NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Collaborating Centre for Nursing and Supportive Care (UK). Irritable Bowel Syndrome in Adults: Diagnosis and Management of Irritable Bowel Syndrome in Primary Care [Internet]. London: Royal College of Nursing (UK); 2008 Feb. (NICE Clinical Guidelines, No. 61.)
Update information March 2017 Recommendation 1.1.1.2 in the short version was updated by NICE with more recent guidance on recognition and referral for suspected cancer. Recommendation 1.1.1.3 in the short version was removed as it was no longer needed after the changes to recommendation 1.1.1.2. February 2015 NICE has made new recommendations relating to the clinical management (dietary and lifestyle advice, and pharmacological therapy) of people with IBS. The recommendations and evidence in sections 3, 7.6, 8.4, 8.5.2 and 9.1 of this guideline that have been highlighted in grey have been stood down and replaced. New recommendations on dietary and lifestyle advice, and pharmacological therapy, can be found in the irritable bowel syndrome in adults update CG61.1. September 2012 A recommendation in this guideline (see pages 28 and 37) has been partially updated by recommendation 1.1.2.1 in 'Ovarian cancer' (NICE clinical guideline 122, 2011).

Irritable Bowel Syndrome in Adults: Diagnosis and Management of Irritable Bowel Syndrome in Primary Care [Internet].
Show detailsINTRODUCTION AND BACKGROUND
Irritable bowel syndrome (IBS) is a chronic, relapsing and often life-long disorder. It is characterised by the presence of abdominal pain associated with defaecation, or a change in bowel habit together with disordered defaecation (constipation or diarrhoea or both), and the sensation of abdominal distension. Symptoms sometimes overlap with other gastrointestinal disorders such as non-ulcer dyspepsia, or with coeliac disease. Diagnosis of IBS has proven difficult historically for many reasons, not least that traditionally an exclusion diagnostic approach has been selected by clinicians. Each year, typically approximately 10% of the population will experience IBS symptoms, with up to half of these presenting to primary care clinicians. In reviewing the literature, it is clear that in the absence of gold standard diagnostic criteria, several criterion referenced diagnostic tools have emerged over the last two decades. These have been used in both prevalence and incidence studies, and have proven to be useful for clinicians in enabling them to provide a diagnosis for those patients presenting with IBS symptoms. These criteria have also allowed for standardisation of IBS diagnosis in research.
Definition
For the purpose of this guideline, IBS is defined using the Rome II criteria, used mainly in the context of research. The Rome group is a pan-European clinician group that have met for the last decade, seeking to provide both clarity and direction for clinicians and patients alike.
The Rome II criteria characterises IBS as:
The IBS population
IBS most commonly affects people between the ages of 20 and 30 years and is twice as common in women as in men. The prevalence of the condition in the general population in the UK is estimated to lie somewhere between 10 and 20%. Recent trends indicate that there is also a significant prevalence of IBS in older people; therefore, IBS diagnosis should be a consideration when an older person presents with unexplained abdominal symptoms. Because incidences of other conditions with similar symptoms are higher in the elderly population, use of certain diagnostic tests is warranted. Co-morbid conditions and poly-pharmacy are common in this patient population. The true prevalence of IBS in the whole population may be higher than estimated, because it is thought that many people with IBS symptoms do not seek medical advice; NHS Direct online data suggest that 75% of people using this service rely on self-care. In England and Wales, the number of people consulting for IBS is extrapolated to between 1.6 and 3.9 million. Evidence suggests that age and race have no consistent effect on the incidence of symptoms. Healthcare professionals need to be sensitive to and take into consideration cultural, ethnic and communication needs of people for whom English is not a first language or who may have cognitive and/or behavioural disabilities. Appropriate action should be taken to facilitate effective consultation.
Investigations commonly requested by clinicians
Primary care investigations are likely to include: routine blood tests such as full blood count, urea and electrolytes, liver function tests; tests for thyroid function, tissue transglutaminase anti-endomysial antibodies (test for coeliac disease); inflammatory markers, stool microscopy; urinary screen for laxatives; and lactose tolerance testing. Other investigations such as gut transit studies (radiological tests to measure the time required for food to move through the digestive tract) and sigmoidoscopy (endoscopy of the lower part of the bowel) are routinely performed in secondary care. Determining the criteria for such requests and appropriate referral into secondary care will be addressed in the guideline.
The need for effective diagnosis – clarifying concepts
IBS is associated with a disproportionately high prevalence of abdominal and pelvic surgery, although the cause of this has not been established. Diagnostic test methodology has traditionally been applied when comparing a new or alternative test with the acknowledged gold standard reference.
Gold standard reference points aim to represent the ‘truth’, and when a test is carried out there are four possible outcomes. These are:
- True positive (detects disease when present)
- False positive (detects disease when it is absent)
- True negative (can identify absence of disease)
- False negative (can identify someone as being disease free when they have it).
It is widely acknowledged within the literature that there is no gold standard reference for the diagnosis of IBS, which means that comparison of definitive diagnostic tests remains difficult. Diagnostic criteria in themselves can be seen as having enormous value, and these could be directly compared in other disease areas to the gold standard reference. In this narrative review of 170 studies/papers, comparisons of criteria are made against a definitive diagnosis of IBS through clinician expertise, augmented by a whole battery in many cases of diagnostic investigations.
In measuring accuracy of diagnostic test/criteria, two measures are used. These are sensitivity and specificity.
Sensitivity
This is a measure (usually expressed as a % of the total population that the test is applied to) that indicates how good the test is in identifying people with the disease.
Specificity
This is a measure (usually expressed as a % of the total population that the test is applied to) that indicates how good the test is in identifying people without the disease.
Problems associated with using these as single measures are acknowledged, as they are difficult to interpret for individual patients. For example, if a test has a sensitivity of 85%, what if I am one of the 15% that the test has failed to identify. In real world situations, what patients and clinicians generally want to know is ‘If this test is positive, does it mean that I have a positive diagnosis?’ or; ‘If this test is negative, does this mean that I do not have the disease?’
What may be more useful is for these single measures to be expressed as a probability; a likelihood of accuracy. Again this is expressed as a %, with positive tests measured against a whole study population who had the test. For example, 37 positive results out of 100 would be a 37% prediction, expressed as a positive predictive value (PPV) of 37% (Dawes et al, 2005). This can be viewed from the reverse perspective; how many negative tests were recorded out of the total study population who had the test. For example 63 negative results out of 100 would be a 63% prediction, expressed as negative predictive value (NPV) of 63%. Using real data (Steurer 2002), of 1000 women who received a positive mammogram result, 90 actually had breast cancer, meaning that the PPV for mammography is 9%. Converted to probability, this means that women have a 1 in 11 chance of having breast cancer if they have a positive mammogram result. Of the 12,102 negative mammogram results, 12,090 did not have breast cancer (meaning that 12 did have breast cancer). This means that the NPV for mammography is 99.9%. Converted to a probability, this means that women have a 1 in 1000 chance of having breast cancer if they receive a negative mammogram result. This is extremely useful to clinicians in trying to establish risk and/or probability of a disease in a particular individual, enabling them to articulate this to the person seeking consultation.
Odds ratios
This is another way of measuring test accuracy (see Appendix 2 of this chapter). Its real value is in estimating test accuracy. This is calculated using test Likelihood Ratio’s (LR) by taking the positive LR and dividing this by the negative LR. Likelihood ratios are useful in estimating the value of diagnostic tests, and as a general principle, the higher the likelihood ratio the more useful that test will be. A high odds ratio is an indicator of a good diagnostic test.
A main aim of the guideline
One of the main aims of this guideline is to identify diagnostic criteria for people presenting with symptoms suggestive of IBS and to ensure that primary care clinicians and people who may have IBS have a reference tool that is both sensitive and specific, with high predictive value of the syndrome. This is an area of healthcare practice which is currently absent, and creates great uncertainty for both clinicians and people who may have IBS.
OBJECTIVES
- To determine the effectiveness of diagnostic criteria for people with IBS.
SELECTION CRITERIA
The selection criteria for this systematic narrative review was to analyse all relevant literature related to diagnosis of IBS. Due to the absence of a gold standard reference for this disease, diagnostic review methodology was not applicable. On this basis, the GDG accepted that a systematic narrative review was the best way of measuring current practice against peer reviewed literature. This review formed the basis for GDG consensus discussions and recommendations for diagnosis of IBS. Studies identified were then quality assessed. Studies included in the review importantly had to have used a criterion referenced diagnostic tool, studies that failed to do so were excluded from the review. This ensured that all relevant studies provided the evidence base in validating a diagnostic tool, enabling primary care clinicians to make a positive IBS diagnosis around symptom recognition.
Types of studies
All published literature on IBS diagnosis was included. This resulted in a large search and, post-sifting, a large number papers being reviewed for potential inclusion in the review.
SEARCH STRATEGY FOR IDENTIFICATION OF THE LITERATURE
Searches were performed on the following core databases: MEDLINE, EMBASE, CINAHL and The Cochrane Library (1966 to current day with guidance from the GDG). Additional databases were not searched for this review. A sensitive search strategy was employed, as recommended by Haynes and Wilczynski (2004) in determining optimal search strategies for retrieving scientifically strong studies of diagnosis. The search strategies are listed in Appendix B.
METHODOLOGY
The benefits of a systematic narrative review of the clinical evidence in the absence of diagnostic test studies are highlighted by Oxman and colleagues. Applying the quality assurance principles advocated by Oxman (1994), a valid review article can provide the best possible source of information that can lay a foundation for clinical decisions to be made. There is argument that focused narrative reviews for individual outcomes, in this case, IBS diagnosis, are more likely to provide valid results that are useful for clinicians.
STRUCTURE OF THE REVIEW
Having provided the background and context for this review, diagnostic criteria are presented that emerge from the systematic narrative review of the literature. Data is presented in three main sections of the review.
In the first section, the evidence relating to the use of criterion based tools in the diagnosis of IBS is presented and discussed. The effectiveness data for each tool is summarized in Table 1 and specificity, sensitivity and positive predictive value of the criteria are reported where available. Studies included in the table illustrate how research has validated the use of these tools, where the criteria used is matched against a clinical reference standard (in all studies this was an expert gastroenterologist) by using specificity, sensitivity and positive predictive value data. The excluded studies are listed in Appendix E and are excluded on the basis that no criterion reference tool was used. This systematic narrative review is followed by a description of an interactive exercise used by the GDG to assess the strengths and weaknesses of each of the identified tools.
Table 1
Summary Table of Diagnostic Papers with diagnostic data provided (reference standard was expert gastroenterologist diagnosis).
In the second section, the evidence relating to the utility of tests to exclude alternative diagnoses is presented and discussed. This is followed by a review of the economic literature for diagnostic testing and an adaptation of one of the cost-effectiveness models identified in the economic literature review.
IBS DIAGNOSTIC CRITERIA
The use of diagnostic criteria has merged over the last three decades, with leading GI specialists such as Manning and Kruis leading the way. Such diagnostic criteria were forerunners to a consensus process amongst leading clinicians which became known as the Rome process. Rome III is the latest iteration and builds on the validated work from authors, in particular the Manning criteria.
Pre-Rome
The first paper to address diagnostic criteria for IBS was a working team report published in 1989 for the 1988 International Congress of Gastroenterology in Rome, Italy. This is acknowledged as the Rome criteria.
Establishment of Rome Committee Process
Following the 1989 publication, a committee was set up the same year to develop for the first time a classification system for all the 21 functional gastro intestinal disorders (FGID). This report was published in 1990 heralding the beginning of the Rome Criteria process. The criteria for IBS in 1989 did not feature pain as a symptom, which is now a current ROME criteria requirement for the diagnosis of IBS.
Rome I
From 1990–1995, seven committees formed to elaborate on the 1990 classification system. Knowledge of this classification system was quite limited, since the journal had a small circulation and was not listed in MEDLINE. The committee however were able to publish a book which featured the updated Rome I criteria in 1992 and it was the first time that pain was required for the diagnosis.
Rome II
By 1995, interest had grown from both clinicians and the pharma industry. Funding was secured from industry to support the development of Rome II. The number of committees was expanded, with wider international contributions forming the basis of this updated set of criteria. Emerging from this process, the criteria were available from 1999 and first published in 2000.
Rome III
Because of the success of the Rome II process, funding support from industry was forthcoming to maintain this consensus process. A co-ordinating committee was formed in 2001 for Rome III (Drossman, Corazziari, Delvaux, Spiller, Talley, Thompson and Whitehead). Work began in May 2003, leading to publication of new criteria in 2007.
Kruis criteria
The aim of the original study was to create a scoring system for IBS diagnosis incorporating history, physical examination and some basic investigations (ESR and blood count).
1Validated criterion reference tool reviewed and acknowledged as used within practice over the last 3 decades.
Kruis patient questionnaire
Download PDF (45K)
Kruis clinician questionnaire
Download PDF (39K)
Manning criteria
The patient should present with at least 2 of the following symptoms for an IBS diagnosis to be made:
- Onset of pain associated with more frequent bowel movements
- Onset of pain associated with more loose bowel movements
- Relief of pain with defaecation
- Abdominal distension
- Sensation of incomplete evacuation with defaecation
- Passage of mucus.
Rome Criteria
At the 13th International Congress of Gastroenterology in Rome in 1988 a group of physicians defined criteria to more accurately diagnose IBS. The Rome criteria are:
The patient should present with 3 months of continuous or recurring symptoms of abdominal pain or irritation that:
- May be relieved with a bowel movement
- May be coupled with a change in frequency, or
- May be related to a change in the consistency of stools.
Two or more of the following are present at least 25 percent (one quarter) of the time:
- A change in stool frequency (more than 3 bowel movement per day or fewer than 3 bowel movements per week)
- Noticeable difference in stool form (hard, loose and watery stools or poorly formed stools)
- Passage of mucous in stools
- Bloating or feeling of abdominal distention
- Altered stool passage (e.g. sensations of incomplete evacuation, straining, or urgency).
Rome I criteria (1992)
The patient should present with at least 3 months of continuous or recurrent symptoms for an IBS diagnosis to be made:
Abdominal pain or discomfort, which is:
- Relieved with defaecation
- and/or associated with altered bowel frequency
- and/or associated with altered stool consistency
- and/or two or more of the following, on at least 1/4 of days:
- Altered stool frequency
- Altered stool form
- Altered stool passage (straining, urgency or tenesmus)
- Passage of mucus
- Usually with bloating or a feeling of abdominal distension.
Rome II criteria
The Rome II Criteria, published in 2000, were developed by 10 multinational working teams that collaborated over 4 years to arrive at a consensus for symptom-based diagnostic standards.
Twelve weeks* or more in the past 12 months of abdominal discomfort or pain that has 2 out of 3 features:
- Relieved with defaecation
- Associated with a change in frequency of stool
- Associated with a change in consistency of stool.
*The twelve weeks need not be consecutive
The following are supportive, but not essential to the diagnosis. One or more are usually present. They add to the clinician’s confidence that the intestine is the origin of the abdominal pain. The more of these symptoms that are present, the greater the confidence with an IBS diagnosis:
- Abnormal stool frequency (> 3/day or < 3/week)
- Abnormal stool form (lumpy/hard or loose/watery) > 1/4 of defaecations
- Abnormal stool passage (straining, urgency or feeling of
- incomplete evacuation) > 1/4 of defaecations
- Passage of mucus > 1/4 of defaecations
- Bloating or feeling of abdominal distension > 1/4 of days.
Rome III Diagnostic Criteria*
Download PDF (216K)
RESULTS OF THE REVIEW AND DISCUSSION
Of the 170 papers reviewed, 45 were excluded as no diagnostic criteria were used in the study population, or they were discussion/professional papers highlighting aspects of care relating to IBS, of which diagnosis was mentioned. Of the remaining 125 papers, 18 studies provided useful data which has been extracted and presented in Table 1. The remaining papers all provided useful background information on the use of diagnostic criteria; many studies used the reference criteria as a way of measuring prevalence and incidence of IBS in general populations. Literature was drawn from a wide international base, with Europe, North America and South East Asia providing the main source of data.
Issues to consider
From this extensive review of the literature, a number of key observations have emerged which the GDG will need to consider in moving towards consensus opinion as to how IBS is diagnosed; which criteria to use in diagnosing IBS and how this potentially will move clinicians away from an exclusion diagnostic approach towards positive diagnosis of IBS, management and patient follow-up. Of significance is the potential cost saving to the NHS of tests that are routinely requested but prove to be of little added value.
Diagnostic thresholds
Clinicians need to be able to determine whether a person has IBS (or not). Balance between missing the diagnosis and over diagnosing is a possibility because criteria may be too vague. Thresholds can be set across different parameters. These include:
- Severity of bowel symptoms
- Symptom count – either all symptoms given equal weight (e.g. Manning – 2 or more symptoms being given equal weight) or identified symptoms being considered as essential with others considered as accessory symptoms (Rome I)
- Duration threshold in combination with symptom count (Rome II) and symptom frequency appears to be highly relevant (Mearin 2003)
- Rome II requires the presence of both abdominal pain and changes in bowel habit and duration of symptoms (at least 12 weeks in last 12 months)
- Rome considers abdominal pain and changes in bowel habit independently and no minimum duration of symptoms
- Kruis developed according to symptoms and evaluation of lab tests
- Following a positive diagnosis of IBS based on clinical criteria clinicians can be reassured that the diagnosis is durable. (Adeniji 2004, follow-up of 196 patients with a diagnosis of IBS between 1989–92 35/86 pts had had further diagnostic investigations without diagnosis changing)
- Prevalence of IBS measured across a New Zealand population cohort (N=980) using 2 of the manning criteria emerged as 18.1%. This decreased to 10.3% if 3 or more of the Manning criteria were used to identify an IBS diagnosis. This then fell to 3.3% when 4 or more of the criteria were used (Barbezat 2002).
A key question for the GDG was “Should a positive diagnostic approach include severity threshold or disabling threshold?”
What happens to patients who do not meet Rome II?
- How important is diagnostic precision for clinicians (this is a different priority for research)? Is the priority to exclude structural cause and/or red flags for symptoms?
- Would treatment be different if patients were diagnosed using Manning or Rome? Does this matter?
- Do they have alternative diagnosis (eg FBD) and/or go for lots of investigations and then turn out to be IBS?
Clinician ignorance of IBS diagnostic criteria
- An Italian study (Bellini 2005) of 28 generalist GPs – 17 judged knowledge of IBS to be insufficient but only three thought that further education might be useful. Ten GPs were familiar with Rome II prior to the study; 19 agreed with Rome II criteria when they used them as part of the study. They reported satisfactory management in approximately 60% of patients.
- Important symptoms for this group – Primary symptoms: changes in bowel habit, abdominal pain relieved by evacuation, bloating. Secondary symptoms: difficult or incomplete evacuation, passing mucous with BM.
- Following clinical evaluation GPs ordered further investigations in large numbers of patients. GPs with more than 20 yrs experience requested less investigations than younger colleagues (p= 0.001). 168/229 patients had routine bloods, 30 – abdominal ultrasound, 87 FOB, 83 faecal analyses, 81 – Thyroid Function, 70 – lower GI endoscopy.
- All agreed that counselling, reassurance, information about natural course of condition and suggestions for coping strategies were first step in management. Patients with diarrhoea were prescribed wider ranging therapeutic interventions and perceived to be in more need of further investigations than those with constipation.
- In 145/229 cases referral to at least one specialist was made (GI specialist most common but gynaecological referral for 19% of women). Referral did not vary with clinical presentation and the most common reasons for referral were diagnostic confirmation, patient need for reassurance and patient request.
- Patients frequently attribute food intolerance (37%) and stress (43%) as the causes of IBS. GPs consider stress (71%), fibre deficiency (83%) and disturbed motility (62%) as most important factors (Bijkerk 2003).
- Helpful clues to aid diagnosis: symptoms chronic or recurrent; pain is variable in location and timing; D and C may alternate; onset sometimes follows GI tract infection; findings on physical examination are usually normal except for abdominal tenderness (Paterson 1999, Canadian IBS position statement).
- USA Primary Care practice based diagnostic evaluations differ significantly from speciality expert opinion based guidelines. Implementation of speciality guidelines in Primary Care would increase utilisation but with limited improvement of diagnostic outcomes (Yawn 2001).
- Patients under 50 yrs of age who meet Manning and have no red flags require no investigations (Paterson et al 1999 Canadian IBS position statement).
- Patient expectations: reassurance, counselling, pharmacotherapy, diagnostic tests and referral to specialist. Dietary interventions were considered less important. Most people with IBS in this study stated that improvement in worst symptom should be target of treatment. Global improvement and QoL were considered much less important as treatment goal.
- A British study in general practice (n=400) Gladman and Gorard (2003) Sent a questionnaire to a random selection of 200 GPs and 200 clinician members of British Society of Gastroenterologists asking about their knowledge of functional GI disorders and their knowledge and use of Manning and Rome criteria for diagnosis. 68/137 GPs believed functional GI disorders were psychosomatic compared to 36/167 of consultants (p=<0.001). Consultants believed that understanding had increased over last 20 years; 50%GPs believed it had not changed. Both believed diagnosis and management had not improved in past 20 years. Only 29/137 GPs had heard of Manning; 16/137 of Rome compared to 134/166 and 139/167 Consultants respectively (p=0.0001). Only 18 GPs used either Manning or Rome in practice and despite increased awareness only 40% consultants used one or other diagnostic criteria in their practice.
Many studies of IBS and developed guidelines to date have been produced by specialists who had seen patients with particularly severe or intractable symptoms. This clinical guideline was developed from a different starting point, with the essential focus being in primary care. All development and implementation must be framed with questions of applicability to General Practice.
The GDG noted that GPs consider Rome II too complicated and more suited for use in secondary care or for research purposes (Thompson 1997; Bellini 2005). The need for a pragmatic useful diagnostic tool was felt to be the most important aspect to the review. As the majority of IBS patients are treated by GPs, any recommendations for the use of diagnostic criteria should ensure that their ease of use by GPs in their practice is established, rather than expecting GPs to use criteria that do not apply in the reality of day-to-day practice (Corsetti and Tack 2004).
What is the role of red flag symptoms alongside diagnostic criteria IBS diagnosis?
- The Manning criteria do not consider red flag symptoms. The addition of red flag symptoms seems to enhance diagnostic accuracy (Paterson 1999, Canadian IBS position statement). The GDG considered this aspect of the review at length, recognising the need for recommendations supporting the IBS algorithm to ensure that red flag symptoms take the patient out of this guideline and into other related NICE guidance.
- The addition of red flags to the Manning criteria increases the PPV of Manning and Rome I and II (Vanner 1999; Hammer 2004).
- Red flag symptoms – these seem to enhance original criteria and importantly relate this guidance to other relevant NICE guidelines, in particular NICE Clinical Guideline 27 ‘Suspected Cancer Referral’ published in 2005.
Discussion
The need for clinicians to be guided in the diagnosis of IBS has emerged as a strong recurrent theme in this systematic narrative review. The seminal work of Manning laid a foundation to enable clinicians to be guided by such criteria in attempting to provide direct answers to patients presenting with a range of symptoms. This work has undoubtedly influenced the development of thinking within the Rome group, and the Rome criteria recently published as Rome III reflects the benefit of validation of the key aspects of the criteria and pragmatic decisions relating to the length of presenting symptom such as pain (6 months). The use of available diagnostic criteria summarised in this review (Table 1) have typically been augmented by further diagnostic investigations that have limited or no benefit and these add considerable costs to the NHS.
The use of consensus agreement regarding the recommendation of single diagnostic criteria serves three main purposes:
- Increased patient confidence through positive diagnosis
- Increased clinician confidence
- Potential for considerable NHS disinvestment in avoiding unnecessary investigations and referrals to multiple specialities.
When looking at combinations of possible criteria used in the available diagnostic tools reviewed, the emergence of Rome III has proven to be timely in relation to guideline development. It features strengths of previous diagnostic criteria, while minimising weaknesses of reviewed tools.
Key questions that emerge from the literature aim at identifying a gold standard or best reference tool. The challenge is that when thresholds differ, results are inconsistent. For clinicians, diagnostic precision of IBS is often of low priority when compared to excluding other structural cause. This is a conceptual misinterpretation which can be explained as under-confident application of clinical examination and clinical history interpretation. Perhaps of significant note, regardless of which criteria were used in included studies in the review, treatment rarely differed against symptom profiles.
Over a decade ago, Jeong and colleagues having identified that Manning had reasonable specificity, called for better diagnostic criteria with improved accuracy to be developed. The road to Rome and the subsequent development of international consensus over the last 15 years has been useful in predetermining consistency in research application. It however, may have distracted from refinement of a tool that is easy and straightforward to use for primary care clinicians.
What clearly emerges from the literature is that with careful history and physical examination, positive diagnosis of IBS is possible. This, augmented by simple laboratory investigations to rule out more serious underlying pathology in the absence of red flag symptoms, would seem to be a step forward for both clinicians in diagnostic practice and patients in receiving timely interventions.
Perhaps it is fitting to highlight within this review that clinicians have been seeking to change the way that they think about diagnostic approaches in relation to this chronic syndrome. The Manning (1978) paper titled “Towards a positive diagnosis of Irritable Bowel Syndrome’ clearly indicates a diagnostic aim, this over the last three decades has been lost, with negative diagnosis by ruling out other conditions being the predominant clinician approach. Returning to the original aim of Manning and colleagues by seeking to influence the behaviour of primary care clinicians in the way that they think and approach diagnosis of people presenting with IBS symptoms is an important objective of this guideline.
GDG interpretation of the review and application of the guideline
General practitioner (GP) training has focussed on the importance of what happens within a typical patient consultation. This is usually recorded and analysed to enable new GPs to reflect on the detail within the consultation, in particular the quality of verbal and non-verbal behaviour, the sequencing of questions and information gathered to enable diagnosis. This is based around simulation and objective structured clinical examination methodology and has effectively enabled GP trainees to experience and develop understanding related to the importance of clinical history prior to physical examination. Using this approach, the NCC-NSC planned an interactive session for the GDG to fully engage with relevant issues. This was felt to be important in demonstrating that in guideline development, the GDG had explored the utility of different criteria that would then inform any consensus recommendations and lay the foundation for a positive implementation experience.
In order to test the utility of different criteria, NCC-NSC staff ran an interactive diagnostic simulation with members of the GDG. A number of typical IBS patient profiles were written by the technical team, which were then shared with four subgroups of the GDG. Details of the patient profiles are listed in Appendix 3 to this chapter.
Sub group constituency:
- Primary or secondary care doctor and/or
- Primary or secondary care nurse and/or
- Allied health professional (eg. Dietician or pharmacist) and/or
- Patient representation allocated to comment and input across the four groups.
One patient profile randomly selected from the total number of prepared patient profiles were randomly allocated to each of the GDG subgroups. Each group then had to discuss the use of allocated diagnostic criteria and elect one member of the group to role-play a GP consultation, responding to the simulated patient profile. Group A were asked to use Kruis Criteria; Group B were asked to use Manning criteria; Group C were asked to use Rome criteria;Group D were asked to use Rome II criteria.
Each group selected a physician to role-play the consultation, timed at a typical 8 minute general practitioner consultation. Two groups selected their GP member, with the other group selecting the GDG clinical lead who is a Gastroenterologist. Members of the NCC-NSC team role-played the four different patients. ROME III at the time of the patient simulation was unpublished, and therefore was unable to be used in this exercise. During each of the four role-plays, GDG members were asked to observe the consultation and record their observations. These typically related to the ease and logical progression of the consultation, shaped by the diagnostic criteria. This simulation enabled the GDG to both interpret the evidence and evaluate how easily the criterion reference based tool could work within a busy primary care environment.
The NCC-NSC technical team transcribed the detail within each of the GP consultations and recorded the information gathered from the patient using each of the three criteria referenced tools. The content was analysed and grouped in emerging themes (see Table 1) to enable the group to fully understand what was possible in recreating the primary care consultation. Typically patients are reticent to come and see a primary care clinician with issues relating to bowel habit/function, and this reticence was simulated with behaviours that demonstrated both hesitancy and embarrassment.
Observations were recorded from three main areas for feedback:
- How GDG members felt each of the diagnostic criteria worked in this simulated patient consultation.
- How the NCC team member felt when role playing the IBS patient, in relation to the sequencing of ideas and extracting of important patient information, facilitating an effective diagnosis.
This was a powerful exercise in embedding the evidence review into a simulated patient-clinician exchange. The importance of ensuring that the guideline recommendations are able to be effectively implemented into routine primary care is clearly important in ensuring that current variations in diagnosing IBS are addressed.
Outcomes from the evidence review and diagnostic criteria simulated exercise were:
- A strong evidence base for the use of diagnostic criteria with good predictive value.
- Agreement of evidence based positive diagnostic criteria for use in primary care which reflects current evidence.
- A contemporised Manning criteria which are consistent with ROME III criteria.
- The decision to refer to agreed criteria as ‘Positive Diagnostic Criteria’.
GDG DISCUSSION
Following the simulated consultation, members of the GDG discussed the high importance of good communication in establishing the clinician-patient relationship. Typically, the evidence demonstrates that diagnostic tendency within primary care is for an exclusion diagnostic approach which is experienced as a negative diagnosis by people with IBS. This can be time consuming, sub-optimal and cost inefficient in relation to unnecessary investigations that are likely to add little or no benefit to predictive value, and can mean that patients are subject to inappropriate referrals to other specialities such as gynaecology. The GDG reflected the importance of language used in the first meeting between people with IBS symptoms and primary care clinicians, supporting the positive diagnostic approach of ‘you have IBS’ as opposed to ‘all investigations are negative and you have nothing else wrong with you, it must be IBS’. This exclusion diagnosis approach is widely reported as typical in the patient population, supported by both patient representatives on the GDG.
Themes emerging from the exercise and focussed GDG discussion
- IBS is a lifelong condition that needs to be managed effectively.
- Symptoms that are most crucial in diagnosis are pain/discomfort relieved by bowel movement, bloating (more common in women; men describe it as abdominal tension) and disordered bowel habit. It was noted that language in the Manning criteria needed to be contemporised; this was discussed and agreed, e.g. pain was contemporised to pain/discomfort.
- Pain/discomfort induced by eating is also common symptom.
- Extra-colonic symptoms were commonly reported in secondary care – with good discussion around their prevalence in primary care.
- The severity of the condition may or may not be useful as a threshold. Whilst high sensitivity maybe attractive, it is important not to miss patients by having too high an exclusion criteria, as reported in the evidence for Rome II, and supported by the simulated consultation feedback and analysis.
- IBS co-exists with other conditions. The possibility of missing inflammatory bowel disease initially would not be perceived by the GDG as problematic
- There is clear evidence supporting diagnostic criterion based reference tools, but their use in practical clinical settings has been reported to be difficult, this was noted by the group and it was felt that published criteria in this guideline should reflect the validated tools, but ordered in such a way that ensures that the tool is intuitive for clinicians to use. It should also facilitate the type of discussion that enables a full history to emerge.
- The individual patient story is very important, emphasising the need for the primary care clinician to focus on the most severe symptom while also establishing other related symptoms.
Published evidence from the diagnostic tools has shaped recent diagnostic approaches for IBS. Whorwell (2006) refers to this as a diagnostic triad, seen below:
- Pain/discomfort – quality and quantitySite of pain: in IBS it can be anywhere in the gut. If the site of pain varies it is unlikely to be cancer (tumour fixed). Need to distinguish this IBS pain discomfort from that caused by gall bladder disease. IBS patients do not tolerate abdominal surgery well.
- Bowel habit – quality and quantityGiving patients’ descriptive examples (e.g. like porridge, rabbit pellets) and using the Bristol Stool Form Scale helps. Incomplete evacuation is reported, creating rectal hypersensitivity. Urgency is increased in Diarrhoea; prevalence for those incontinent is 20% (patients often do not disclose unless asked directly).
- Bloating in women(Absence of bloating in women = red flag) Less common in men, although they may report that the abdomen is tight/hard.
The diagnostic triad clearly reflects the valuable work published by Manning and the Rome group. It also highlights the importance of the extra-colonic features that maybe reported by people presenting with IBS symptoms, typically these include nausea, low back pain, bladder symptoms and thigh pain.
The GDG agreed that primary care clinicians should ask open questions to establish the multiple features of the syndrome, recognising that a potential conflict may exist within Primary Care in terms of the time available to the clinician in exploring the whole range of presenting symptoms.
Diagnostic certainty
In establishing the sensitivity and specificity of different diagnostic criteria by using a clinical reference standard (expert gastroenterologist diagnosis), looking at a pragmatic diagnostic reference tool appears to be of great value to the primary care clinician. The advent of ROME III during the development of this guideline was both timely and beneficial in shaping the and further strengthening the diagnostic criteria agreed within final recommendations. Of equal value, is the provision of clear economic evidence relating to supplementary diagnostic tests.
UTILITY OF TESTS TO EXCLUDE ALTERNATIVE DIAGNOSES
In order to determine the utility of diagnostic tests used to exclude alternative diagnoses in people meeting symptom based criteria for IBS, we needed evidence on the pre-test probability of organic GI disease in people meeting IBS diagnostic criteria and the accuracy of diagnostic tests in identifying organic GI disease. A published systematic review by Cash (2002) was identified which considered the utility of diagnostic tests by evaluating the evidence in these two areas. The selection criteria for the review were:
- Use of a cohort of IBS patients explicitly diagnosed via symptom based criteria (a priori).
- Performance of common diagnostic tests with either blinded comparison with gold standard.
- Results quantified as normal or abnormal with abnormal test resulting in alternative diagnosis of organic disease.
Six studies were included in the Cash (2002) systematic review and these were quality assessed using eight quality criteria (Hamm 1999; Tolliver 1994; Pimental 2000; Sanders 2001; Francis 1996; MacIntosh 1992). All were prospective cohorts of consecutive patients. All were in secondary care, except Hamm (1999) which did not state whether the participants were in primary or secondary care. The patients in Hamm (1999) were all enrolled in a treatment trial. One study had a control group of healthy volunteers (Sanders 2001). In addition to these six studies our search identified two further studies which had been published since the systematic review and which met the inclusion criteria (Sanders 2003; Pimentel 2003). Each study used different criteria for recruiting patients. These are summarised as follows:
- Referral for abdominal pain not previously evaluated (Tolliver 1994)
- Diagnosis of IBS made at first attendance and evaluated within 6 months (Francis 1996)
- Enrolment in treatment trial i.e. not all recent diagnosis (Hamm 1999)
- Referral for altered bowel habit or requesting investigation to reassure following clinical diagnosis of IBS (Sanders 2001)
- Referral for breath testing (Pimentel 2000)
- All patients attending gastroenterology practice (MacIntosh 1992)
- Primary care attendees, including people entering GP surgery for any reason (Sanders 2003)
- Advertisement within community and IBS support groups (Pimentel 2003).
The study characteristics and results are summarised in Tables 3 to 10 below for each class of diagnostic test. The number of abnormal test results is reported alongside the alternative diagnoses resulting from these tests. Where the tests were not given to the whole study cohort this has been noted. For lactose intolerance and bacterial overgrowth we have noted in Table 4 10 whether the diagnosis was confirmed by an improvement in symptoms following treatment, as an abnormal hydrogen breath test result does not provide a definitive diagnosis of either condition.
Table 3
Colonic evaluation.
Table 4
Lactose intolerance.
Table 5
Thyroid function.
Table 6
Stool tests.
Table 7
Other laboratory tests.
Table 8
Coeliac screening.
Table 9
Ultrasound.
Table 10
Bacterial overgrowth.
Table 2 is reproduced from Cash (2002) and summarises the evidence on the pre-test probability of organic GI disease from the 6 studies included in the systematic review. This is compared to general population data presented by Cash (2002), although it was not clear how the general population sample was defined. In addition to the data presented by Cash, Sanders (2003) reported a general population prevalence of 1% for coeliac disease in people recruited from a UK primary care setting and a prevalence of 3.3% in people meeting IBS diagnostic criteria.
Table 2
Pre-test probability of organic GI disease in people meeting symptom based criteria for IBS and in the general population.
The evidence on the clinical utility of tests for alternative diagnoses in patients meeting IBS diagnostic criteria can be summarised as follows:
- The pre-test probability of organic disorders, including colon cancer, inflammatory bowel disease, thyroid disease and lactose malabsorption was no different in IBS populations when compared to the general population.
- In the IBS population, common investigations including endoscopy of the colon, ultrasound, stool ova and parasite testing, faecal occult blood, thyroid function testing and hydrogen breath testing for lactose intolerance and bacterial overgrowth were unlikely to lead to the diagnosis of organic disease. Rectal biopsy was also demonstrated to be ineffective.
“It is amazing to see the expensive, dangerous and extensive workups to which healthy patients are subjected by physicians searching for an organic cause in patients who obviously suffer from IBS.” Jeong et al (1993). Repeated testing can also undermine patient confidence in a positive IBS diagnosis.
ECONOMIC EVIDENCE ON DIAGNOSTIC TESTS TO IDENTIFY ALTERNATIVE DIAGNOSES
Of the four included studies, two consider the cost-effectiveness of screening for coeliac disease in the IBS population, one considers the cost-effectiveness of endoscopy in the IBS population and one considers the cost-effectiveness of diagnostic strategies for IBD in patients who do not meet the Rome criteria for IBS. The characteristics of the included studies are given in Appendix C. All four were model based economic evaluations with two considering the short-term diagnostic period (Suleiman 2001; Dubinsky 2002) and two considering patient outcomes over longer time-frames of 10 years or more (Spiegel 2004; Mein 2004). The quality of each study has been critically appraised using a validated check-list for economic analyses and details are provided in Appendix D. Due to variation in the interventions and populations considered, each study will be discussed separately.
Mein (2004)
The primary aim of this study was to assess the cost-effectiveness of coeliac disease testing in patients with suspected irritable bowel syndrome in the US health care system. This was done by using a decision tree to estimate the number of coeliac disease cases detected, QALYs gained and costs resulting from three testing strategies and comparing these to no testing. The three strategies were; tissue transglutaminase antibody (TTG), antibody panel, or upfront endoscopy with biopsy. All positive serological tests were followed by endoscopy with small bowel biopsy and the potential complications of this procedure were accounted for. All positive upfront endoscopies were assumed to be confirmed by an antibody panel. Long-term treatment costs were assumed similar between patients with IBS and those diagnosed with coeliac disease. The increase in health-related quality of life associated with correctly diagnosing coeliac disease in patients with suspected IBS and initiating a gluten-free diet was estimated indirectly by comparing utility estimates for treated and untreated coeliac disease and IBS measured in different populations. This is less reliable than direct utility measurement as it combines estimates from different populations. However, the uncertainty surrounding this parameter has been adequately examined in a sensitivity analysis. The authors stated that they used conservative assumptions to deliberately bias the model against testing for coeliac disease. These included assuming no reduction in resource use or increase in life-expectancy following correct diagnosis of coeliac disease and initiation of treatment. The base case prevalence for coeliac disease in patients with suspected IBS was 3% and was varied from 1 to 5% in a sensitivity analysis.
The probabilistic model estimated that testing with TTG would detect 28 out of 30 cases present in a population of 1000 individuals but testing with a full antibody panel would only detect one further case. The median incremental cost per case detected was $6,700 (interquartile range $4,800 – $9,700) for TTG vs no testing and $12,300 ($8,900 – $17,700) for antibody panel vs no testing. The incremental cost per case detected for antibody panel vs TTG was $167,000 ($110,000 – $279,000). The incremental cost per QALY was $11,200 ($7,200 – $17,900) for TTG vs no testing and $20,900 ($13,500 – $34,300) for antibody panel vs no testing. The incremental cost per QALY for antibody panel vs TTG was $287,000 ($99,400 – $675,000). The upfront biopsy strategy resulted in a lower QALY gain and higher costs than TTG testing and was therefore dominated by TTG testing. In the one-way deterministic sensitivity analysis, reducing the prevalence to 1% increased the cost per QALY of TTG vs no testing from $7,400 to $19,900 and decreasing the utility gain associated with treatment from 0.024 to 0.01 increased the cost per QALY to $17,900. This demonstrates that whilst the cost-effectiveness results are sensitive to changes in these parameters, the TTG testing strategy is still cost-effective compared to no testing at the thresholds considered ($50,000 to $100,000 per QALY).
This study provided evidence that TTG testing followed by confirmatory endoscopy with biopsy would be cost-effective in patients with suspected IBS in the US health-care system. We converted the cost per QALY directly from 2003 US$ to 2006 UK£ using Health Care Purchasing Power Parity rates (2003 PPP rates UK/US = 2317/5711, OECD 2006) and Hospital and Community Health Services Pay and Pricing Index (2006/2003 = 241.3/224.8 (Netten 2006) and this gave a cost per QALY for TTG vs no testing of £4,900. This is a crude estimate as it assumes that each component of the total cost has an equal weighting in both counties, which may not be true due to differences in the health care systems between the US and UK. However, the relatively low value of this estimate compared to typical UK thresholds of £20,000 to £30,000 per QALY, suggests that this intervention may also be cost-effective from a UK NHS and PSS perspective.
Spiegel (2004)
This aim of this study was to assess the cost-effectiveness of screening for coeliac disease in patients fulfilling the Rome II criteria for diarrhoea predominant IBS (IBS-D) in the US health care system. A strategy of screening for coeliac disease using serological tests followed by confirmatory endoscopy with biopsy was compared with a strategy of initiating IBS therapy without screening for coeliac disease. This was done using a decision tree to estimate the number of patients receiving appropriate therapy for either IBS or coeliac disease, the number of missed coeliac disease diagnoses and the number of patients for whom IBS treatment was delayed due to coeliac disease testing. It was assumed that 1 in 4 clinicians eventually test for coeliac disease in patients who do not respond to empiric IBS treatment, resulting in an average diagnostic delay of 6 months. A Markov model was then used to estimate transitions between states of symptomatic improvement and remission once patients have begun treatment for either IBS or coeliac disease. The analysis was based on a generic serological test using data which reflected the range of serological tests available (anti-EMA and anti-TTG IgA antibodies). The results are presented in terms of the cost per additional patient with symptomatic improvement after 10 years. The authors state that the model was deliberately biased in favour of IBS treatment without testing for coeliac disease in order to place the burden of proof for cost-effectiveness on coeliac testing. This was done by using estimates from the unfavourable end of the range presented in the literature for the following parameters; coeliac disease prevalence, sensitivity and specificity of tests for coeliac disease, rate of coeliac disease testing in patients not responding to empiric IBS therapy, IBS treatment effectiveness and cost. For example, IBS treatment was assumed to be effective in 75% of patients based on the effectiveness of alosetron but the cost of therapy was assumed to be $45 per month which is similar to the cost of loperamide. The model also assumed that 30% of the population with coeliac disease had “latent” or “potential” coeliac disease which would not be detected by small bowel biopsy but would have the potential to benefit from a gluten free diet. It also assumed that 5% of the population with coeliac disease would have concurrent IgA deficiency which would render serological screening for IgA antibodies ineffective. These assumptions were based on limited data but were included to bias the model against testing for coeliac disease and their impact was explored through sensitivity analyses.
The deterministic base case model estimated that testing for coeliac disease resulted in 51.6% of the cohort achieving symptomatic improvement at 10 years, whilst initiating IBS therapy without testing for coeliac disease resulted in 50.9% of the population achieving symptomatic improvement. The incremental cost was $77 per patient resulting in a cost per additional symptomatic improvement after 10 years of $11,000. The probabilistic model resulted in a median cost per symptomatic improvement of $12,983 (95% CI: Dominating to $41,031). The results were sensitive to the prevalence of coeliac disease in the population considered. The cost-effectiveness ratio was under $50,000 when the prevalence was >1% and screening dominated no screening (resulted in more health gain at reduced cost) when the prevalence was over 8%.
These results were difficult to interpret as they were presented for the US health care system and did not provide benefits measured in QALYs. The aim of a Markov model is usually to determine the proportion of time a patient spends in each health state over the duration of the model and to use this to estimate their aggregate health gain over the time-horizon considered. This analysis did not present results in terms of the time spent in the symptom remission state, but instead presented the results in terms of the number of patients in this state at the end of the model, which may not accurately reflect the amount of health benefit accrued over the duration of the model. It was therefore less useful in determining whether testing for coeliac disease is cost-effective compared to no testing than the evidence provided by Mein (2004).
Dubinsky (2002)
This study examined the cost-effectiveness of initial serodiagnostic screening followed by standard invasive testing, compared to standard testing alone in patients presenting with symptoms suggestive of IBD from a third-party payer perspective in the US health care system. The authors state that the population considered by this analysis was patients presenting with symptoms which did not meet the Rome I criteria for IBS. As the aim of this review is to consider the cost-effectiveness of testing for alternative diagnoses in patients meeting the diagnostic criteria for IBS, following the application of a criterion based reference tool, this study was not directly relevant to the target population. We would expect the patient population meeting the diagnostic criterion for IBS to have a lower prevalence of IBD. As the analysis considered a wide range of prevalence values (5% to 75%) in a sensitivity analysis, the results for the lower end of this prevalence range were considered to have some relevance to the target population.
The decision analytic model considered six alternative diagnostic strategies. Two levels of serodiagnostic screening were evaluated. In the primary screening strategy (PR 1) patients received a primary assay followed by a gold standard invasive diagnostic test if the primary screen was positive or if a negative primary screen was followed by persistent symptoms. In the sequential screening strategy (SS 1) a positive primary assay was followed by a confirmatory assay and if this was positive it was followed by a gold standard test. Negative results followed by persistent symptoms were investigated using the gold standard test as in the primary screen. These were compared to gold standard testing upfront (GS 1) which consisted of colonoscopy with biopsies and histological examination as well as a barium upper GI series and small bowel follow-through. Three additional strategies were also considered in which the first three strategies were extended to include a second gold standard test in patients with persistent symptoms following the first gold standard test (PS 2, SS 2 and GS 2). The proportion of patients returning with persistent symptoms due to IBS not meeting the Rome I criteria or other causes of symptoms was assumed to be 50% based on expert opinion and varied from 0 to 100% in a sensitivity analysis. A decision tree model was used to estimate the accuracy and cost of each of the six strategies. No costs or health benefits following diagnosis were estimated. An incremental cost-effectiveness analysis was also presented which compared the relative cost-effectiveness of the six competing strategies.
In the basecase model all of the serodiagnostic strategies had lower costs and higher diagnostic accuracy than the gold standard strategies. The SS 1 strategy had the lowest cost and a diagnostic accuracy of 96.95%. The SS 2 strategy cost $20.30 more per patient but had a slightly higher diagnostic accuracy of 97.90% resulting in a cost per % increase in accuracy of $2,137. The SS 1 strategy dominated all other strategies by having higher accuracy and lower cost and also resulted in the lowest number of invasive procedures out of all six strategies (610 for SS 1 vs 1000 for GS 1 and 1010 for GS 2). In the cost sensitivity analysis, standard invasive testing was more cost-effective when the costs of testing were varied outside of the plausible range considered by the sensitivity analysis.
Standard invasive testing was more cost-effective when the prevalence of IBD was varied to >76%, or when the proportion of patients with persistent symptoms was varied to over 89%. These results suggest that serodiagnostic screening for IBD in patients with “atypical” IBS symptoms would be less costly and more effective than immediate gold standard invasive testing. These results are based on cost data from the US and the conclusions may be different in a UK analysis if the relative costs of invasive and non-invasive testing are significantly different. Whilst these results apply to patients with “atypical” IBS who do not meet the Rome I criteria, the sensitivity analyses carried out demonstrate that they will apply equally to groups with lower prevalence rates of IBD, provided that less than 89% of patients are given the gold standard test after returning with persistent symptoms following a negative serodiagnostic test. This study does not address whether further testing in patients returning with persistent symptoms is beneficial but assumes that this occurs in practice regardless. This number may be higher or lower in the group meeting the diagnostic criteria for IBS depending on the confidence placed on the positive diagnosis. This study did not address whether these strategies for diagnosing IBD are cost-effective compared to a strategy of initiating IBS treatment, following a positive IBS diagnosis, without excluding IBD. It therefore did not demonstrate the cost-effectiveness of serological testing for IBD in patients meeting diagnostic criteria for IBS.
Suleiman (2001)
The aim of this study was to assess the incremental cost-effectiveness of endoscopic procedures in the work-up for IBS. It did not consider the incremental cost-effectiveness of a specific test for an alternative diagnosis in patients with IBS, but considered the increase in diagnostic probability achieved by using various sequences of tests to exclude alternative diagnoses. These tests included; hydrogen breath test to exclude lactase deficiency or bacterial overgrowth, flexible sigmoidoscopy and colonoscopy to exclude inflammatory colitis, diverticular disease and colon cancer, and small bowel follow-through to exclude small bowel cancer. A decision tree was used to estimate the probability of IBS in the remaining population following each diagnostic test. Various sequences of tests were considered but each began with a clinical history, physical examination and laboratory tests. The costs of further testing following false positive tests and the costs and health impact of delayed diagnosis of IBS or alternative diagnoses were not considered. The authors presented incremental cost-effectiveness ratios (ICERs) for flexible sigmoidoscopy and colonoscopy in terms of the incremental cost per 1% increase in IBS probability, but these figures considered the cost in the individual and did not take into account the number of patients who would be tested at each stage of the diagnostic sequence. The authors also presented average cost-effectiveness ratios, “ACERs” which gave the cost of the whole diagnostic sequence in a cohort of patients divided by the number of correct diagnoses.
The model demonstrated that lower ACERs are achieved by using flexible sigmoidoscopy after rather than before hydrogen breath testing and small bowel follow-through. The same was found for colonoscopy in the absence of a previous flexible sigmoidoscopy. The results demonstrated that carrying out colonoscopoy without flexible sigmoidoscopy at the end of the diagnostic sequence would result in a lower ACER than carrying out colonosocopy following flexible sigmoidoscopy at the end of the diagnostic sequence.
The relevance of these results to this review was limited as the study did not consider the incremental cost-effectiveness of testing for a specific alternative diagnosis in patients meeting IBS diagnostic criterion. However, it did demonstrate that the cost of diagnostic testing can be reduced by using more costly interventions at the end of a diagnostic sequence without changing the number of correct diagnoses. The clinical outcomes did not vary when the ordering of tests was varied due to the assumption that each test is independent of the next. In practice this may not be strictly true and there may be some dependence resulting in slightly different clinical outcomes depending on the test sequencing. However, it is still likely that lower costs would be achieved in practice by using more costly invasive investigations at the end of the diagnostic sequence as this would reduce the number of people who require these invasive tests. This would also minimise adverse health outcomes due to complications.
Summary
There was some relevant published literature concerning the cost-effectiveness of screening for coeliac disease in patients with suspected IBS. The study by Mein (2004) provided a cost per QALY for coeliac testing vs no testing from a US perspective. Whilst this could not be applied directly to the population under consideration, due to differences in the health care systems between the US and the UK, the low cost per QALY suggested that this intervention may also be cost-effective from a NHS and PSS perspective. The studies by Dubinsky (2002) and Suleiman (2001) did not consider directly whether further diagnostic testing would be cost-effective in patients meeting diagnostic criteria for IBS compared to no further diagnostic testing. They did provide some evidence that where diagnostic testing does take place, it is cost-effective to use less costly and less invasive tests first in the diagnostic sequence with positive results confirmed by standard invasive testing compared to invasive testing early in the diagnostic sequence.
Having considered the evidence on the clinical utility of diagnostic tests in patients meeting IBS diagnostic criteria, the GDG decided that there was insufficient evidence of clinical utility to warrant further economic analysis on the cost-effectiveness of diagnostic testing, except for serological testing for coeliac disease. It was not considered necessary to carry out further economic analysis to estimate the cost-effectiveness of routine laboratory investigations, such as FBC, ESR and CRP. They are unlikely to result in a significant cost burden for the NHS and it would be difficult to estimate their cost-effectiveness due to their non-specific nature which means that an abnormal result may be due to a variety of causes. Therefore, further economic modelling focused on the cost-effectiveness of serological testing for coeliac disease.
Cost-effectiveness of screening for coeliac disease in patients meeting IBS diagnostic criteria – adaptation of a published economic evaluation
Further analysis was carried out to adapt the cost-effectiveness estimate provided by Mein (2004) to make it more applicable to the NHS in England and Wales. UK specific data was obtained for the prevalence of undiagnosed coeliac disease, diagnosis costs, and HRQoL and ongoing resource use for individuals with IBS. A discounting rate of 3.5% was applied to both costs and QALYs in line with the NICE reference case for cost-effectiveness analysis (NICE 2007). Mein (2004) did not consider the additional cost of gluten-free foods in their analysis, but as gluten-free foods can be prescribed through the NHS this cost was also considered in our analysis. Mein (2004) did not allow for any increased life-expectancy that may result from adherence to a gluten-free diet in patients diagnosed with coeliac disease. This was considered to be overly conservative as one of the main aims of adherence to a gluten-free diet in coeliac disease is to reduce the risk of malignant diseases associated with coeliac disease such as Non-Hodgkin Lymphoma (West 2004). The model was therefore adapted to include an estimated survival difference between patients with diagnosed and undiagnosed coeliac disease. The economic model reported by Mein (2004) compared serological testing for IgA tissue transglutaminase (TTG) antibodies against a strategy of no testing. However IgA EMA testing is more commonly used in the UK than IgA TTG so this was used in the UK adaptation and TTG was considered in a sensitivity analysis.
The prevalence of undiagnosed coeliac disease in patients meeting IBS diagnostic criteria was taken from a cross-sectional study conducted in a UK primary care setting (Saunders 2003). The study population was randomly sampled from all adults entering the GP premises on study days. A subgroup of individuals meeting the ROME II diagnostic criteria for IBS was identified. The prevalence of undiagnosed coeliac disease was 3.3% (4/123) in individuals who fulfilled the ROME II criteria for IBS. This estimate was used in the model as the prevalence of undiagnosed coeliac disease in patients meeting IBS diagnostic criteria. The prevalence from the primary care sample as a whole (1%) was used in a sensitivity analysis as the expected lower limit for the prevalence in the IBS population.
Sensitivity and specificity values for IgA EMA were taken from a published health technology assessment which included a systematic review of autoantibody testing in children with type I diabetes (Dretzke 2004). This systematic review included studies carried out in symptomatic populations or populations at a higher risk of developing coeliac disease but not exclusively type I diabetes. The sensitivity and specificity estimates used in the model were the Q values (overall best test performance with equal sensitivity and specificity) from the well-described studies as given in Table 17 of Dretzke (2004).
The NHS cost of an IgA EMA antibody test was also taken from Dretzke (2004). The cost of esophagogastroduodenoscopy (EGD) with biopsy to confirm coeliac disease was taken from the NHS references costs (2005–06) for day case endoscopic procedures on the stomach or duodenum (Department of Health 2006). The cost for an EGD with complications was assumed to be equal to the NHS reference cost for the same procedure as a non-elective in patient (average length of stay of 1 day). The cost of care for IBS was taken from a study by Akehurst (2002) which estimated the NHS costs for IBS patients and matched controls. As in the cost-effectiveness analysis by Mein (2004), it was assumed that the NHS costs of managing coeliac disease are equal to the costs of managing IBS except that there is the additional cost of gluten free foods on prescriptions. This may be an overestimate if IBS-like symptoms are reduced when patients with coeliac disease are established on a gluten-free diet.
The NHS cost of supporting a gluten-free diet by providing foods on prescription was calculated by estimating the total cost of gluten-free foods prescribed by the NHS in England in 2005 (£21.2million) from the Prescription Cost Analysis (NHS Health and Social Care Information Centre 2006) and the number of people diagnosed with coeliac disease based on a population for England of 50.4million and a prevalence for diagnosed coeliac disease of 0.26%.(Fowell 2006). This gave an annual cost of £162 per diagnosed case of coeliac disease.
The health utility of IBS was taken from the study by Akehurst (2002) which estimated health utility for IBS patients using the EQ-5D. Mein (2004) attempted to estimate the utility gain associated with diagnosing coeliac disease in patients with IBS-like symptoms. However, this estimate was considered to be unreliable as it was calculated by comparing utility values for health states estimated in different populations. No direct evidence was available on the utility gain achieved by diagnosing and treating coeliac disease in patients with IBS-like symptoms. O’Leary (2002) found that coeliac patients with IBS-like symptoms had a lower HRQoL than those without symptoms, but these symptoms were equally common in coeliac patients who did and didn’t adhere to a gluten-free diet. Casellas (2005) found that recently diagnosed patients who had not started a gluten-free diet had a lower quality of life and a higher prevalence of IBS-like symptoms compared to patients who had been established on a gluten-free diet, but the study design was cross-sectional, so it was not possible to say from this whether the diet itself provided an improvement in quality of life. In the basecase analysis it was assumed that the gluten-free diet did not provide any gain in health utility, so the only benefit was from improved survival. A threshold analysis was carried out to assess the size of health utility gain that would need to be achieved by adherence to a gluten-free diet, in order to give a cost per QALY under £20,000, when assuming that the gluten-free diet does not provide a survival gain.
There is evidence that patients with coeliac disease have a significantly higher than expected mortality (SMR = 2.0, p<0.0001) (Corrao 2001) and that mortality risk is significantly increased for patients with a diagnostic delay of over 12 months but is not significantly increased when it is less than this. Mortality is also significantly higher than expected (SMR 2.5, p<0.0001) in patients with severe symptoms of malabsorption such as diarrhoea or weight loss but not significantly increased in patients with milder symptoms which may be seemingly unrelated to coeliac disease. There is evidence that survival is significantly reduced in the first 3 years after diagnosis but not beyond. The timing of the observed excess mortality may be due to excess deaths in patients who had extended diagnostic delay and who were not diagnosed until after symptoms had become severe. It may be possible to prevent the excess mortality in patients with IBS–like symptoms by prompt diagnosis through serological screening. In order to estimate the survival gain associated with prompt diagnosis we have assumed that undiagnosed cases have a reduced survival compared to diagnosed cases. We have taken the survival ratio for coeliac patients compared to the general public and used this to estimate the reduction in mortality avoided by prompt diagnosis. This is equivalent to a relative reduction in cumulative survival of around 2% over the first 3 years of the model for patients with coeliac disease presenting with IBS-like symptoms whose coeliac disease remains undiagnosed. This may have underestimated the survival gain associated with diagnostic testing, as the SMR in the whole coeliac population is lower than in those patients with extended diagnostic delay, but it may also have overestimated the survival gain as prompt diagnosis may not result in a complete reduction of mortality to general population levels. These survival ratios were applied to UK life-tables (Office for National Statistics 2006), assuming a male to female ratio of 1:2, and gave an estimated difference in expected life-years of 1.4 LYs for patients with diagnosed and undiagnosed coeliac disease. Once discounting was applied to the expected survival this difference was reduced to 0.54 discounted LYs. A sensitivity analysis was also carried out using the upper 95% CI of the survival ratio which resulted in a lower estimated survival gain of 0.31 discounted LYs.
The parameter values used in the UK adaptation are summarised in Table 11 alongside those used by Mein (2004) in the US basecase. Univariate sensitivity analysis was used to determine whether the deterministic estimate of cost-effectiveness was sensitive to changes in the UK specific parameters. This included a threshold analysis on the utility gain associated with establishing a gluten-free diet and the cost of prescribing gluten-free foods on the NHS. The parameter values used in the sensitivity analysis are also given in Table 11. All costs were uplifted to 2005/06 values where applicable and the uplifted values are included in Table 11 in italics.
Table 11
Parameters used in Mein (2004) analysis and in the UK adaptation.
A probabilistic sensitivity analysis (PSA) was carried out to estimate the uncertainty in the cost-effectiveness estimate due to the uncertainty in the model input parameters. We characterised the parameter uncertainty by using a probability distribution to describe each of the parameters, details of which can be found in Appendix H. We sampled randomly from these distributions 1000 times and estimated the model outputs (incremental costs and incremental QALYs) for each set of sampled parameters and used these to estimate the uncertainty surrounding the cost per QALY estimate. We based our PSA on 1000 samples of the parameter distributions. The results are presented as a cost-effectiveness acceptability curve (CEAC) which shows the proportion of samples that resulted in a cost per QALY value below various thresholds. It should be noted that the PSA does not account for uncertainty around the model assumptions and these have been explored separately using univariate sensitivity analysis.
The deterministic results for the UK basecase estimate are given in Table 12. The serological testing strategy identified 32 out of 33 prevalent cases of coeliac disease in the cohort of 1000 patients with IBS symptoms for a diagnosis cost of £36,300, giving a cost per correctly diagnosed case of coeliac disease of £1,122. These diagnoses resulted in an additional 43.4 LYs (undiscounted) over the lifetime of the cohort which is equivalent to 11.69 QALYs (discounted). This was associated with a further £122,300 (discounted) of treatment costs, including gluten-free products for patients diagnosed with coeliac disease, over the lifetime of the cohort. The overall cost per QALY for serological testing compared to no testing was £13,560 for a life-time horizon.
Table 12
Deterministic basecase results for 1000 patients meeting IBS diagnostic criteria. Costs, LYs and QALYs are discounted at 3.5% per annum.
The mean cost per QALY over the 1000 samples carried out for the probabilistic analysis was £14,300. The CEAC in Figure 1 shows the probability that the cost per QALY is under various cost per QALY thresholds given the uncertainty in the parameters used to estimate cost-effectiveness. It shows that the cost per QALY had an 80% probability of being under £20K per QALY and a 96% probability of being under £30K per QALY under the basecase assumptions.
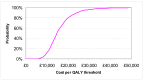
Figure 1
Cost-effectiveness acceptability curve (CEAC) for coeliac disease testing in patients with IBS-like symptoms compared to no testing.
The univariate sensitivity analysis in Figure 2, shows that the cost per QALY estimate was not particularly sensitive to the age of the patient at presentation. This may be because younger patients have a longer life-expectancy, but this increases both their lifetime cost of care and their survival gain from preventing excess mortality. Cost-effectiveness was not significantly impacted by higher testing costs, higher costs for ongoing IBS / coeliac disease management, lower health state utility values for patients with IBS / coeliac disease or lower sensitivity and specificity values for serological testing. Using a zero discounting rate lowered the cost per QALY as the majority of the survival benefit was gained over the long-term whilst the upfront diagnosis costs occurred early in the model.
Figure 2
Univariate sensitivity analysis results for coeliac disease testing in patients with IBS-like symptoms compared to no testing.
The cost-effectiveness estimate was sensitive to the survival gain attributed to identifying patients with coeliac disease and establishing them on a gluten-free diet, as this was the only benefit included in the basecase model. Using the lower estimate of survival benefit increased the cost per QALY to £23,000. The threshold analysis on utility gain demonstrated that establishing patients with coeliac disease on a gluten-free diet would need to produce a utility gain of 0.011 in order for the cost per QALY to remain under £20,000, when assuming that there is no survival gain. This is a small utility gain compared to the difference in health utility between IBS patients and matched controls (0.135, Akehurst 2002). These two sensitivity analyses on the survival and QALY gain demonstrated that whilst there is some uncertainty surrounding the expected benefits of identifying individuals with coeliac disease and initiating a gluten-free diet, testing is likely to be cost-effective in patients with IBS-like symptoms, so long as there is a small improvement in quality of life or a small reduction in mortality risk as a result of a correct diagnosis of coeliac disease.
The threshold analysis on the cost of prescribing gluten-free foods shows that up to £263 per patient per annum could be spent on gluten-free foods before the cost per QALY reached the threshold of £20,000. The estimated cost for providing gluten-free foods on prescription is based on the total costs of prescriptions for gluten-free foods in 2005 and an estimate of the prevalence of diagnosed coeliac disease. Using the lower estimate of prevalence from Fowell (2006) gave a higher cost of £234 per patient per annum, which based on our threshold analysis, would still provide a cost per QALY under £20,000.
We have estimated the cost-effectiveness of testing with EMA compared to no testing as this is the test most commonly available to primary care clinicians in the UK. However, TTG is also available in some areas of the NHS. Sensitivity and specificity values were available for TTG (96%, 95% CI of 92%–98%) from the Dretzke (2004) HTA, but a direct cost estimate for TTG was not available. In the economic analysis conducted as part of the HTA, the cost of TTG was assumed equal to the cost of testing for anti-gliadin antibodies (AGA) as these tests use similar techniques. The cost for these tests was estimated to be slightly higher than the cost of EMA. We carried out a sensitivity analysis to see whether testing using TTG would also be cost-effective when using the evidence on test cost and accuracy from the HTA (Dretzke 2004). The slightly lower accuracy for TTG resulted in a slightly lower QALY gain of 11.53 per 1000 people tested, for testing compared to no testing. The slightly higher test cost (£14 compared to £12) resulted in a slightly higher total cost £164,683 per 1000 people tested. The overall cost per QALY for TTG compared to no testing was therefore, £14,283. This suggests that testing with TTG would also be cost-effective compared to no testing. We have not carried out an analysis to consider which antibody test is the more cost-effective test to use as we did not feel that the cost data was sufficiently robust to allow a reliable comparison. In addition, TTG is a relatively new technology and the evidence base may have improved since the searches carried out by Dretzke (2004). There are also other factors that must be taken into account when deciding which tests should be available to primary care clinicians in the NHS, which we have not considered. Therefore we did not feel that our cost-effectiveness analysis was sufficiently robust to recommend the use of either test in preference to the other. However, there is good evidence that using either of these tests is cost-effective compared to no testing in people with IBS.
SUMMARY OF THE EVIDENCE
There is a good evidence base for the application of diagnostic criteria in the diagnosis of people presenting with IBS symptoms, allowing primary care clinicians to make a positive diagnosis with confidence. This is illustrated in Table 1. This has potential to change the current approach to diagnosis, avoiding unnecessary diagnostic tests of limited or in many cases no value. Economic analysis supported by GDG interpretation demonstrates that only four investigations from the included studies for this review are of use to the clinician, in either augmenting their positive diagnosis of IBS or related co morbidity such as Coeliac disease. The cost-effectiveness of two different antibody tests for coeliac disease (EMA and TTG) was considered as both are available within the NHS but access to these tests varies across the NHS. We did not consider which of the two antibody tests is most cost-effective as there was insufficient evidence on the relative cost to make a fair assessment of the incremental cost-effectiveness. The GDG recognised the potential need to clarify which is the better diagnostic test and determine which test was more cost effective, but it was agreed that this was not a clinical priority for this guideline.
There was limited evidence on the clinical utility of routine laboratory investigations such as a FBC, ESR or CRP. However, GDG consensus was that these low cost tests were clinically useful in supporting a positive diagnosis and were unlikely to significantly increase the cost burden to the NHS.
For all other diagnostic investigations (ultrasound, sigmoidoscopy, colonosocopy, barium enema, thyroid function test, faecal ova and parasite tests, faecal occult blood test, hydrogen breath test) the GDG felt that there was insufficient evidence of their clinical utility to support the routine use of these tests in individuals meeting the IBS diagnostic criteria who did not have any red flag symptoms. They also felt that repeated testing could undermine confidence in the positive diagnostic approach. Whilst the cost-effectiveness of these tests was not explicitly estimated, the GDG felt that they were unlikely to be cost-effective given the lack of evidence of clinical utility in this population. The GDG recognized the importance of early and appropriate investigations in individuals with red flag symptoms. They were also mindful that the recommendations should not discourage the use of investigations in patients who do not meet the IBS diagnostic criteria or who have symptoms suggestive of organic disease.
The clinical significance of this review is two fold. Patient experience is often determined by the first exposure to healthcare, and the use of diagnostic criteria offers people who may have IBS the potential for symptom based condition to be diagnosed and managed confidently from the first consultation. The potential for cost saving is a real possibility, by determining the small number of investigations which offer primary care clinicians added benefit to confirm their clinical diagnosis. Identifying tests which are routinely requested but have little or no diagnostic value has real potential for disinvestment within the NHS. The validation of the ‘Positive Diagnostic Criteria’ is a clear step towards addressing the current variations in diagnostic practice within primary care for people presenting with IBS symptoms. Contemporised language previously used in the Manning criteria aligns closely to ROME III criteria. The diagnostic criteria recommended in this guideline implicitly acknowledges their contribution, in strengthening a contemporary IBS diagnostic approach.
GDG COMMENTARY
Duration of symptom profile
Having reviewed the evidence and analysed application of the criterion referenced diagnostic tools, duration of symptom profile was recognised to be an important aspect to consider in making recommendations for practice. Three, six and twelve month durations were all discussed and the consensus of the group was that a duration of 6 months was the most appropriate.
Entry filter for use of the diagnostic tool
Primary care clinicians should consider assessment for IBS if the patent reports any of the following symptoms for at least 6 months:
- Change in bowel habit
- Abdominal pain/discomfort and or bloating.
Positive Diagnostic Criteria
For a positive diagnosis to be made, the patient must present with at least 3 of the agreed diagnostic criteria. Language used in the tool was contemporised as follows:
Supportive investigations
Appropriate investigations identified were:
Follow-up
Once a positive diagnosis has been made, patient follow up is a key aspect of longer term management in managing and evaluating the response to first line therapy interventions. Giving patients the opportunity either to re-attend as required or possibly making regular appointments was discussed. It was agreed by the GDG that follow up should be explicitly stated within the recommendations, and in the absence of any evidence supporting this, consensus agreement would be used.
EVIDENCE STATEMENTS
- There are two published studies providing evidence on the cost-effectiveness of screening for Coeliac disease in patients with suspected IBS although only one presented the results in terms of the cost per QALY gained. This study provided a cost per QALY for celiac testing vs no testing from a US perspective. This published decision analytic model was adapted to consider the cost-effectiveness of serological screening for coeliac disease from a UK perspective. This showed that antibody testing (EMA or TTG) is likely to be cost-effective in patients with IBS-like symptoms when taking into account the potential for improved survival or a modest gain in quality of life following diagnosis.
- There is evidence from published literature that where diagnostic testing does take place, it is cost-effective to use less costly and less invasive tests first in the diagnostic sequence with positive results confirmed by standard invasive testing compared to invasive testing early in the diagnostic sequence.
RECOMMENDATION
Healthcare professionals should consider assessment for IBS if the person reports having had any of the following symptoms for at least 6 months:
- Abdominal pain or discomfort
- Change in bowel habit.
RECOMMENDATION
All people presenting with possible IBS symptoms should be asked if they have any of the following “red flag” indicators and should be referred to secondary care for further investigation if any are present (see ‘Referral guidelines for suspected cancer’, NICE clinical guideline 27, for detailed referral criteria where cancer is suspected).
- Unintentional and unexplained weight loss
- Rectal bleeding
- A family history of bowel or ovarian cancer
- A change in bowel habit to looser and/or more frequent stools persisting for more than 6 weeks in a person aged over 60 years.
RECOMMENDATION
All people presenting with possible IBS symptoms should be assessed and clinically examined for the following “red flag” indicators and should be referred to secondary care for further investigation if any are present (see ‘Referral guidelines for suspected cancer’, NICE clinical guideline 27, for detailed referral criteria where cancer is suspected).
- Anaemia
- Abdominal masses
- Rectal masses
- Inflammatory markers for inflammatory bowel disease.
If there is significant concern that symptoms may suggest ovarian cancer, a pelvic examination should also be considered.
RECOMMENDATION
A diagnosis of IBS should be considered only if the person has abdominal pain or discomfort that is either relieved by defaecation or associated with altered bowel frequency or stool form. This should be accompanied by at least two of the following four symptoms:
- Altered stool passage (straining, urgency, incomplete evacuation)
- Abdominal bloating (more common in women than men), distension, tension or hardness
- Symptoms made worse by eating
- Passage of mucus.
Other features such as lethargy, nausea, backache and bladder symptoms are common in people with IBS, and may be used to support the diagnosis.
RECOMMENDATION
In people who meet the IBS diagnostic criteria, the following tests should be undertaken to exclude other diagnoses:
- Full blood count (FBC)
- Erythrocyte sedimentation rate (ESR) or plasma viscosity
- C-reactive protein (CRP)
- Antibody testing for coeliac disease (endomysial antibodies [EMA] or tissue transglutaminase [TTG]).
RECOMMENDATION
The following tests are not necessary to confirm diagnosis in people who meet the IBS diagnostic criteria:
- Ultrasound
- Rigid/flexible sigmoidoscopy
- Colonoscopy; barium enema
- Thyroid function test
- Faecal ova and parasite test
- Faecal occult blood
- Hydrogen breath test (for lactose intolerance and bacterial overgrowth).
RECOMMENDATION
Follow-up should be agreed between the healthcare professional and the person with IBS, based on the response of the person’s symptoms to interventions. This should form part of the annual patient review. The emergence of any ‘red flag’ symptoms during management and follow-up should prompt further investigation and/or referral to secondary care.
Appendix 1. Sensitivity and specificity values offered as Odds Ratios
Table 3. Summary of Studies Validating Standard Diagnostic Criteria
Appendix 2. Comparison table for Rome Criteria
Table
A Symposium: Diagnostic Criteria for IBS/Hammer and Talley.
Appendix 3. PATIENT PROFILES FOR SIMULATED GP CONSULTATION
IBS GDG MEETING 30th November- 1st December 2006
Patient 1
Female, aged 37yrs, married 2 children.
Recurrent abdominal discomfort approximately a week out of every month – sometimes worse pre-menstrual, worse if eats cauliflower or spicy foods.
Tired and lethargic – has tried different diets to help energy levels but nothing works.
Bowel – change in bowel habits, had diarrhoea after holiday abroad now seems to have constipation followed by diarrhoea.
Patient 2
Male, aged 44 yrs, divorced, 4 children, 2 ex wives.
Frequent abdominal pain, most days of the week.
Bloating worse by end of day with increased flatulence.
Constipation, thinks there has been a little rectal bleeding but not sure. Worse since new job – very stressful over last six months.
Social life diminished because embarrassed to go out, becoming increasingly depressed.
Worried he may have something serious.
Patient 3
Female, aged 51yrs, single.
Diarrhoea on and off for last 2 years.
Abdominal pain.
Back ache.
Nausea.
Weight loss.
2 x visit to Doctors with urinary symptoms – no UTI but symptoms recur intermittently describes herself as fed up – not depressed.
Patient 4
Female, aged 24yrs
Altered bowel habit – diarrhoea and constipation – changes all the time feels she never empties her bowels, passes mucus in diarrhoea, pale bulky stools when constipated. Has had ‘sensitive tummy’ since she was a child.
Mother has long term problems with constipation. Abdominal pain better after bowel movement.
Some foods make it worse she wonders if she has food allergy – sometimes gets a rash and frequently has mouth ulcers.
Drinks a lot of milk when ‘off’ food.
Footnotes
- 1
In the absence of a gold standard, the reference standard was expert gastroenterologist diagnosis.
- INTRODUCTION AND BACKGROUND
- OBJECTIVES
- SELECTION CRITERIA
- SEARCH STRATEGY FOR IDENTIFICATION OF THE LITERATURE
- METHODOLOGY
- STRUCTURE OF THE REVIEW
- IBS DIAGNOSTIC CRITERIA
- RESULTS OF THE REVIEW AND DISCUSSION
- GDG DISCUSSION
- UTILITY OF TESTS TO EXCLUDE ALTERNATIVE DIAGNOSES
- ECONOMIC EVIDENCE ON DIAGNOSTIC TESTS TO IDENTIFY ALTERNATIVE DIAGNOSES
- SUMMARY OF THE EVIDENCE
- GDG COMMENTARY
- EVIDENCE STATEMENTS
- Sensitivity and specificity values offered as Odds Ratios
- Comparison table for Rome Criteria
- PATIENT PROFILES FOR SIMULATED GP CONSULTATION
- Diagnosis - Irritable Bowel Syndrome in AdultsDiagnosis - Irritable Bowel Syndrome in Adults
Your browsing activity is empty.
Activity recording is turned off.
See more...
