal of General Internal Medicine. 1997;12:567–80. [PMC free article: PMC1497162] [PubMed: 9294791]
- Walker R. W., McLarty D. G., Kitange H. M., Whiting D., Masuki G., Mtasiwa D. M., Machibya H., Unwin N., Alberti K. G. Stroke Mortality in Urban and Rural Tanzania. Lancet. 2000;355:1684–87. [PubMed: 10905244]
- Whiting D. R., Hayes L., Unwin N. C. Challenges to Health Care for Diabetes in Africa. Journal of Cardiovascular Risk. 2003;10:103–10. [PubMed: 12668907]
- WHO (World Health Organization). 1998. Population Ageing—A Public Health Challenge. Geneva: WHO.
- ———. 1999. "Diagnosis and Classification of Diabetes Mellitus." In Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications. Geneva: WHO.
- ———. 2000. The World Health Report 2000—Health Systems: Improving Performance. Geneva: WHO.
- ———. 2003. The World Health Report 2002—Reducing Risks, Promoting Healthy Life. Geneva: WHO. [PubMed: 14741909]
- Wojtaszewski J. F., Hansen B. F., Gade J., Kiens B., Markuns J. F., Goodyear L. J., Richter E. A. Insulin Signaling and Insulin Sensitivity after Exercise in Human Skeletal Muscle. Diabetes. 2000;49(3):325–31. [PubMed: 10868952]
- Yudkin J. S. Insulin for the World's Poorest Countries. Lancet. 2000;355:919–21. [PubMed: 10752719]
- Zouvanis M., Pieterse A. C., Seftel H. C., Joffe B. I. Clinical Characteristics and Outcome of Hyperglycaemic Emergencies in Johannesburg Africans. Diabetic Medicine. 1997;14:603–6. [PubMed: 9223400]
- Cardiovascular complications of diabetes mellitus in sub-Saharan Africa.[Circulation. 2005]Cardiovascular complications of diabetes mellitus in sub-Saharan Africa.Kengne AP, Amoah AG, Mbanya JC. Circulation. 2005 Dec 6; 112(23):3592-601.
- [Diabetes mellitus in sub-Saharan Africa: epidemiological aspects and management issues].[Med Trop (Mars). 2007][Diabetes mellitus in sub-Saharan Africa: epidemiological aspects and management issues].Gning SB, Thiam M, Fall F, Ba-Fall K, Mbaye PS, Fourcade L. Med Trop (Mars). 2007 Dec; 67(6):607-11.
- Review Type 2 diabetes mellitus and obesity in sub-Saharan Africa.[Diabetes Metab Res Rev. 2010]Review Type 2 diabetes mellitus and obesity in sub-Saharan Africa.Tuei VC, Maiyoh GK, Ha CE. Diabetes Metab Res Rev. 2010 Sep; 26(6):433-45.
- Review Diabetes in Africa. Pathogenesis of type 1 and type 2 diabetes mellitus in sub-Saharan Africa: implications for transitional populations.[J Cardiovasc Risk. 2003]Review Diabetes in Africa. Pathogenesis of type 1 and type 2 diabetes mellitus in sub-Saharan Africa: implications for transitional populations.Osei K, Schuster DP, Amoah AG, Owusu SK. J Cardiovasc Risk. 2003 Apr; 10(2):85-96.
- Review Descriptive epidemiology of cardiovascular risk factors and diabetes in sub-Saharan Africa.[Prog Cardiovasc Dis. 2013]Review Descriptive epidemiology of cardiovascular risk factors and diabetes in sub-Saharan Africa.Mensah GA. Prog Cardiovasc Dis. 2013 Nov-Dec; 56(3):240-50.
- Diabetes Mellitus - Disease and Mortality in Sub-Saharan AfricaDiabetes Mellitus - Disease and Mortality in Sub-Saharan Africa
Your browsing activity is empty.
Activity recording is turned off.
See more...
