NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Zhu MX, editor. TRP Channels. Boca Raton (FL): CRC Press/Taylor & Francis; 2011.
21.1. INTRODUCTION
21.1.1. TRP Channels in Caenorhabditis elegans
Transient receptor potential (TRP) family channels are conserved from Caenorhabditis elegans to humans. About 28 TRP members have been identified in mammals. On the basis of their sequence homology and functional similarity, these channels are further divided into seven subfamilies. Accumulating evidence shows that mammalian TRP channels are broadly involved in regulating sensory physiology, as they are important for sensing a wide variety of physical and chemical cues from both intracellular and extracellular sides.1
Through genetic screening and database searching, 17 TRP channels have been identified in C. elegans, and they cover all of the seven TRP subfamilies.2 The phylogenic relationship between each C. elegans TRP channel and some of their general functions are summarized in Figure 21.1. TRP channels in C. elegans may also form homo- or heterotetramers just like their mammalian homologs. Each worm TRP subunit is also believed to contain six transmembrane segments (S1–S6) with a putative pore-forming loop between S5 and S6. Interestingly, many C. elegans TRP channels have been shown to play important roles in sensory transduction, including chemosensation, mechanosensation, osmosensation, and proprioception,2 suggesting that TRP channels play an evolutionarily conserved role in sensory physiology in the animal kingdom.

FIGURE 21.1
TRP channels in C. elegans. Dendrogram plot of 17 C. elegans TRP channels using ClustalW algorism. The evolutionary distance between each TRP channel is indicated by the branch length in point accepted mutation (PAM) units. Some general functions of specific (more...)
As a powerful genetic model organism, C. elegans offers a number of benefits in characterizing the function and regulation of TRP channels in vivo. First, all TRP subfamilies are present in C. elegans, and many of them display high homology to their vertebrate counterparts. Second, multiple mutant strains are available for each C. elegans TRP channel. Third, the short generation time (∼3 days) and availability of rich genetic tools, together with a simple and well-characterized nervous system, make C. elegans a valuable system to characterize gene functions in vivo, particularly those with a role in the nervous system.
21.1.2. Physiological Roles of C. elegans TRP Channels
Among the three C. elegans TRPC members, TRP-1 and TRP-2 are widely distributed in multiple types of neurons,3,4 while TRP-3 is enriched in sperm.5 Consistent with its expression pattern, TRP-3 is required for sperm-egg interactions, and TRP-3 null mutant worms are infertile.5 By contrast, TRP-1 and TRP-2 mutant worms are superficially wild type. However, these mutant worms exhibit impaired neuronal Ca2+ transients and are defective in their response to nicotine.4 Interestingly, ectopic expression of human TRPC3 rescues the nicotine-dependent behavior in TRP-2 mutant worms,4 implying a conserved role of TRPC channels in nicotine-dependent behavior. As is the case with mammalian TRPC channels, the activation of C. elegans TRPC channels also appear to depend on Gq/11-coupled receptors and PLCβ.4
The TRPV channel OSM-9 was identified in a genetic screen for mutants with a defective response to osmotic shock and odorants.3,6 Subsequent homology cloning identified four other osm-9/capsaicin receptor-related (ocr) genes, ocr-1 to ocr-47. C. elegans TRPV channels are primarily expressed in sensory neurons.2 OSM-9 and OCR-2, the best-characterized TRPV channels in C. elegans, regulate chemosensation, osmosensation, and mechanosensation.3,8,9 This polymodal feature of OSM-9 and OCR-2 is observed in their mammalian homologs, such as TRPV1. Several C. elegans TRPV channels have been suggested to form heterotetramers,7,10 a feature that is also shared by many mammalian TRP channels. Furthermore, OSM-9 and OCR-2 are regulated by polyunsaturated fatty acids (PUFAs) like some Drosophila and mammalian TRP channels.11–13 However, while mammalian TRPV1-4 are heat-activated channels,14 C. elegans TRPV channels have not been found to display temperature sensitivity.
Four TRPM channels are present in C. elegans: GON-2, GTL-1, GTL-2, and CED-11. GON-2 (abnormal gonad development) is mainly expressed in the gonad and intestine, where it regulates gonad development and Mg2+ uptake, respectively.15,16 GTL-1 (gontwo like) is highly expressed in the intestine of C. elegans, where it plays an essential role in regulating Mg2+ homeostasis and defecation rhythm together with GON-2.16
TRP-4, the sole TRPN/NOMPC channel in C. elegans, is mainly expressed in a subgroup of sensory neurons and interneurons.17,18 Similar to its Drosophila and zebrafish homologs, TRP-4 is involved in mechanosensation and is required for detecting mechanical attributes from the bacteria lawn on which worms navigate.18,19 More interestingly, TRP-4 also regulates proprioception by modulating the worm’s body posture through the stretch-sensitive neuron DVA.18
The C. elegans genome encodes two TRPA channels, TRPA-1 and TRPA-2. TRPA-1 displays about 40% similarity to mouse and Drosophila TRPA1, while TRPA-2 is more distantly related and lacks the long ankyrin-like repeats found in TRPA1.20 TRPA-1 is widely expressed in many cell types, including neurons, muscles, and epithelium.20 In OLQ and IL1 but not ASH neurons, TRPA-1 is involved in head withdrawal and nose touch responses.20 Calcium imaging experiments show that in OLQ neurons TRPA-1 is involved in nose touch-evoked Ca2+ transients, suggesting that TRPA-1 functions in mechanosensation.20
PKD-2 (polycystic kidney disease-related), the sole TRPP channel in C. elegans, shares 33% sequence identity and 52% similarity with its human homolog PKD2 (also called TRPP2).21 PKD-2 is colocalized with the TRPP1 protein LOV-1 in male-specific neurons where it regulates male mating behavior.21 Cilia targeting of PKD-2 is required for its normal function.21
CUP-5 (coelomocyte uptake-defective) is the C. elegans homolog of TRPML (mucolipin) and is mainly expressed in coelomocytes and some head neurons.22 Similar to its mammalian homolog, CUP-5 is important for the biogenesis of late endosomes and lysosomes.23 In addition, cup-5 mutant worms display an abnormal accumulation of apoptotic cells,24 a phenomenon that has also been observed in mucolipidosis type IV (MLIV) patients and a Drosophila model of MLIV.25
In summary, TRP channels in C. elegans are expressed in a wide range of cell types and tissues and play diverse functions, ranging from sensory perception to intracellular homeostasis. Overall, the physiological functions of many C. elegans TRP channels are well correlated with their vertebrate counterparts.
21.2. IDENTIFICATION OF TRP CHANNELS IN C. ELEGANS
A number of C. elegans TRP channels were cloned through classic forward genetic screens for mutants defective in behavior and development. These include OSM-9, GON-2, PKD-2, and CUP-5. For example, mutant alleles of osm-9, the first characterized C. elegans TRPV homolog, were isolated in a genetic screen for mutants that cannot avoid high osmotic stress, and the osm-9 gene was then identified through positional cloning.3,6 In this screen, wild-type N2 worms were mutagenized with ethyl methanesulfonate (EMS), causing random mutations across the genome at a rate of 5 × 10–4 to 5 × 10–2 per gene.26 The progeny of thousands of F1 animals were then assayed for osmotic avoidance response. Defective F2 single worms were cloned out for further analysis. After confirmation of the phenotype, the responsible gene was mapped to a small region of a defined chromosome with phenotypic markers.3,6 Single nucleotide polymorphism (SNP) markers are now more commonly used to map mutations. Subsequently, overlapping cosmids covering the mapped interval can be introduced individually or in combination into the mutant as a transgene through germ line transformation. Cosmids that rescue the behavioral defect must contain the gene, which can be further identified by genomic sequencing of the molecular lesions in the mutated gene followed by direct rescue of the mutant phenotype with the wild-type gene through germ line transformation.3 Recent advances in deep-sequencing technologies have shown that mutant genes can be directly identified through whole-genome sequencing at an affordable cost for organisms like C. elegans, whose genome size is about one-thirtieth of that of mammals.27 It is anticipated that this approach will be widely utilized for cloning in the future.
Subsequent database searching identified four C. elegans homologs of osm-9: ocr-1 to ocr-4. The TRPM members GTL-1, GTL-2, and CED-11 were identified through the same strategy following the positional cloning of the first C. elegans TRPM member GON-2.2 Members in the other three TRP subfamilies (TRPC, TRPN, and TRPA) were all identified by homology cloning.2
21.3. GENETIC CHARACTERIZATION OF TRP CHANNELS IN C. ELEGANS
Several downstream effectors of TRP channels have been identified through classic forward genetic screens. For example, an unexpected role of the TRPV channels OSM-9 and OCR-2 in serotonin synthesis has been revealed by screening EMS-mutagenized worms. These worms carry a GFP transgene driven by the promoter of tph-1, a gene essential for the synthesis of serotonin.28 By screening ∼6500 worms, Zhang et al. isolated several mutant strains that displayed greatly reduced GFP expression specifically in the ADF serotonin neurons. One mutant strain each was mapped to ocr-2 and osm-9.28 Thus, in ADF neurons, the TRPV channels OSM-9 and OCR-2 act upstream of serotonin synthesis. This illustrates the power of forward genetic screens in revealing new functions of TRP channels. Forward genetic screens also provide a useful tool to dissect the signaling molecules downstream and upstream of C. elegans TRP channels. For instance, cup-5-null mutant worms are lethal.29 Schaheen et al. carried out a genetic screen for mutants that suppress the lethal phenotype of cup-5 worms. MRP-4, an ABC transporter, has been identified as the downstream suppressor of CUP-5.29 Using a similar approach, several suppressors of gon-2 have been isolated. One such suppressor has been cloned and called gem-4 (gon-2 extragenic modifier), a gene that is a member of the conserved copine family of calcium-dependent phosphatidylserine-binding proteins.30 GEM-4 is predicted to be membrane associated and may potentially inhibit GON-2 activity. Suppressor screens may identify novel genes that functionally interact with TRP channels.
Reverse genetics has also been applied to study TRP channels in C. elegans, including TRP-1-4, OCR-1-4, GTL-1, and TRPA-1. The first step in reverse genetics is to obtain mutant alleles of the studied gene based on its sequence information. The most commonly used method is chemical-induced gene deletion. A large pool of wild-type worms is mutagenized by EMS (∼13% of EMS-induced mutations are deletions) or trimethylpsoralen (TMP)/UV (∼50% TMP/UV-induced mutations are deletions).26 The deletion events can be readily detected by PCR with primers corresponding to the genes of interest.26 Two consortiums (http://celeganskoconsortium.omrf.org/ and http://www.shigen.nig.ac.jp/c.elegans/index.jsp) have been formed to generate deletion alleles for all worm genes, which are freely available to the research community. In addition, classic homologous recombination techniques have also been developed to knockout and knockin genes in C. elegans.31,32 These approaches may become widely used in the future.
In the past decade, the development of RNA interference (RNAi) techniques has offered a powerful tool to study gene functions.33 Sequence-defined double-stranded RNAs (dsRNA) cause potent and long-lasting degradation of endogenous mRNA of the corresponding gene.33 Currently, dsRNA can be introduced into C. elegans via three routes: microinjection, soaking, and bacterial feeding.34 The choice of method depends on the experimental requirement. In general, microinjection and soaking of dsRNA are applied to small-scale experiments and can produce a potent gene knockdown effect. Bacterial feeding is less labor intensive and thus a better fit for large-scale experiments, though its knockdown effect is sometimes less effective. In this protocol, bacteria produce dsRNA from a plasmid that carries a DNA fragment of the gene of interest under the control of an IPTG-inducible T7 promoter at both 5′ and 3′ ends, which can be directly fed to worms.34 A brief protocol is described as follows:
- Inoculate the bacterial strain HT115 carrying an RNAi plasmid overnight.
- Seed about 300 μL liquid culture on regular NGM plates containing 25 μg/ mL carbenicillin and 1 mM IPTG.
- Let the plate sit overnight at room temperature to induce the expression of double-strand RNA.
- Transfer several (∼10) young adult worms to RNAi plates to lay eggs for 5 hours.
- After eggs hatch and reach L4 stage, transfer them to new RNAi plates and let them grow for 1–2 days.
Mutant worms isolated through reverse genetic screens or worms treated with RNAi can then be subjected to phenotypic analysis using a host of behavioral and functional assays as described below.
21.4. BEHAVIORAL CHARACTERIZATION OF C. ELEGANS TRP CHANNELS
Multiple behavioral assays have been developed to test the in vivo functions of TRP channels in C elegans. Here we give a brief description of these assays.
Chemotaxis assay: C. elegans TRPV channels are mainly involved in chemosensation, osmosensation, and mechanosensation.35 Among the best characterized TRPVs are OSM-9 and OCR-2, and several behavioral assays have been performed to study their functions. In osm-9 or ocr-2 mutant worms, the AWA and ASH neuron-mediated sensory functions are largely defective, including chemotaxis to odors and nose touch and high osmotic stress-induced avoidance responses.3,7 Additionally, they are important for social feeding behavior mediated by the ASH and ADL neurons.8 Chemotaxis assay was originally developed by Ward and later modified by Bargmann et al.36,37 Briefly, 10 mL chemotaxis agar (1.6% agar, 5 mM K3PO4 [pH 6.0], 1 mM CaCl2, 1 mM MgCl2) is poured into a 10-cm petri dish. Test compound and control (∼1–2 μL) are placed on opposing sides of the agar surface. A group of adult worms is washed three times with S Basal (0.1 M NaCl, 0.05 M K3PO4, 5 μg/mL cholesterol) to remove bacteria and then placed at the center of the test plate with an equal distance to the test compound and control. At different time points, the number of worms at the test compound area and control area are counted to determine the chemotaxis index (CI). In many cases, a small drop of NaN3 (1M, 1–2 μL) is also applied to both spots to paralyze worms once they reach there, which greatly facilitates counting.
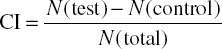
Nose touch response: The sensory neurons ASH, OLQ, and FLP mediate the nose touch response in C. elegans with ASH playing a dominant role.38 Wild-type worms respond to gentle nose touch by initiating robust backward movement,38 and several TRP channel mutants are defective in this response.7,20 In this assay, an eyelash hair is placed right in front of the path of a forward-moving worm as shown in Figure 21.2b. A nose-on collision with the eyelash usually stops the worm from moving forward and also evokes backward movement. Testing plates should contain a thin layer of OP50 bacteria because the nose touch response on food is much more robust. However, too many bacteria may allow the worm to crawl through under the hair. osm-9, ocr-2, and TRPa-1 mutant worms all show defects in this response.

FIGURE 21.2
Behavior tests of TRP channels in C. elegans. (a) Chemotaxis assay in which a group of worms is placed at the middle line of the plate, while test compound and control diluent are at opposite sides. The number of worms at both sites is counted at different (more...)
High-osmolarity avoidance assay: To test osmotic avoidance behavior, the osmotic ring assay is typically used for population analysis, while the droplet assay is usually employed for testing individual worms. When the nose of a worm reaches high-smolarity environment, it usually stops forward movement and moves backward immediately. Unseeded NGM plates are used for these assays. For the ring assay, wild-type worms are usually retained within the high-osmolarity ring for at least 10 min, while most osm-9 and ocr-2 mutant worms escape, as they are severely defective in this avoidance behavior. To make a high-osmolarity ring barrier, one can soak a test tube cap in 20–50 μL 1–4 M glycerol or fructose solution and then place the open end on the plate to print a ring.39 In order to better visualize the ring, a trace amount of bromophenol blue may be included in the solution. After three washes with S basal solution, young adult worms are placed at the center of the ring as shown in Figure 21.2c. The number of worms that have crawled through or are retained within the ring is counted 10 min after the onset of the assay, and the escape rate can be calculated. In the droplet assay, a high-osmolarity solution is absorbed into a capillary pipette. A small drop of solution is then gently dipped close to the nose tip of a forward-moving worm as shown in Figure 21.2d. After contacting the droplet, wild-type worms typically pause and initiate backward movement immediately. A robust avoidance response is observed in wild-type worms but is absent in osm-9 or ocr-2 mutants.
Defecation cycles: In the TRPM subfamily, little is known about GTL-2 and CED-11, while GON-2 and GTL-1 are highly expressed in the intestine and required for maintaining normal defecation cycles.40,41 Defecation occurs rhythmically every 40–45 s with little variance.42 Each cycle consists of four well-defined stages: (1) posterior body-wall muscle contraction (pBoc), (2) relaxation, (3) anterior body-wall muscle contraction (aBoc), and (4) expulsion.40,42 This behavior can be readily observed under a stereoscope. At the cellular level, IP3 receptor (IP3R)-dependent calcium oscillation in intestine cells is critical for this rhythmic contraction.41,43 Defecation cycles are disrupted in gon-2; gtl-1 double mutant worms. Consequently, these animals are constipated. Ca2+ oscillation is disrupted in the mutant.41
Locomotion assay: Unlike TRP-3, which is specifically expressed in sperm and required for sperm-egg fusion during fertilization, TRP-1 and TRP-2 are expressed in neurons, suggesting that they may be involved in behavioral control. Although TRP-1 and TRP-2 mutant worms are superficially wild type, an unexpected role of these two TRPC channels has been revealed in nicotine-dependent behavior. Similar to rodent models, worms display acute, adaptation, sensitization, and withdrawal responses to nicotine.4 Nicotine effects can be quantified by measuring the locomotion velocity in response to nicotine treatments. This can be achieved by counting the body bending frequency through human observation or, ideally, using an automated worm tracking system. Interestingly, in TRP-1 and TRP-2 mutant worms, these nicotine-dependent behavioral responses are abolished.4 Moreover, 2-APB, a nonspecific TRPC channel blocker, completely blocks nicotine-dependent behavior in C. elegans, supporting a critical role of TRP-1 and TRP-2 in nicotine dependence.4 For practical assays, fresh nicotine or other types of drugs can be dissolved in warm nematode growth media (NGM) and poured into petri dishes. Alternatively, nicotine solution may be directly spread on the surface of NGM plates, which should be allowed to diffuse throughout the plate overnight before use. As nicotine is light-sensitive, assay plates should be kept in the dark and used within a week. C. elegans TRPN (TRP-4) channels are likely mechanosensitive. The TRP-4 mutant worms are defective in locomotion behavior. These worms exhibit a loopy body posture during locomotion.18 To quantify this phenotype, locomotion behavior is recorded using an automated worm tracking system, and the body posture of the worm is determined by examining the video clips.18 To do so, the worm body is artificially divided into 12 segments, and the bending angle between each segment can be quantified accordingly. Worms reduce their locomotion velocity upon encountering a bacteria lawn, the so-called basal slowing response that is mediated by the mechanosensory dopamine neurons CEP, ADE, and PDE. These neurons sense the mechanical attributes from the bacteria lawn.44 The TRP-4 mutant worms are defective in basal slowing response, suggesting that TRP-4 may be the mechanosensitive channel in dopamine neurons. An automated worm tracker can also be used to quantify this behavioral response.
Foraging assay: The TRPa-1 mutant worms display defective head foraging movement and nose touch response.20 Foraging behavior is manifested by oscillating nose movement when worms explore their environment on food. This behavior requires the function of the OLQ and IL1 mechanosensory neurons.45 The TRPa-1 mutant worms display a reduced rate of foraging behavior. In response to touch delivered by a hair pick on the top of the head or anterior segment of a forward-moving worm, the worm usually initiates backward movement, and the foraging behavior is inhibited during backward movement.20 The inhibition of foraging in wild-type worms reaches ∼80%, whereas only about 30% inhibition was observed in TRPa-1 mutants, suggesting that TRPA-1 functions in mechanosensation.20
Male mating behavior: PKD-2, the sole TRPP member in C. elegans, plays an important role in male mating behavior together with the C. elegans polycystin-1 homolog LOV-1. Male mating behavior consists of a series of stereotypic steps executed by male worms upon encountering hermaphrodites, including response to contact, backing, turning, location of vulva, spicule protraction, and sperm transfer.46 This behavior can be quantified by human observation under a stereoscope. The pkd-2 mutant males display a strong phenotype at the step of location of the vulva. PKD-2 is expressed in male-specific sensory neurons that are considered both chemosensory and mechanosensory.21
21.5. FUNCTIONAL CHARACTERIZATION OF TRP CHANNELS IN C. ELEGANS
Functional assays are needed to understand the molecular and cellular details of TRP channels that cannot be obtained through behavioral analysis. As most TRP channels are nonselective cation channels, two major assays are constantly employed to study various aspects of these channels: Ca2+ imaging and electrophysiological recording.
The optical transparency of the worm body makes it possible to perform calcium imaging in a noninvasive manner. To achieve noninvasive recording, genetically encoded Ca2+ indicators (e.g., G-CaMP and cameleon) are expressed as a transgene in defined cell types using cell-specific promoters.47,48 Cameleon is a FRET-based sensor, and upon calcium binding, the ratio of YFP/CFP fluorescence of cameleon increases.48 This ratiometric readout of [Ca2+]i is largely insensitive to illumination intensity and uneven tissue distribution of the sensor. G-CaMP is a nonratiometric sensor that is based on a single GFP variant whose fluorescence intensity increases upon calcium binding.47 Nonratiometric [Ca2+]i measurement is sensitive to light intensity and uneven intracellular distribution of the sensor. Li et al. co-expressed a red fluorescence protein (DsRed) as an internal reference marker together with G-CaMP in the same cells, thereby enabling ratiometric imaging.
In vivo Ca2+ imaging experiments have been performed to record the function of many C. elegans TRP channels. Typically, worms are glued on a 2% agarose pad with Nexaband cyanoacrylate glue.18 For cameleon imaging, CFP bleed-through is a concern for calculating the ratio change and should be subtracted. The ratio change is tabulated as
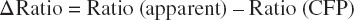
where

where I indicates the fluorescence intensity and Ratio (CFP) is typically assumed to be 0.6.19
Several C. elegans TRP channels have been functionally expressed in heterologous systems, such as HEK293 cells and CHO cells. In these cases, it is plausible to study these channels with chemical Ca2+ indicators. For example, C. elegans TRPC channels have been successfully expressed in HEK293T cells. Store-and receptor-operated Ca2+ entry have been measured in TRPC channel-expressing HEK293T cells.5,18
Another important method to study ion channels is patch-clamp recording. The advantages of electrophysiological recordings over Ca2+ imaging include (1) outstanding temporal resolution, (2) higher sensitivity, (3) direct measurement of biophysical properties of ion channels, and (4) easy access to the intracellular content of recorded cells. However, owing to the small size of most C. elegans cells, especially neurons where most TRP channels are expressed, whole-cell patch-clamp recording of ion channel activity in their native environment is technically challenging. In the past decade, conventional whole-cell voltage-clamp and current-clamp techniques have been developed to record C. elegans neurons.49 This approach can be applied to study TRP channels in vivo. The general setup is similar to that used for conventional brain slice recording. General information about whole-cell patch-clamp techniques has been discussed in many textbooks and reviews.50–52 Here we just describe some special aspects pertaining to whole-cell recording of C. elegans cells.
Prior to recording, worms are glued with Histoacryl Blue (B. Braun, Melsungen, Germany) on a cover slip coated with Sylgard 184 (Dow Corning, Midland, MI). Glue is typically applied with a mouth pipette pulled from borosilicate glass. If the region to be recorded is on the ventral side of the worm, glue should be applied to the dorsal side, and vice versa. After the worm is glued, the cuticle is cut open with a sharp glass capillary. Intrinsic hydrostatic pressure within the worm body forces out part of the intestine, gonad, and eggs. These tissues may be removed. A small drop of collagenase (0.4 mg/mL in bath solution) is often applied to briefly digest tissues to facilitate access of recording pipettes. In most cases, cells of interest are marked by a transgene expressing fluorescent proteins. This allows for easy identification of cells for recording.
Alternatively, ion channels can be recorded from primary cultured C. elegans embryonic cells. Cultured embryonic cells typically maintain many in vivo features. For example, they retain the expression of some cell-specific markers,53,54 and importantly, may undergo partial differentiation in culture.53 A detailed protocol of culturing C. elegans embryonic cells has been described.55 Briefly, a large quantity of C. elegans eggs can be isolated by lysing adult worms with bleach-based buffer (for 25 mL lysis buffer, mix 5 mL fresh Chlorox bleach, 1.25 mL 10 N NaOH, and 18.75 mL sterile H2O). A seven-minute treatment is usually sufficient to break 70% of adult worms. The lysis reaction can then be terminated by adding egg buffer (118 mM NaCl, 48 mM KCl, 2 mM CaCl2, 2 mM MgCl2, 25 mM Hepes, pH 7.3, 340 mOsm). After several rounds of centrifugation and resuspension in egg buffer, pellets are resuspended and centrifuged in 30% sucrose to separate eggs from debris. Isolated eggs are digested for ∼20 min with 4 mg/mL chitinase (Sigma) to remove eggshell. After digestion, cells are washed and dissociated using a syringe needle in L-15 culture medium (in 500 mL L-15 medium [Gibco], add 50 mL heat-inactivated Fetal Bovine Serum, 7.7 g sucrose, and 5 mL 1:100 Pen/Strep). Dissociated cells are plated on polylysine-pretreated cover slips and cultured at room temperature. Practically, transgenic worms expressing fluorescent protein markers in specific cell types are used, allowing for easy recognition of cells of interest for recording. Conventional patch-clamp techniques can be directly applied to these cultured embryonic cells. The C. elegans TRPM channels GON-2 and GTL-1 have been studied using this approach.16,41 One disadvantage of this approach is that cultured neurons have lost their native synaptic connections, and some of their neuronal properties may not be preserved.
As discussed previously, several C. elegans TRP channels have been functionally expressed in heterogonous systems.3,5,18,35 For these TRPs, heterologous systems offer a powerful tool to study the biophysical properties of these channels in vitro.
21.6. CONCLUSION
The power of C. elegans genetics provides a unique opportunity to study gene functions in vivo. All TRP channel subfamilies are present in the C. elegans genome, and importantly, many C. elegans TRP channels share similar functions and modes of regulation with their vertebrate counterparts.2 Thus, characterization of C. elegans TRP channels may provide valuable insights into the function and regulation of their vertebrate homologs. Genetic screens in C. elegans have identified several endogenous regulators and effectors of TRP channels. More signaling molecules upstream and downstream of TRP channels are expected to be identified through genetic approaches in C. elegans. Owing to technical reasons, most studies of C. elegans TRP channels were performed using genetic and behavioral approaches. Electrophysiological recording results of TRP channels in C. elegans are rather limited. Thus, the biophysical properties of most C. elegans TRP channels remain to be determined. The recent development of in situ patch-clamp recording techniques in C. elegans will foster a thorough understanding of these channels.
ACKNOWLEDGMENTS
We thank the members of the Xu lab for the insightful comments. Work in the lab is supported by the NIGMS, NIDA, and Pew Scholar Award (X. Z. S. X.).
REFERENCES
- 1.
- Clapham D.E. TRP channels as cellular sensors. Nature. 2003;426(6966):517–524. [PubMed: 14654832]
- 2.
- Xiao R, Xu X.Z. Function and regulation of TRP family channels in C. elegans. Pflügers Archiv. 2009;458(5):851–860. [PMC free article: PMC2857680] [PubMed: 19421772]
- 3.
- Colbert H.A, Smith T.L, Bargmann C.I. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. Journal of Neuroscience. 1997;17(21):259–269. [PMC free article: PMC6573730] [PubMed: 9334401]
- 4.
- Feng Z. et al. A C. elegans model of nicotine-dependent behavior: regulation by TRP-family channels. Cell. 2006;127(3):621–633. [PMC free article: PMC2859215] [PubMed: 17081982]
- 5.
- Xu X.Z, Sternberg P.W. A C. elegans sperm TRP protein required for sperm-egg interactions during fertilization. Cell. 2003;114(3):285–297. [PubMed: 12914694]
- 6.
- Colbert H.A, Bargmann C.I. Odorant-specific adaptation pathways generate olfactory plasticity in C. elegans. Neuron. 1995;14(4):803–812. [PubMed: 7718242]
- 7.
- Tobin D. et al. Combinatorial expression of TRPV channel proteins defines their sensory functions and subcellular localization in C. elegans neurons. Neuron. 2002;35(2):307–318. [PubMed: 12160748]
- 8.
- de Bono M. et al. Social feeding in Caenorhabditis elegans is induced by neurons that detect aversive stimuli. Nature. 2002;419(6910):899–903. [PMC free article: PMC3955269] [PubMed: 12410303]
- 9.
- Kahn-Kirby A.H. et al. Specific polyunsaturated fatty acids drive TRPV-dependent sensory signaling in vivo. Cell. 2004;119(6):889–900. [PubMed: 15607983]
- 10.
- Jose A.M. et al. A specific subset of transient receptor potential vanilloid-type channel subunits in Caenorhabditis elegans endocrine cells function as mixed heteromers to promote neurotransmitter release. Genetics. 2007;175(1):93–105. [PMC free article: PMC1774992] [PubMed: 17057248]
- 11.
- Chyb S, Raghu P, Hardie R.C. Polyunsaturated fatty acids activate the Drosophila light-sensitive channels TRP and TRPL. Nature. 1999;397(6716):255–259. [PubMed: 9930700]
- 12.
- Hwang S.W. et al. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(11):6155–6160. [PMC free article: PMC18574] [PubMed: 10823958]
- 13.
- Watanabe H. et al. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424(6947):434–438. [PubMed: 12879072]
- 14.
- Venkatachalam K, Montell C. TRP channels. Annual Review of Biochemistry. 2007;76:387–417. [PMC free article: PMC4196875] [PubMed: 17579562]
- 15.
- Sun A.Y, Lambie E.J. gon-2, a gene required for gonadogenesis in Caenorhabditis elegans. Genetics. 1997;147(3):1077–1089. [PMC free article: PMC1208235] [PubMed: 9383054]
- 16.
- Teramoto T, Lambie E.J, Iwasaki K. Differential regulation of TRPM channels governs electrolyte homeostasis in the C. elegans intestine. Cell Metabolism. 2005;1(5):343–354. [PMC free article: PMC2241660] [PubMed: 16054081]
- 17.
- Walker R.G, Willingham A.T, Zuker C.S. A Drosophila mechanosensory transduction channel. Science. 2000;287(5461):2229–2234. [PubMed: 10744543]
- 18.
- Li W. et al. A C. elegans stretch receptor neuron revealed by a mechanosensitive TRP channel homologue. Nature. 2006;440(7084):684–687. [PMC free article: PMC2865900] [PubMed: 16572173]
- 19.
- Kindt K.S. et al. Dopamine mediates context-dependent modulation of sensory plasticity in C. elegans. Neuron. 2007;55(4):662–676. [PubMed: 17698017]
- 20.
- Kindt K.S. et al. Caenorhabditis elegans TRPA-1 functions in mechanosensation. Nature Neuroscience. 2007;10(5):568–577. [PubMed: 17450139]
- 21.
- Barr M.M, Sternberg P.W. A polycystic kidney-disease gene homologue required for male mating behaviour in C. elegans. Nature. 1999;401(6751):386–389. [PubMed: 10517638]
- 22.
- Fares H, Greenwald I. Regulation of endocytosis by CUP-5, the Caenorhabditis elegans mucolipin-1 homolog. Nature Genetics. 2001;28(1):64–68. [PubMed: 11326278]
- 23.
- Treusch S. et al. Caenorhabditis elegans functional orthologue of human protein h-mucolipin-1 is required for lysosome biogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(13):4483–4488. [PMC free article: PMC384773] [PubMed: 15070744]
- 24.
- Hersh B.M, Hartwieg E, Horvitz H.R. The Caenorhabditis elegans mucolipin-like gene cup-5 is essential for viability and regulates lysosomes in multiple cell types. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(7):4355–4360. [PMC free article: PMC123652] [PubMed: 11904372]
- 25.
- Venkatachalam K. et al. Motor deficit in a Drosophila model of mucolipidosis type IV due to defective clearance of apoptotic cells. Cell. 2008;135(5):838–851. 2008. [PMC free article: PMC2649760] [PubMed: 19041749]
- 26.
- Jansen G. et al. Reverse genetics by chemical mutagenesis in Caenorhabditis elegans. Nature Genetics. 1997;17(1):119–121. [PubMed: 9288111]
- 27.
- Sarin S. et al. Caenorhabditis elegans mutant allele identification by whole-genome sequencing. Nature Methods. 2008;5(10):865–867. [PMC free article: PMC2574580] [PubMed: 18677319]
- 28.
- Zhang S. et al. Caenorhabditis elegans TRPV ion channel regulates 5HT biosynthesis in chemosensory neurons. Development. 2004;131(7):1629–1638. [PubMed: 14998926]
- 29.
- Schaheen L, Patton G, Fares H. Suppression of the cup-5 mucolipidosis type IV-related lysosomal dysfunction by the inactivation of an ABC transporter in C. elegans. Development. 2006;133(19):3939–3948. [PubMed: 16943270]
- 30.
- Church D.L, Lambie E.J. The promotion of gonadal cell divisions by the Caenorhabditis elegans TRPM cation channel GON-2 is antagonized by GEM-4 copine. Genetics. 2003;165(2):563–574. [PMC free article: PMC1462791] [PubMed: 14573470]
- 31.
- Berezikov E, Bargmann C.I, Plasterk R.H. Homologous gene targeting in Caenorhabditis elegans by biolistic transformation. Nucleic Acids Research. 2004;32(4):e40. [PMC free article: PMC390312] [PubMed: 14982959]
- 32.
- Vazquez-Manrique R.P. et al. Improved gene targeting in C. elegans using counter-selection and Flp-mediated marker excision. Genomics. 2009;95(1):37–46. [PubMed: 19747540]
- 33.
- Fire A. et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391(6669):806–811. [PubMed: 9486653]
- 34.
- Ahringer J. Reverse genetics. In WormBook. ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.47.1.
- 35.
- Kahn-Kirby A.H, Bargmann C.I. TRP channels in C. elegans. Annual Review Physiology. 2006;68:719–736. [PubMed: 16460289]
- 36.
- Ward S. Chemotaxis by the nematode Caenorhabditis elegans: identification of attractants and analysis of the response by use of mutants. Proceedings of the National Academy of Science of the United States of America. 1973;70(3):817–821. [PMC free article: PMC433366] [PubMed: 4351805]
- 37.
- Bargmann C.I, Hartwieg E, Horvitz H.R. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell. 1993;74(3):515–527. [PubMed: 8348618]
- 38.
- Kaplan J.M, Horvitz H.R. A dual mechanosensory and chemosensory neuron in Caenorhabditis elegans. Proceedings of the National Academy of Science of the United States of America. 1993;90(6):2227–2231. [PMC free article: PMC46059] [PubMed: 8460126]
- 39.
- Hart A.C. Behavior. In WormBook. 2006. ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.87.1.
- 40.
- Kwan C.S. et al. TRPM channels are required for rhythmicity in the ultradian defecation rhythm of C. elegans. BMC Physiology. 2008;8:11. [PMC free article: PMC2409367] [PubMed: 18495023]
- 41.
- Xing J. et al. Highly Ca2+-selective TRPM channels regulate IP3-dependent oscillatory Ca2+ signaling in the C. elegans intestine. Journal of General Physiology. 2008;131(3):245–255. [PMC free article: PMC2248719] [PubMed: 18299395]
- 42.
- Thomas J.H. Genetic analysis of defecation in Caenorhabditis elegans. Genetics. 1990;124(4):855–872. [PMC free article: PMC1203977] [PubMed: 2323555]
- 43.
- Dal Santo P. et al. The inositol trisphosphate receptor regulates a 50-second behavioral rhythm in C. elegans. Cell. 1999;98(6):757–767. [PubMed: 10499793]
- 44.
- Sawin E.R, Ranganathan R, Horvitz H.R. C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron. 2000;26(3):619–631. [PubMed: 10896158]
- 45.
- Hart A.C, Sims S, Kaplan J.M. Synaptic code for sensory modalities revealed by C. elegans GLR-1 glutamate receptor. Nature. 1995;378(6552):82–85. [PubMed: 7477294]
- 46.
- Barr M.M, Garcia L.R. Male mating behavior. In WormBook. 2006. pp. 1–11. [PMC free article: PMC4780960] [PubMed: 18050467]
- 47.
- Nakai J, Ohkura M, Imoto K. A high signal-to-noise Ca2+ probe composed of a single green fluorescent protein. Nature Biotechnology. 2001;19(2):137–141. [PubMed: 11175727]
- 48.
- Miyawaki A. et al. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature. 1997;388(6645):882–887. [PubMed: 9278050]
- 49.
- Lockery S.R, Goodman M.B. Tight-seal whole-cell patch clamping of Caenorhabditis elegans neurons. Methods in Enzymology. 1998;293:201–217. [PubMed: 9711611]
- 50.
- Sakmann B, Neher E. Patch clamp techniques for studying ionic channels in excitable membranes. Annual Review of Physiology. 1984;46:455–472. [PubMed: 6143532]
- 51.
- Sakmann B, Neher E. Single-channel Recording. 2nd ed. New York: Plenum Press; 1995.
- 52.
- Walz W. In Neuromethods. 2nd ed. Totowa, NJ: Humana Press; 2007. Patch-clamp analysis: advanced techniques.
- 53.
- Bianchi L, Driscoll M. Culture of embryonic C. elegans cells for electro-physiological and pharmacological analyses. In WormBook. 2006. pp. 1–15. [PMC free article: PMC4781032] [PubMed: 18050435]
- 54.
- Bianchi L. et al. The neurotoxic MEC-4(d) DEG/ENaC sodium channel conducts calcium: implications for necrosis initiation. Nature Neuroscience. 2004;7(12):1337–1344. [PubMed: 15543143]
- 55.
- Christensen M. et al. A primary culture system for functional analysis of C. elegans neurons and muscle cells. Neuron. 2002;33(4):503–514. [PubMed: 11856526]
- Review The TRP superfamily of cation channels.[Sci STKE. 2005]Review The TRP superfamily of cation channels.Montell C. Sci STKE. 2005 Feb 22; 2005(272):re3. Epub 2005 Feb 22.
- Review TRP channels: an overview.[Cell Calcium. 2005]Review TRP channels: an overview.Pedersen SF, Owsianik G, Nilius B. Cell Calcium. 2005 Sep-Oct; 38(3-4):233-52.
- Review [Recent advances on TRP channels].[Sheng Li Ke Xue Jin Zhan. 2008]Review [Recent advances on TRP channels].Han CY, Wang XL. Sheng Li Ke Xue Jin Zhan. 2008 Jan; 39(1):27-32.
- Review Functionality of the TRPV subfamily of TRP ion channels: add mechano-TRP and osmo-TRP to the lexicon![Cell Mol Life Sci. 2005]Review Functionality of the TRPV subfamily of TRP ion channels: add mechano-TRP and osmo-TRP to the lexicon!Liedtke W, Kim C. Cell Mol Life Sci. 2005 Dec; 62(24):2985-3001.
- Review Role of Transient Receptor Potential Channels in Acute and Chronic Itch.[Itch: Mechanisms and Treatment...]Review Role of Transient Receptor Potential Channels in Acute and Chronic Itch.Wilson SR, Bautista DM. Itch: Mechanisms and Treatment. 2014
- Studying TRP Channels in Caenorhabditis elegans - TRP ChannelsStudying TRP Channels in Caenorhabditis elegans - TRP Channels
Your browsing activity is empty.
Activity recording is turned off.
See more...
