NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd edition. Boston: Butterworths; 1990.

Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd edition.
Show detailsDefinition
Mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC) were first introduced by Wintrobe in 1929 to define the size (MCV) and hemoglobin content (MCH, MCHC) of red blood cells. Termed red cell indices, these values are useful in elucidating the etiology of anemias. Red cell indices can be calculated if the values of hemoglobin, hematocrit (packed cell volume), and red blood cell count are known. With the general availability of electronic cell counters, red cell indices are now automatically measured in all blood count determinations.
Variation in the size of red cells (anisocytosis) can be quantified and expressed as red cell distribution width (RDW) or as red cell morphology index. The RDW is more widely available and is discussed in this chapter. The size distribution of a population of cells is graphically represented by the red cell histograms (Price–Jones curves) (see Figure 152.1). Similar histograms are also available for white blood cells and platelets.
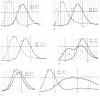
Figure 152.1
Red cell histograms in various conditions. (A) Heterozygous beta thalassemia. (B) Poor iron utilization (R-E block, chronic disease). (C) Iron deficiency anemia. (D) Dimorphic anemia—iron deficiency, recent transfusion. (E) Macrocytic anemia (liver (more...)
MCV defines the size of the red blood cells and is expressed as femtoliters (10−15; fl) or as cubic microns (μm3). The normal values for MCV are 87 ± 7 fl.
MCH quantifies the amount of hemoglobin per red blood cell. The normal values for MCH are 29 ± 2 picograms (pg) per cell.
MCHC indicates the amount of hemoglobin per unit volume. In contrast to MCH, MCHC correlates the hemoglobin content with the volume of the cell. It is expressed as g/dl of red blood cells or as a percentage value. The normal values for MCHC are 34 ± 2 g/dl.
RDW represents the coefficient of variation of the red blood cell volume distribution (size) and is expressed as a percentage. The normal value for RDW is 13 ± 1.5%.
Technique
Red cell indices MCV, MCH and MCHC are calculated from hemoglobin, hematocrit, and red blood cell count as follows:

Most clinical laboratories now use automated machines to perform blood counts (commonly called CBC) that include red cell indices as part of the profile. Two types of automated machines are generally used. Instruments like the Coulter S model employ the principle of electric impedance; others, like the Hemalog System Analyzer, use optical methods in performing cell counts. Most of the automated machines give the following values: white cell count, red cell count, platelet count, hemoglobin, hematocrit, MCV, MCH, and MCHC. Newer machines, capable of calculating RDW or red cell morphology index, mean platelet volume, absolute lymphocyte count, and differential white cell count ate now being used in many clinical laboratories. These instruments are also capable of producing histograms.
While the automated cell counters are fast, convenient, and precise, certain conditions can interfere with machine calculations and result in spurious values. It is important that clinicians become familiar with the more common causes of spurious results with electronic counters (Table 152.1):
Table 152.1
Spurious Results with Automated Cell Counters.
- In red cell agglutination, doublet erythrocytes are counted as one, and larger clumps are not counted as red blood cells at all. This leads to a "decrease" in red cell count and a falsely elevated MCV. Determination of the hemoglobin value is not affected. Prewarming the sample eliminates these spurious values.
- In hyperglycemia, red cells are transiently hypertonic in relation to the isotonic diluting fluid, resulting in swollen cells and an elevated MCV. This can be avoided if some time is allowed for equilibration after dilution.
- Hemoglobin is quantified based on its absorption characteristics. Conditions such as hyperlipidemias, hyperbilirubinemia, a very high white blood cell count, and high serum protein can interfere with this measurement and result in falsely elevated hemoglobin values.
- Presence of immunoglobulins or fibrinogen precipitated by low temperatures in the blood sample leads to interference with cell counts, resulting in spuriously increased white blood cell count and sometimes small elevations in hemoglobin, hematocrit, red blood cell count, and a slight decrease in MCV. Prewarming the sample to 37°C will correct the artificial values.
- When the values of hemoglobin, red cell count, and MCV are affected, MCH and MCHC also become abnormal, since these indices are calculated and are not directly measured.
Sometimes a set of spurious values may be the first clue to an otherwise unsuspected clinical condition (e.g., the combination of low hematocrit, normal hemoglobin, and high MCV and MCHC is characteristic of cold agglutinins). The MCV, since it is an average value, can be normal in the presence of two different cell populations (e.g., dimorphic anemias, red cell fragmentation with reticulocyte response). It is, therefore, important to examine the peripheral smear in the evaluation of anemias. When available, RDW is a good indicator of the degree of anisocytosis. Similarly, the red cell histogram, which offers a graphic depiction of red cell size distribution, will reveal anisocytosis even when the MCV is normal.
Basic Science
During erythropoiesis, the process of erythroid maturation involves a progressive condensation of nuclear chromatin (termed nuclear maturation) and finally its extrusion from the cell, the synthesis of hemoglobin in the cytoplasm (termed cytoplasmic maturation), and a concomitant reduction in cell size due to division and water loss.
Defects in nuclear maturation, as seen in megaloblastic anemias due to folate or B12 deficiency, result in large oval erythrocytes (macroovalocytes) with a normal hemoglobin content. The MCV and MCH are increased, while the MCHC remains normal. There is anisocytosis, and RDW is often increased. In the macrocytosis of liver disease, where there is no defect in nuclear maturation, the cells are large due to an excess red cell membrane. These cells are round, rather than oval, and the RDW is normal.
Defective hemoglobin synthesis results in small cells (low MCV) with or without anisocytosis. In heterozygous β-thalassemias, the cells are uniformly small (low MCV; RDW tends to be normal), whereas in iron deficiency, anisocytosis (increased RDW) may be the first laboratory abnormality, even before anemia and microcytosis are seen.
In abnormalities involving nuclear maturation, hemoglobin production proceeds normally, while cell division lags behind, ultimately leading to a larger than normal cell. In contrast, when there is defective and delayed synthesis of hemoglobin, the continued cell division leads to microcytosis.
Clinical Significance
Anemias may be classified based on their etiology (e.g., hemolytic, hemorrhagic, etc.), erythropoietic response (e.g., hypoproliferative, ineffective), or cell morphology (e.g., macrocytic, microcytic-hypochromic).
Red cell indices are valuable in the morphologic classification of anemias. Since different etiologic factors result in characteristically different red cell morphology, the clinician can properly plan the management of a patient with an anemia if he can interpret the blood counts and peripheral blood smear well.
Anemias are classified, according to the size of the red cell, as being normocytic (normal MCV), macrocytic (increased MCV), or microcytic (decreased MCV). Microcytic anemias were also often described as being hypochromic based on peripheral smear examination and MCHC when this value was determined manually. MCHC as measured by the electronic machines is mostly normal in microcytic anemias, however, and the value of MCH closely parallels the value of MCV. The optical properties of the small, thin microcytes make them appear hypochromic on the blood smear, while the hemoglobin concentration remains in the normal range (microcytic, normochromic anemias).
There are no hyperchromic anemias. In spherocytosis, the MCHC is increased due to loss of membrane and the consequent spherical shape assumed by the cell.
The general availability of RDW as a measure of anisocytosis helps further in the evaluation of anemias based on morphology (see Table 152.2). Significant anisocytosis often leads to an increased RDW, whereas in its absence the RDW remains normal.
Table 152.2
MCV and RDW in the Evaluation of Anemias.
It should be pointed out again that an evaluation of anemias is not complete without the careful examination of a well-prepared peripheral blood smear. Red cell indices, RDW, and red blood cell histograms will not help identify conditions such as red cell inclusions (e.g., malarial parasites) or membrane abnormalities such as spherocytosis that might be responsible for the anemia.
References
- Bessman JD, Gilmer PR, Gardner FH. Improved classification of anemias by MCV and RDW. Am J Clin Pathol. 1983;80:322–26. [PubMed: 6881096]
- Bessman JD, Gilmer PR, Gardner FH. Too early to put down RDW for discriminating iron deficiency and thalassemia. Am J Clin Pathol. 1986;86:693–5. [PubMed: 3776927]
- Cornbelt J. Spurious results from automated hematology cell counters. Lab Med. 1983;14:509–14.
- Gottfried EL. Erythrocyte indexes with the electronic counter. N Engl J Med. 1979;300:1277. [PubMed: 431694]
- Johnson CS, Tegos C, Beutler E. Thalassemia minor: routine erythrocyte measurements and differentiation from iron deficiency. Am J Clin Pathol. 1983;80:31–36. [PubMed: 6858962]
- McClure S, Custer E, Bessman JD. Improved detection of early iron deficiency anemia in non-anemic subjects. JAMA. 1985;253:1021–23. [PubMed: 3968826]
- Payne BA, Pierre RV, Morris MA. Use of instruments to obtain red blood cell profiles. J Med Tech. 1985;2:379–88.
- Rose MS. Epitaph for the MCHC. Br J Med. 1971;4:169. [PMC free article: PMC1799061] [PubMed: 5113025]
- Williams WJ. Examination of the blood. In: Williams WJ, Beutler E, Erslev AJ, Lichtman MA, eds. Hematology, 3d ed. New York: McGraw-Hill, 1983;9–14.
- Wintrobe MM. Principles of hematologic examination. In: Wintrobe MM, ed. Clinical hematology, 8th ed. Philadelphia: Lea & Febiger, 1981;7–19.
- [Clinical reference values for laboratory hematology tests calculated using the iterative truncation method with correction: Part 1. Reference values for erythrocyte count, hemoglobin quantity, hematocrit and other erythrocyte parameters including MCV, MCH, MCHC and RDW].[Rinsho Byori. 1990][Clinical reference values for laboratory hematology tests calculated using the iterative truncation method with correction: Part 1. Reference values for erythrocyte count, hemoglobin quantity, hematocrit and other erythrocyte parameters including MCV, MCH, MCHC and RDW].Shiga S, Koyanagi I, Kannagi R. Rinsho Byori. 1990 Jan; 38(1):93-103.
- The prognostic roles of red blood cell-associated indicators in patients with resectable gastric cancers.[Transl Cancer Res. 2020]The prognostic roles of red blood cell-associated indicators in patients with resectable gastric cancers.Cui MT, Liang ZW, Sun YZ, Wu J, Lu H, Wang WJ, Xu MD, Jiang M, Li W, Qian J, et al. Transl Cancer Res. 2020 Apr; 9(4):2300-2311.
- Improving the diagnosis of myelodysplastic syndrome by red blood cell parameters.[Clin Transl Oncol. 2023]Improving the diagnosis of myelodysplastic syndrome by red blood cell parameters.Zhu Y, Han S, Chen X, Wu S, Xiong B. Clin Transl Oncol. 2023 Oct; 25(10):2983-2990. Epub 2023 Apr 21.
- Review Hemoglobin and Hematocrit.[Clinical Methods: The History,...]Review Hemoglobin and Hematocrit.Billett HH. Clinical Methods: The History, Physical, and Laboratory Examinations. 1990
- Review The red blood cell distribution width.[J Emerg Med. 1991]Review The red blood cell distribution width.Evans TC, Jehle D. J Emerg Med. 1991; 9 Suppl 1:71-4.
- Red Cell Indices - Clinical MethodsRed Cell Indices - Clinical Methods
Your browsing activity is empty.
Activity recording is turned off.
See more...