Over the past decade, noninvasive imaging of the peripheral arteries has evolved into a highly reliable alternative to invasive digital subtraction angiography. Both magnetic resonance angiography (MRA) and computed tomography angiography (CTA) are capable of identifying the location and grade of peripheral arterial disease (PAD) with high accuracy, and both modalities can be used to manage the entire spectrum of patients with suspected or known PAD. Although atherosclerotic PAD is the underlying cause of symptoms in the vast majority of patients, noninvasive cross-sectional imaging is also well suited for identification of alternative non-atherosclerotic causes such as vasculitis and other less frequent causes.
Keywords:
Magnetic resonance angiography (MRA), Computed tomography angiography (CTA), Peripheral arterial disease, Intermittent claudication, Chronic critical ischemia, Atherosclerosis, Non-atherosclerotic peripheral arterial diseaseLearning Objectives
To describe the most commonly used clinical classification systems for peripheral arterial disease
To describe the technical principles of both MRA and CTA of peripheral arteries
To learn about the diagnostic accuracy of both MRA and CTA for detection of peripheral arterial disease
To name several causes of non-atherosclerotic peripheral arterial disease
Technical advances over the past decade have facilitated fast and robust noninvasive imaging of the peripheral vascular tree in routine clinical practice for the entire spectrum of peripheral arterial disease (PAD). Both magnetic resonance angiography (MRA) and computed tomography angiography (CTA) are highly accurate methods that enable high-fidelity depiction of arterial anatomy, atherosclerotic plaque, and narrowing of the peripheral vasculature from the aorta down to the feet. In addition, due to their ability to depict extra-arterial anatomy of the entire lower extremity, both methods are well suited for the detection of non-atherosclerotic peripheral arterial disease.
Below, we first detail the clinical context and technical background of both MRA and CTA since high-quality peripheral vascular imaging demands careful attention to proper patient positioning and acquisition. We include suggestions for imaging protocols for both modalities. Subsequently, we discuss the most common clinical applications of both MRA and CTA. We conclude with a short discussion of the clinical efficacy of both methods.
20.1. Epidemiology and Different Manifestations of Peripheral Arterial Disease
Peripheral arterial disease refers to conditions affecting blood flow to the lower extremities due to obstruction of some part of the arterial system from the infrarenal aorta or further distally. Total disease prevalence based on objective testing has been evaluated in several epidemiologic studies and is in the range of 3–10%, increasing to 15–20% in persons over 70 years [1].
Although atherosclerosis is the underlying cause in the vast majority of cases, there are many diseases that can cause PAD (see below). It is important to keep in mind that causes of arterial obstruction vary primarily as a function of age. Identification of the underlying cause necessitates careful review of the patients’ history, symptoms, risk factors, and other medical conditions. In patients below 45 years of age, one should always consider non-atherosclerotic causes of PAD such as vasculitis, fibromuscular dysplasia, popliteal entrapment, cystic adventitial disease, and other uncommon entities, especially if there are little or no risk factors for atherosclerosis.
20.2. Clinical Background and Classification Systems
There are various classification systems for PAD. Clinically the primary distinction is between patients with intermittent claudication (IC) and patients with the more severe form of PAD, chronic critical ischemia (CCI). The former is usually limited to “single-level” disease, i.e., a stenosis or short occlusion in the iliac or femoral arteries. IC is a lifestyle limiting disease, and first-line treatment consists of treatment of risk factors and supervised exercise therapy [1]. There is a relative indication for invasive therapy, and the risk for major complications such as amputations is very low. This is opposed to patients with CCI, who have high-grade stenoses and/or occlusions at multiple levels of the vascular tree. In CCI resting perfusion is inadequate to meet basic metabolic demand, and patients suffer from rest pain and sometimes even tissue loss. Patients with CCI have an absolute indication for invasive treatment to restore adequate perfusion to the tissues subtended by the stenosed or occluded arteries. If perfusion is not restored, major complications such as permanent loss of function and amputation can result.
The simplest and most commonly used system to classify chronic hypoperfusion of the lower extremity is that described by Fontaine in 1954, who distinguishes four categories of PAD (Table ). A more elaborate system with six categories is the one described by Rutherford (Table ). The distinction between the two systems is primarily based on the addition of objective findings such as Doppler signals, arterial brachial index (ABI), and pulse volume recordings, in addition to anamnestic pain-free walking distance. Rutherford also described a system for classification of acute limb ischemia (Table ). There are various other clinical classification systems, all refinements of the systems by Fontaine and Rutherford. Hardman et al. review these additional PAD classification systems in [2].
The Fontaine classification system for peripheral arterial disease as first described in [28]
Rutherford classification for chronic limb ischemia [29]
Rutherford classification for acute limb ischemia
20.3. Technical Background of Peripheral MRA and Imaging Protocol
Magnetic resonance angiography of the peripheral vascular tree (pMRA) is a highly reliable method to identify arterial stenoses and obstructions, and several meta-analyses reported high sensitivities and specificities for the detection of angiographically proven arterial narrowing [3, 4]. Currently, two methods are used: contrast-enhanced pMRA and non-contrast-enhanced pMRA. Contrast-enhanced techniques are most commonly used although the latter method is potentially more attractive because no injection of contrast agent is needed.
20.3.1. Contrast-Enhanced Techniques
The typical pMRA imaging protocol consists of imaging of three consecutive fields of view (FOV) in rapid succession during infusion of a gadolinium-based contrast agent. Imaging parameters and spatial resolution are optimized for each FOV to balance the required time to complete the acquisition versus the level of detail required to optimize detection of arterial narrowing with high sensitivity and specificity.
The arterial system is imaged with a heavily T1-weighted sequence during the first arterial passage of the contrast agent. Because the length of the peripheral vascular tree exceeds the FOV of the MRI scanner, it takes three to four acquisitions to depict the abdominal aorta and the lower extremities. In patients with IC a “top-to-bottom” approach whereby the acquisition commences in the abdomen followed by acquisitions of the upper and lower leg stations usually suffices to obtain the necessary information for clinical decision-making. Occasionally, venous enhancement is encountered in the lower leg station that may hamper detection of stenosis in the lower leg arteries. Although this may degrade image quality, it almost never leads to a study that yields insufficient information for clinical management since treatment is primarily focused on the aortoiliac and superficial femoral arteries. In patients with CCI, this is usually supplemented with a dedicated dynamic acquisition with a separate contrast agent injection to depict lower leg arterial anatomy. High-fidelity depiction of the outflow vasculature is essential in patients with CCI because treatment options may involve percutaneous transluminal angioplasty (PTA) or bypass surgery involving lower leg or pedal arteries.
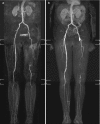
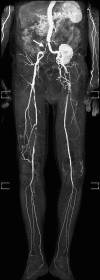
Injection of contrast agent renders the vascular system bright, while signal from the background is suppressed due to the short repetition time (TR). The vascular tree is typically displayed using maximum intensity projections (MIP) to provide a complete overview of the vascular system at a glance. In order to obtain sufficient background suppression to identify small peripheral arteries, it is essential to suppress signal from fat, since this has the lowest T1 of any tissue in the body. The most commonly used strategy to suppress fat is to use subtraction. This approach requires acquisition imaging the peripheral vascular tree without the injection of contrast agent with identical imaging parameters [5]. The acquired images are subtracted from the images with contrast agent with the aim to suppress signal from fat and background tissue (Fig. ). Advantages of this approach are the high vessel-to-background contrast and the simplicity (i.e., it can be applied on virtually any MRI scanner). Disadvantages are the √2 drop in signal to noise and the additional time it takes to acquire the images without contrast agent. Also, the resulting subtracted images may suffer from misregistration artifacts in case of patient motion between the acquisition with and without contrast agent of the same anatomical location. To avoid false-positive diagnosis of stenosis, contrast-enhanced source images always need to be evaluated. A second approach to suppress signal from fat is to combine the acquisition with Dixon-based water-fat decomposition techniques [6]. In contrast to subtraction, there is no need to acquire to same anatomy twice. Instead, images are acquired with two instead of one echoes for every TR, which allows to decompose the signal of protons in water and fat in each voxel. Subsequently, images can be reconstructed in which only the water component is shown and the signal from fat is disregarded (Fig. ).
Maximum intensity projection (MIP) of three-station MRA of the peripheral arteries in a 67-year-old female. Image on the left (a) is obtained using subtraction. Image on the right (b) is obtained using a non-subtracted Dixon technique
20.3.2. Non-contrast-Enhanced Techniques
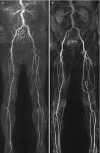
There has been considerable interest in non-contrast or native MR angiography in recent years. The excellent review by Lim and Koktzoglou [7] will provide the interested reader with a technical overview. An in-depth discussion of the different techniques is beyond the scope of this chapter because of the wide variety of methods offered by different vendors. To date most experience in the peripheral vasculature has been obtained with quiescent interval single-shot magnetic resonance angiography (QISS). QISS angiography is particularly useful for imaging the lower extremities because the technique allows for large anatomical coverage in short imaging times (Fig. ). Also, good results have been obtained versus standard of reference techniques [8].
Maximum intensity projection (MIP) images of non-contrast MRA (a) and corresponding contrast-enhanced MRA (b) in a 66-year-old male with intermittent claudication. The number and severity of arterial stenoses correspond well between the two methods. (more...)
20.4. Technical Background of Peripheral CTA and Imaging Protocol
Multi-detector CTA (MDCTA) has revolutionized vascular imaging due to its larger volumetric extent of coverage, rapid acquisition speeds, and high spatial resolution. Modern multi-detector CT scanners have tube rotation speeds as low as 0.28 s and slice thickness of 0.33 mm allowing submillimeter isotropic imaging, which is comparable to the gold standard digital subtraction angiography (DSA). Multi-detector CTA produces a larger volume of coverage per gantry rotation permitting rapid acquisitions from the abdomen to the toe in a matter of seconds. One advantage of this, in addition to patient comfort, is the potential for lowering contrast doses; however, contrast timing also becomes more critical with these devices due to higher acquisition speeds.
20.4.1. Technical Aspects
Image quality in CT angiography is dependent on both spatial resolution and temporal resolution. Axial in-plane spatial resolution within the scan plane is increased by smaller field of view, larger matrix size, and smaller focal spot. Through plane z-axis resolution is improved by having a greater number of smaller detectors. High isotropic spatial resolution with modern MDCTA has regular square-shaped voxels of 0.3–0.4 mm or less, allowing full reconstruction of the entire 3D dataset in any arbitrary orientation, making this technique arguably more powerful than DSA [9]. Noise, caused by random fluctuations in radiation exposure, is another important factor affecting image quality in MDCTA, reducing overall image contrast, and can be remedied by increasing tube current/voltage or increasing voxel size. Finally, image contrast which is the difference in intensity between one tissue and another can be improved by lower kVp but is also amplified in MDCTA by use of an iodinated contrast agent.
20.4.2. Scanner Design
Modern CT scanners have a wide array of detectors, with as many as 500–600 in a single row, which absorb the X-ray exposure from the X-ray tube during a single rotation. This allows greater anatomic coverage at high spatial resolution. This is combined with spiral CT mode, where the table and patient are moved steadily through the scanner gantry, as the tube is rotated around the patient. The relationship between patient and gantry motion is referred to as pitch, which is defined as table movement (mm) per tube rotation divided by collimator width (mm). A higher pitch, which is frequently used with CTA of the peripheral arteries, results in lower z-axis resolution but faster coverage and lower radiation exposure. Dual source CT technology consists of two X-ray tubes orientated at right angles to each other and two corresponding sets of detectors. This design improves temporal resolution and overall image quality. Dual-energy CT is when each of the two X-ray tubes on the dual source CT scanner emits X-rays at different energy levels and is detected by the corresponding detector array. The unique absorptive spectra allow discrimination of different tissues. In peripheral CTA, this may have a role in improved characterization of pathology in the vessel wall. Other manufacturers offer alternative scanner designs with rapid kV switching or dual-layer detector technology. Apart from the increased temporal resolution, the improved capability to discriminate tissues is similar.
20.4.3. Radiation Dose
CT is responsible for a significant share of overall radiation dosage to the patient population in medical imaging and MDCTA of the peripheral vasculature, with its extensive anatomic coverage, may result in higher than normal radiation exposure [10]. Radiation dose is dependent on tube current (mAs) and tube voltage (kV). Increasing mAs may improve image quality by increasing contrast to noise and lowering overall noise; however, radiation dose doubles with a doubling of mAs. Lower kV results in lower radiation exposure, but higher kV may be required to attain adequate penetration of the X-ray beam in larger patients. Adaptive scanning, whereby tube current is either reduced or switched off during rotations over sensitive body parts, such as the breast, can result in significant decreases in radiation dose. It has also been shown that peripheral CTA at tube currents as low as low as 50 mAs results in decreased radiation dose without a compromise in diagnostic efficacy [11].
20.4.4. Contrast Administration
With the more rapid acquisition speeds of MDCTA, contrast administration becomes challenging, and timing takes on added importance [12, 13]. High-quality CTA typically necessitates contrast densities of greater than 200 HU in vessels of interest. There are several factors affecting contrast enhancement and time to peak following contrast administration including iodine content [14], injection rate, and patient’s cardiac status. In general, higher injection rates will produce a higher peak of enhancement with a tighter bolus; injection rates of 4–5 mL/s are typical with CTA. Similarly, higher iodine content contrast medium will produce a greater enhancement peak, although when adjusted for iodine delivery rate differences are minimized. One disadvantage of modern scanners is that the scanner may “overshoot” the contrast bolus so that for peripheral CTA it may be necessary to slow down the acquisition by slowing the table speed [15].
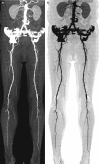
Accurate contrast timing is typically achieved with either bolus tracking or test bolus techniques. With bolus tracking, scan acquisition is triggered when contrast density reaches a predefined threshold (e.g., 150 HU in abdominal aorta for peripheral CTA), as defined by operator (Fig. ). The test bolus technique involves injecting a small dose of contrast (e.g., 20–30 mL) first to accurately measure the contrast transit time beforehand.
Scout image of the abdomen and lower extremities is used for planning the CT scan (a). Source CT images from CTA study of the abdomen, pelvis, and lower extremities following intravenous injection of iodinated contrast (b). Bolus tracking technique where (more...)
20.4.5. Lower Extremity CTA Technique
A scout view is typically carried out initially. A pre-contrast scan from the upper abdomen to the feet is optional and may be desirable in patients who may have extensive calcifications (e.g., diabetics, dialysis-dependent chronic renal failure) or those with metal in soft tissues (e.g., prior surgery, trauma with metallic foreign body) [16]. Bolus tracking is usually used for contrast timing in CTA of lower extremities. A region of interest (ROI) is placed in the abdominal aorta at the level of the celiac origin, and the scan acquisition is triggered when the density within the vascular lumen reaches more than 150 HU. Contrast agent is injected at 5 mL/s via a large bore (i.e., 18G) intravenous cannula. Images are acquired in a cranial to caudal direction with slice thickness of 1 mm or less and collimator thickness of 0.4–0.6 mm.
Typically, a slower scan time is preferable for lower extremity CTA so as not to overshoot the contrast bolus. Table pitch of 1.1, gantry rotation of 0.37 s, table increment of 21.1 mm/360° rotation, and table speed of 63 mm/s will have a scan time of 23 s; a fast contrast injection is preferred. If there is suspected severe disease with slow flow, then a slower contrast injection bolus and longer scan time may be preferred. This can be achieved by reducing the table speed through the scanner, i.e., the pitch, and by slowing the gantry rotation time.
Dual-energy MDCTA may have advantages at separating calcium from contrast in the vessel lumen. Subtraction of both datasets will produce a contrast only luminal image. Additionally, the calculation of a “virtual” non-contrast dataset will avoid the need for a separate acquisition and may reduce overall radiation exposure. Dual-energy MDCTA has shown high sensitivity for detecting stenosis compared to the gold standard DSA [17].
Dynamic CT, whereby multiphase CT acquisitions are acquired to follow contrast filling and drainage in a time-resolved manner, may have benefits for evaluating the infra-popliteal vessels, particularly in patients with slow or asymmetric flow. Dynamic CTA has been shown to have higher contrast and diagnostic confidence compared to conventional CTA [18]. Limitations of this technique include higher radiation dose and increased volumes of contrast.
20.4.6. Image Processing
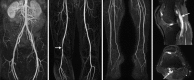
With fully isotropic submillimeter spatial resolution, it is possible to reconstruct the acquired CTA 3D dataset in any anatomic orientation. The 3D data is reconstructed with multiplanar reformatted (MPR), maximum intensity projection (MIP) and volume rendered (VR) algorithms (Fig. ). Basic axial, coronal, and sagittal MPR images are reconstructed automatically at the scanner immediately following the CT acquisition. Image data is then transferred to dedicated post-processing software where specific MIP and VR images are created. Curved MPR images of the vessel may be useful to accurately quantify the degree of stenosis and interrogate the vessel wall (Fig. ).
Coronal MIP images with positive (a) and negative (b) contrast from a patient with significant infra-popliteal peripheral vascular disease. Note incomplete bone removal in the pelvis, which is a limitation of automatic image post processing. In this case (more...)
Curved MPR displays the vessel along its entire length and allows accurate measure of stenosis and depiction of vessel wall, if needed
20.4.7. Diagnostic Accuracy
The diagnostic accuracy of CTA for detection and grading of peripheral arterial disease is high and similar to that of MRA. Several meta-analyses have been performed, and all of these report uniformly high values for sensitivity, specificity, and accuracy in patients with intermittent claudication [19–21]. There is a relative lack of data on the diagnostic accuracy of CTA in patients with CCI.
20.5. Clinical Applications
20.5.1. Atherosclerotic Peripheral Arterial Disease
In patients with suspected or known PAD, it is essential to determine and describe the exact location of arterial and extent of arterial lesions. Almost invariably, PAD affects the aorta and iliac and lower extremity arteries. In patients with diabetes mellitus, PAD typically presents with lower leg arterial involvement, while the proximal arteries are relatively spared. Apart from the aortic arch branch vessel origins, the upper extremity is almost never affected. The degree of stenosis is usually described as more or less than 50% luminal narrowing, and occlusions need to be mentioned separately. There is no consensus on how stenosis should be measured (i.e., percentage reduction of luminal diameter or percentage reduction in cross-sectional area); therefore, most observers use visual analysis to estimate the former. Both methods are highly accurate if images of good quality are available. Regardless of which method is used, sensitivity, specificity, and accuracy for detection of angiographically proven stenosis are generally in the range of 90–100% [3, 4, 19–21] with MRA and CTA.
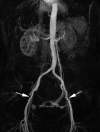
In patients with known stents or metal implants, image quality may be degraded with both MRA and CTA. For pMRA examinations are especially important that the requesting physicians note this on the study request in order to avoid false impression of stenosis. An example of stent artifacts is shown in Fig. .
There is signal loss in the right common iliac artery due to the presence of a stainless steel stent (arrow). Artifacts can be recognized by abrupt caliber change of arterial lumen. In this patient it is not possible to assess the degree of stenosis with (more...)
20.5.2. Aneurysmal Disease
Aneurysm is defined as a focal enlargement of an artery to more than 1.5 times its normal diameter. For the aorta the general cutoff value that warrants follow-up is 3.0 cm and for the iliac arteries 1.8 cm. Measurements of aneurysms should always be performed perpendicular to the center lumen line and should include any mural thrombus as well as the arterial wall (Fig. ). True aneurysms involve the intima, media, and adventitial layers of the vessel. Aneurysm is categorized as false when fewer than three layers are involved.
Partial volume maximum intensity projection of bilateral common iliac artery aneurysms in a 68-year-old male (a). Diameter measurements of aneurysms should always be performed perpendicular to the center lumen line to avoid over- or underestimation of (more...)
20.5.3. Non-atherosclerotic Peripheral Arterial Occlusive Disease
Although atherosclerosis is by far the most common cause of PAD, there are other causes as well. Any disease process that leads to narrowing or occlusion of peripheral arteries may lead to the typical history and complaints of IC or CCI. Non-atherosclerotic causes of PAD should be considered in young patients or patients without typical risk factors. Depending on the underlying disease process, involvement of specific anatomic sites or characteristic angiographic findings may be seen.
20.5.3.1. Vasculitis
Vasculitis refers to the process of acute or chronic inflammatory changes of small, medium, or large arteries as well as veins. In addition to signs of vascular narrowing or occlusion, patients typically present with systemic signs such as fever, malaise, weight loss, and abnormal laboratory tests such high erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), as well as leukocytosis with granulocytosis, thrombocytosis, and normochromic/normocytic anemia. The latter reflect the acute phase response. It is important to understand that there are no universally accepted diagnostic criteria for large-vessel vasculitides, including giant cell arteritis and Takayasu arteritis. More information about the diagnostic aspects can be found in the excellent review by Keser and Aksu [22]. Both CTA and MRA can suggest the diagnosis of vasculitis, but nowadays whole-body PET imaging is often used in the diagnostic workup. There is a large list of inflammatory arteriopathies that may lead to symptoms of PAD. Below, we discuss the commonly encountered diseases.
20.5.3.2. Takayasu Arteritis
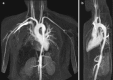
Takayasu arteritis (TA) is a large vessel vasculitis that may affect the aorta, its main branches, as well as the upper extremities. Occasionally, the iliac arteries are involved. The disease mainly affects younger women and can go unrecognized for a prolonged period due to its relatively low prevalence. Besides the typical history of vague, non-specific complaints, TA is characterized by a specific morphological pattern of arterial narrowing; stenoses typically have an elongated “hourglass” aspect (Fig. ). This is in contrast to the more serrated and abrupt appearance of atherosclerotic stenoses. Both MRA and CTA are excellent diagnostic tools for diagnosis of TA [23].
Coronal (a) and sagittal (b) maximum intensity projections of thoracic MR angiogram show typical smooth elongated “hour-glass” narrowing of the descending thoracic aorta in a 22-year-old female patient with Takayasu disease. PET scanning (more...)
20.5.3.3. Thromboangiitis Obliterans
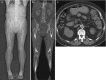
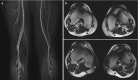
Thromboangiitis obliterans (TAO) is an uncommon segmental inflammatory arteriopathy that affects medium- and small-sized arteries, veins, and nerves of the arms and legs. The typical clinical scenario is that of a young male heavy smoker [24]. The main differential diagnosis in case of distal lower extremity involvement is atherosclerotic PAD in diabetes mellitus. However, the presence of diabetes rules out the diagnosis of TAO. Angiographically, TAO is characterized by short segmental occlusions of arteries or veins by inflammatory thrombi. Often, these occlusions are bridged by corkscrew collaterals. This is known as Martorell’s sign (Fig. ).
MR angiogram of a 29-year-old male patient with symptoms of critical ischemia in the left lower leg. The patient was a heavy smoker. Left panel (a) shows maximum intensity projection with impression of distal lower leg arterial occlusion and moderate (more...)
20.5.3.4. Fibromuscular Dysplasia
Fibromuscular dysplasia (FMD) is a noninflammatory arteriopathy that usually affects the medium-sized arteries such as the renal, carotid, and iliac arteries in young Caucasian women. Several subtypes are distinguished [25]. The characteristic angiographic appearance is the so-called string of beads which refers to the presence of multiple stenoses interspersed with aneurysmal dilatations (Fig. ).
A 37-year-old female patient with bilateral intermittent claudication. In both external iliac arteries, typical “string-of-beads” arterial narrowing can be seen (arrows). This finding is highly suggestive of fibromuscular dysplasia
20.5.3.5. Popliteal Entrapment
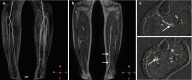
Popliteal artery entrapment refers to abnormalities of the popliteal fossa anatomy, whereby the popliteal artery is displaced medially by the medial head of the gastrocnemius muscle or when it courses anterior to the popliteus muscle [26]. The popliteal artery can be dynamically compressed when the gastrocnemius muscle is actively used. There are four types of popliteal entrapment that may be encountered. Types I–III refer to various degrees of medial displacement by the medial head of the gastrocnemius muscle, and in type IV, both the popliteal artery and vein course anterior to the popliteus muscle. Because the diagnosis of popliteal entrapment relies on precise anatomical knowledge of the popliteal fossa, it is necessary to supplement the MR angiography acquisition with high spatial resolution T2-TSE anatomical images of the popliteal fossa to visualize the relationship between the muscles and the vascular structures (Fig. ).
A 39-year-old male patient with complaints of intermittent claudication of the right leg. MR angiogram in neutral position shows normal caliber of popliteal artery on both sides. Supplemental T2-TSE images in neutral position (left) and during plantar (more...)
20.5.3.6. Cystic Adventitial Disease
Another non-atherosclerotic cause of PAD is cystic adventitial disease (CAD). In CAD, a mucoid cyst develops in the arterial wall which can lead to compression of the lumen and symptoms of PAD. The disease leads to smooth arterial narrowing of the affected artery. The cyst can easily be diagnosed by ultrasonography, CT, or MRI (Fig. ), although MRI is the technique of choice in case of suspected CAD due to its ability to definitively identify the mucoid cystic source of the arterial narrowing. Although infrequently encountered, treatment consists of simple percutaneous aspiration of the mucoid material which results in immediate relief of symptoms [27].
A 41-year-old male patient with intermittent claudication of the right leg. MR angiogram shows smooth luminal narrowing of the right popliteal artery. The age of the patient in combination with the aspect of the stenosis is highly suggestive for non-atherosclerotic (more...)
Take-Home Messages
MRA and CTA are highly reliable modalities to noninvasively depict the entire peripheral vascular tree.
Knowledge about the technical background of both MRA and CTA is helpful to obtain the best possible results with each modality.
MRA and CTA accurately depict the location and severity of atherosclerotic arterial obstruction.
Noninvasive imaging of the peripheral arteries can help elucidate the cause of symptoms in symptomatic patients with low likelihood of atherosclerotic peripheral arterial disease.
References
- 1.
Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, TASC II Working Group. Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg. 2007;45(Suppl 1):S5–67. [
PubMed: 17223489] [
CrossRef]
- 2.
- 3.
Nelemans PJ, Leiner T, de Vet HC, van Engelshoven JM. Peripheral arterial disease: meta-analysis of the diagnostic performance of MR angiography. Radiology. 2000;217:105–14. [
PubMed: 11012430] [
CrossRef]
- 4.
Menke J, Larsen J. Meta-analysis: accuracy of contrast-enhanced magnetic resonance angiography for assessing steno-occlusions in peripheral arterial disease. Ann Intern Med. 2010;153:325–34. [
PubMed: 20820041] [
CrossRef]
- 5.
Leiner T, Ho KY, Nelemans PJ, de Haan MW, van Engelshoven JM. Three-dimensional contrast-enhanced moving-bed infusion-tracking (MoBI-track) peripheral MR angiography with flexible choice of imaging parameters for each field of view. J Magn Reson Imaging. 2000;11:368–77. [
PubMed: 10767065] [
CrossRef]
- 6.
Leiner T, Habets J, Versluis B, Geerts L, Alberts E, Blanken N, Hendrikse J, Vonken EJ, Eggers H. Subtractionless first-pass single contrast medium dose peripheral MR angiography using two-point Dixon fat suppression. Eur Radiol. 2013;23:2228–35. [
PubMed: 23591617] [
CrossRef]
- 7.
Lim RP, Koktzoglou I. Noncontrast magnetic resonance angiography: concepts and clinical applications. Radiol Clin N Am. 2015;53:457–76. [
PubMed: 25953284] [
CrossRef]
- 8.
Amin P, Collins JD, Koktzoglou I, Molvar C, Markl M, Edelman RR, Carr JC. Evaluating peripheral arterial disease with unenhanced quiescent-interval single-shot MR angiography at 3 T. AJR Am J Roentgenol. 2014;202:886–93. [
PubMed: 24660721] [
CrossRef]
- 9.
Mahesh M, Cody DD. Physics of cardiac imaging with multiple-row detector CT. Radiographics. 2007;27:1495–509. [
PubMed: 17848705] [
CrossRef]
- 10.
Mettler FA, Wiest PW, Locken JA, Kelsey CA. CT scanning: patterns of use and dose. J Radiol Prot. 2000;20:353–9. [
PubMed: 11140709] [
CrossRef]
- 11.
Fraioli F, Catalano C, Napoli A, et al. Low-dose multidetector-row CT angiography of the infra-renal aorta and lower extremity vessels: image quality and diagnostic accuracy in comparison with standard DSA. Eur Radiol. 2006;16:137–46. [
PubMed: 15988586] [
CrossRef]
- 12.
Bae KT. Peak contrast enhancement in CT and MR angiography: when does it occur and why? Pharmacokinetic study in a porcine model. Radiology. 2003;227:809–16. [
PubMed: 12702823] [
CrossRef]
- 13.
Bae KT, Tran HQ, Heiken JP. Uniform vascular contrast enhancement and reduced contrast medium volume achieved by using exponentially decelerated contrast material injection method. Radiology. 2004;231:732–6. [
PubMed: 15163812] [
CrossRef]
- 14.
Fleischmann D. Use of high concentration contrast media: principles and rationale-vascular district. Eur J Radiol. 2003;45(Suppl 1):S88–93. [
PubMed: 12598032] [
CrossRef]
- 15.
Fleischmann D, Rubin GD. Quantification of intravenously administered contrast medium transit through the peripheral arteries: implications for CT angiography. Radiology. 2005;236:1076–82. [
PubMed: 16000649] [
CrossRef]
- 16.
Fleischmann D, Hallett RL, Rubin GD. CT angiography of peripheral arterial disease. J Vasc Interv Radiol. 2006;17:3–26. [
PubMed: 16415129] [
CrossRef]
- 17.
Kau T, et al. Dual-energy CT angiography in peripheral arterial occlusive disease—accuracy of maximum intensity projections in clinical routine and subgroup analysis. Eur Radiol. 2011;21:1677–86. [
PubMed: 21365195] [
CrossRef]
- 18.
Sommer WH, et al. Diagnostic accuracy of dynamic computed tomographic angiographic of the lower leg in patients with critical limb ischemia. Investig Radiol. 2012;47:325–31. [
PubMed: 22543967] [
CrossRef]
- 19.
Heijenbrok-Kal MH, Kock MC, Hunink MG. Lower extremity arterial disease: multidetector CT angiography meta-analysis. Radiology. 2007;245:433–9. [
PubMed: 17848679] [
CrossRef]
- 20.
Met R, Bipat S, Legemate DA, Reekers JA, Koelemay MJ. Diagnostic performance of computed tomography angiography in peripheral arterial disease: a systematic review and meta-analysis. JAMA. 2009;301:415–24. [
PubMed: 19176443] [
CrossRef]
- 21.
Jens S, Koelemay MJ, Reekers JA, Bipat S. Diagnostic performance of computed tomography angiography and contrast-enhanced magnetic resonance angiography in patients with critical limb ischaemia and intermittent claudication: systematic review and meta-analysis. Eur Radiol. 2013;23:3104–14. [
PubMed: 23801421] [
CrossRef]
- 22.
- 23.
Barra L, Kanji T, Malette J, Pagnoux C. CanVasc. Imaging modalities for the diagnosis and disease activity assessment of takayasu’s arteritis: a systematic review and meta-analysis. Autoimmun Rev. 2018;17:175–87. [
PubMed: 29313811] [
CrossRef]
- 24.
- 25.
Narula N, Kadian-Dodov D, Olin JW. Fibromuscular dysplasia: contemporary concepts and future directions. Prog Cardiovasc Dis. 2018;60:580–5. [
PubMed: 29534984] [
CrossRef]
- 26.
Lejay A, Ohana M, Lee JT, Georg Y, Delay C, Lucereau B, Thaveau F, Gaertner S, Chakfé N, Groupe Européen de Recherche sur les Prothèses Appliquées à la Chirurgie Vasculaire (GEPROVAS). Popliteal artery entrapment syndrome. J Cardiovasc Surg. 2014;55:225–37. [
PubMed: 24796917]
- 27.
- 28.
Fontaine R, Kim M, Kieny R. Surgical treatment of peripheral circulation disorders [in German]. Helv Chir Acta. 1954;21:499–533. [
PubMed: 14366554]
- 29.
Rutherford RB, Flanigan DP, Gupta SK, et al. Suggested standards for reports dealing with lower extremity ischemia. J Vasc Surg. 1986;4:80–94. [
CrossRef]
- 30.
Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26:517–38. [
PubMed: 9308598] [
CrossRef]


 1 and
1 and