NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Institute of Medicine (US) Forum on Microbial Threats. Global Climate Change and Extreme Weather Events: Understanding the Contributions to Infectious Disease Emergence: Workshop Summary. Washington (DC): National Academies Press (US); 2008.

Global Climate Change and Extreme Weather Events: Understanding the Contributions to Infectious Disease Emergence: Workshop Summary.
Show detailsGLOBAL CLIMATE CHANGE AND EXTREME WEATHER EVENTS: UNDERSTANDING THE CONTRIBUTIONS TO INFECTIOUS DISEASE EMERGENCE
Humans have long recognized that climatic conditions influence the appearance and spread of epidemic diseases (NRC, 2001). Hippocrates’ observations of seasonal illnesses, in the fifth century B.C.E., formed the basis for his treatise on epidemics. Hippocratic medicine, which attempted to predict the course and outcome of an illness according to its symptoms, also considered winds, waters, and seasons as diagnostic factors. Ancient notions about the effects of weather and climate on disease remain in the medical and colloquial lexicon, in terms such as “cold” for rhinovirus infections; “malaria,” derived from the Latin for “bad air”; and the common complaint of feeling “under the weather.”
Today, evidence that the Earth’s climate is changing (IPCC, 2007b) is leading researchers to view the long-standing relationships between climate and disease from a global perspective. Increased atmospheric and surface temperatures are already contributing to the worldwide burden of disease and premature deaths, and are anticipated to influence the transmission dynamics and geographic distribution of malaria, dengue fever, tick-borne diseases, and diarrheal diseases such as cholera (IPCC, 2007a). Global warming is also accelerating the worldwide hydrological cycle, increasing the intensity, frequency, and duration of droughts; heavy precipitation events; and flooding (IPCC, 2007a). Such extreme weather events have been increasing (IPCC, 2007a) and have been linked to global warming (Hoyos et al., 2006). These weather events may, in turn, contribute to and increase the risk for a wide range of vector- and non-vector-borne diseases in humans, plants, and animals (IPCC, 2007b).
The projected health consequences of future climate change and extreme weather events are predominantly negative.1 The most severe impacts are expected to occur in low-income countries where adaptive capacity is weakest. Developed countries are also vulnerable to the health effects of weather extremes, as was demonstrated in 2003 when tens of thousands of Europeans died as a result of record-setting summer heat waves (Kovats and Haines, 2005). Climate change is expected to reinforce additional contributors to infectious disease emergence including global trade and transportation, land use, and human migration (IOM, 2003).
The Forum on Microbial Threats of the Institute of Medicine (IOM) held a public workshop in Washington, DC, on December 4 and 5, 2007, to explore the anticipated direct and indirect effects of global climate change and extreme weather events on infectious diseases of humans, animals, and plants and the implications of these health impacts for global and national security. Through invited presentations and discussions, invited speakers considered a range of topics related to climate change and infectious diseases, including the ecological and environmental contexts of climate and infectious diseases; direct and indirect influences of extreme weather events and climate change on infectious diseases; environmental trends and their influence on the transmission and geographic range of vector- and non-vector-borne infectious diseases; opportunities and challenges for the surveillance, prediction, and early detection of climate-related outbreaks of infectious diseases; and the international policy implications of the potentially far-reaching impacts of climate change on infectious disease.
Organization of the Workshop Summary
This workshop summary report was prepared for the Forum membership in the name of the rapporteurs and includes a collection of individually-authored papers and commentary. Sections of the workshop summary not specifically attributed to an individual reflect the views of the rapporteurs and not those of the Forum on Microbial Threats, its sponsors, or the IOM. The contents of the unattributed sections are based on presentations and discussions at the workshop.
The workshop summary is organized into chapters as a topic-by-topic description of the presentations and discussions that took place at the workshop. Its purpose is to present lessons from relevant experience, to delineate a range of pivotal issues and their respective problems, and to offer potential responses as discussed and described by workshop participants.
Although this workshop summary provides an account of the individual presentations, it also reflects an important aspect of the Forum philosophy. The workshop functions as a dialogue among representatives from different sectors and allows them to present their beliefs about which areas may merit further attention. The reader should be aware, however, that the material presented here expresses the views and opinions of the individuals participating in the workshop and not the deliberations and conclusions of a formally constituted IOM study committee. These proceedings summarize only the statements of participants in the workshop and are not intended to be an exhaustive exploration of the subject matter or a representation of consensus evaluation.
Workshop Context and Scope
Encouraged by opening remarks from the Forum’s chair, David Relman, and Harvey Fineberg, President of the IOM, workshop presenters and discussants attempted to identify scientific questions that must be answered in order to discern—and, ultimately, to predict—the effects of a changing climate on specific infectious diseases, as well as the technical means to tackle these issues. At the same time, workshop participants grappled with an overarching question: What degree of scientific certainty that global climate change threatens human, animal, and plant health must be achieved before taking actions to mitigate these effects?
The National Research Council (NRC) report Under the Weather: Climate, Ecosystems, and Infectious Diseases (2001) has served as both a springboard and a resource for many discussions, including this workshop. The meeting began with a keynote address by Donald Burke of the University of Pittsburgh, who chaired the interdisciplinary committee that produced that influential report (see Burke in Chapter 1). Its key findings, summarized in Box SA-1, reflect considerable scientific uncertainty regarding the causal relationship between global climate change and infectious disease emergence.2
This nuanced assessment has endured, as demonstrated in the 2007 report of Working Group II of the Intergovernmental Panel on Climate Change (IPCC), whose members studied the influence of climate change3 on biological and social systems (IPCC, 2007a). The report states with “very high confidence” that “climate change currently contributes to the global burden of disease and premature deaths,” but notes that “at this early stage the effects are small but are projected to progressively increase in all countries and regions.”
Physical Evidence of Climate Change
There is little doubt that Earth’s climate is changing as a result of human activities. The IPCC’s Working Group I, which assessed the physical science of climate change, concluded that the “warming of the climate system is unequivocal, as is now evident from observations of increases in global average air and ocean temperatures, widespread melting of snow and ice, and rising global average sea level” and that “most of the observed increase in global average temperatures since the mid-twentieth century is very likely due to the observed increase in anthropogenic greenhouse gas concentrations” (IPCC, 2007b). A more detailed discussion of these findings appears in Appendix SA-1 (see page 43), “A Brief History of Climate Change,” and in Chapter 1.
Several workshop participants remarked on the IPCC’s conclusions and called attention to the following general observations suggestive of the broad, profound, and rapidly accelerating impacts of climate change on Earth’s physical systems:
- Oceans as heat sinks: Energy from global warming has been absorbed almost entirely by ocean waters, and relatively little has contributed to the melting of glacial ice or increases in air temperatures (Barnett et al., 2005; Levitus et al., 2005). To date, the thermal expansion of seawater accounts for about half of the observed rise in sea level. As sea levels rise, coastal flooding occurs more frequently and groundwater becomes increasingly saline.
- Warming at high latitudes: Warming is occurring fastest in boreal and arctic regions,4 where its effects are amplified by the melting of snow, ice, and tundra (which also releases methane, a greenhouse gas), according to speaker Paul Epstein of the Harvard Medical School. Measurements by speaker Compton Tucker of the National Aeronautics and Space Administration (NASA) reveal that Greenland (which he described as a “canary for climate change”) is melting at an accelerating pace that currently results in a net loss of approximately 160 km3 of ice per year (see Tucker in Chapter 3).
- Heat waves: Epstein observed that climate change is not only associated with increases in the extent, breadth, intensity, and frequency of heat waves, but also with disproportionately elevated nighttime temperatures, which have increased twice as fast as average ambient temperatures since 1970. He also noted that as warming increases the levels of atmospheric water vapor, heat waves are more likely to be accompanied by increased humidity (IPCC, 2007b; see also Milly et al., 2005).
- Dwindling freshwater supplies: Warmer temperatures mean less water stored in glaciers and snow cover, which yield freshwater for approximately one-sixth of the world’s population (IPCC, 2007a), according to presenter Sir Andrew Haines of the London School of Hygiene and Tropical Medicine. By 2050, he said, annual river runoffs are predicted to decrease by 10 to 30 percent in midlatitude dry regions and in the dry tropics (Milly et al., 2005).
- Hydrological extremes: Warming of the global climate system accelerates the hydrological cycle, producing more droughts, floods, and other extreme weather events. Warming-induced evaporation causes drought in some places, while higher atmospheric water content leads to more intense downpours elsewhere (Karl and Trenberth, 2003).Epstein remarked that the confluence of trends toward increased interannual variability in precipitation (IPCC, 2001, 2007b), heavier precipitation events (Groisman et al., 2004), and more winter precipitation falling as rain rather than snow (Frederick and Gleick, 1999; Gleick, 2004; Levin et al., 2002) reflects the overall increase in seasonal (and, apparently, day-to-day) hydrological variability. He also noted that successive droughts punctuated by heavy rains not only favor flooding, but may also destabilize ecosystems, creating conditions that may be associated with clusters of mosquito-, rodent-, and water-borne disease outbreaks.5
- Higher winds: Circumpolar westerly winds are accelerating, particularly in the Southern Hemisphere (Gillett and Thompson, 2003; IPCC, 2007a), an effect Epstein described as a key sign of climatic instability.6 Moreover, he said, as temperatures rise and pressure gradients build, winds can be expected to increase in intensity, generating stronger windstorms and altering the movement of weather fronts.
In June 2008, the U.S. Climate Change Science Program and the Subcommittee on Global Change Research released a report entitled Weather and Climate Extremes in a Changing Climate. While the IPCC (2007) report looked at the global effects of climate change on biological and social systems, this report focuses on the effects of climate change in North America, Hawaii, the Caribbean, and the U.S. Pacific Islands. Table SA-1 illustrates observed climate phenomena in the last 50 years and projects the likelihood of continued changes in North America. These phenomena include warmer days and nights, increased precipitation, more intense hurricanes, and larger areas affected by drought.
TABLE SA-1
Observed Changes in North American Extreme Events, Assessment of Human Influence for the Observed Changes, and Likelihood That the Changes Will Continue Through the Twenty-first Century.
Over the last two decades, hydrometeorological disasters (e.g., hurricanes, droughts, floods) have affected a steadily increasing number of people living in vulnerable areas, most of them in developing countries, as shown in Figure SA-1. This development might be more accurately described as “global weirding,” Burke said, in order to capture both the severity and the unpredictability of weather events spawned by global warming. As discussed in subsequent sections of this summary and in Chapter 1, extreme weather conditions increase the risk of transmission for a variety of infectious diseases, including diarrheal diseases, vector-borne diseases, and respiratory infections. Following a weather disaster such as a hurricane, affected areas must often cope with multiple infectious disease outbreaks.

FIGURE SA-1
People affected by hydrometeorological disaster (millions per year). SOURCE: Reproduced from United Nations Development Programme (2007) with permission of Palgrave Macmillan.
Coincident Changes in Climate and Infectious Diseases
There are no appropriate, independent controls for the study of global climate change on Earth, Epstein observed. A wide range of methodologies must be harnessed, therefore, in order to assess changes in biological variables—including the geographic range and incidence of diseases—in relation to changes in temperature and precipitation (see Chapter 1). Information obtained from a variety of monitoring and mapping techniques can be integrated into geographic information systems (GISs) and used to identify and compare physical and biological phenomena. By enabling the overlay of multiple sets of data, GISs also provide contributions to descriptive and mathematical models that may be used to project the biological impacts of various climate change scenarios. Additional methods are used to analyze data gathered across scientific disciplines in order to reveal patterns and emerging trends associated with climate change, calculate rates of change (i.e., in the geographic range, prevalence, and incidence of infectious diseases), and compare these observations with predicted outcomes.
Many of the methodologies used to study the effects of climate change yield correlations, rather than proof of causation, Epstein acknowledged, but he argued that when observational data from multiple sources (1) match model projections, (2) are consistent with each other, and (3) can be explained by plausible biological mechanisms, the preponderance of the evidence warrants further attention and exploration. Moreover, he added, models could be used to test such associations and their apparent underlying mechanisms (see Chapter 1).
In particular, Epstein identified three outcome variables as central to understanding the effect of climate change on the distribution of infectious diseases: shifts in altitude (and latitude), changes in seasonality, and responses to increased weather variability.
Shifts in altitude Many animal and plant species are adapted to specific habitats that occupy a narrow range along altitudinal and latitudinal climatic gradients.7 Increasing temperatures not only melt alpine glaciers and drive the upward migration of plant communities, but also enable insects and other species that serve as infectious disease vectors to occupy higher altitudes (Epstein et al., 1998).8 Such changes in conditions—which are conducive to changes in the ranges of disease agents and vectors—are occurring at high-altitude locations across the globe: in the Andes, the Sierra Nevada, the East African highlands, the European Alps, and the mountainous regions of India, Nepal, and Papua New Guinea, Epstein observed.
Seasonal shifts Climatic warming is expected to lengthen seasonal activity periods for mosquitoes and other insect vectors, thereby increasing opportunities for exposure to infectious diseases such as malaria (Tanser et al., 2003; van Lieshout et al., 2004). Ecological opportunists—including insects and rodents that serve as vectors of, and reservoirs for, infectious diseases—tend to proliferate rapidly in disturbed environments, while large predator species (infectious disease hosts) suffer under unstable environmental conditions, Epstein said.
Responses to increased weather variability Increased climate variability, along with habitat fragmentation and pollution, is likely to alter predator-prey relationships, which in turn influence infectious disease transmission dynamics. Such disequilibrium is thought to have precipitated the 1993 outbreak of a rodent-borne infection, hantavirus pulmonary syndrome, in the Four Corners region of the southwestern United States. That year, early, heavy rains ended an intense drought (during which predator populations declined) and provided new food for rodents, whose populations then expanded rapidly (Calisher et al., 2005; Patz et al., 1996).
While it is anticipated that climate change will influence infectious disease emergence, several workshop participants emphasized that direct causal connections have yet to be established between climate change and infectious diseases, and that accurate predictions of infectious disease behavior cannot yet be made on the basis of climate projections alone.
Climate and Health
“Climate change will affect the health of humans as well as the ecosystems and species on which we depend, and … these health impacts will have economic consequences,” predicts a recent report published by the Center for Health and the Global Environment (2005), edited by Epstein and Evan Mills (see Chapter 1 for the executive summary of this report, Climate Change Futures: Health, Ecological and Economic Dimensions). The report highlights a broad range of known and anticipated health consequences of climate change for humans, animals, and plants. In addition to influencing the location and frequency of infectious disease emergence and outbreaks, these effects include increased pest damage of crop plants, which in turn could contribute to human malnutrition; greater concentrations of pollen and fungi in the air, raising the risk of allergic symptoms and asthma; and higher rates of injury and death due to weather disasters and fires. Indeed, as Epstein (2005) has concluded, “it would appear that we may be underestimating the breadth of biologic responses to changes in climate.”
Figure SA-2 illustrates the multiple pathways by which variations in climate affect the health of humans, animals, and plants. Direct influences include long-term regional changes in average temperature and precipitation, as well as extreme weather events such as floods, droughts, or violent storms. Climate change may also exert health effects indirectly, by altering ecosystems in ways that, for example, affect the geographic distribution or transmission dynamics of infectious diseases.
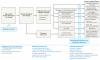
FIGURE SA-2
Potential health effects of climate variability and change. SOURCE: Reprinted with permission from the American Medical Association from Haines and Patz (2004). Copyright 2004. All rights reserved; adapted from Patz et al. (2000).
Direct and Indirect Effects of Climate on Infectious Diseases
Climate exerts both direct and indirect influences on the transmission and geographic distribution of infectious diseases, such as those shown in Table SA-2 (NRC, 2001). Direct effects of climate on infectious disease occur through the following mechanisms:
TABLE SA-2
Examples of Diseases Influenced by Environmental Conditions.
- Pathogen replication rate. This is particularly true of vector-borne diseases of warm-blooded animals, due to the exposure of pathogens to ambient weather conditions for part of their life cycle.
- Pathogen dissemination. This occurs when floods contaminate drinking water reservoirs, resulting in diarrheal diseases, and also when dry winds distribute soil-borne pathogens.
- Movement and replication of vectors and abundance of animal hosts. These include reservoir species for infectious diseases, such as migratory birds that carry avian influenza.
Climate also influences the distribution and transmission of infectious diseases through indirect effects on local ecosystems and human behavior. For example, abundant precipitation provides more and better breeding sites for vector species such as mosquitoes, ticks, and snails, while increasing the density of vegetation beneficial to these organisms (Githeko et al., 2000). Drought, on the other hand, may prompt people to store water in open containers, which also provide ideal breeding environments for mosquitoes.
Climate influences each component of the epidemiological triad of host-vector (see Figure SA-3), pathogen, and environment, which intersect to produce infectious disease. The complex ecologies of vector-borne diseases render them particularly sensitive to variations in temperature, which can alter patterns of disease incidence, seasonal transmission, and geographic range (McMichael et al., 2006; Sutherst, 2004). Some scientists predict that the effects of climate change and variability on vector-borne diseases are likely to be expressed in the form of short-term epidemics, as well as through gradual changes in disease trends (Githeko et al., 2000).
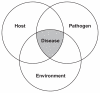
FIGURE SA-3
The epidemiological triad. SOURCE: Reprinted from Snieszko (1974) with permission from Blackwell Publishing Ltd. Copyright 1974.
Climate’s Role in Context
Climate interacts with a range of factors that shape the course of infectious disease emergence, including host, vector, and pathogen population dynamics; land use, trade, and transportation; social, political, and economic systems; human and animal migration; and interventions that control or prevent disease. These interdependent influences—or web of causation—can act together, resulting in outbreaks or epidemics of infectious disease; for example, people and animals (both domesticated and wild), if forced by climate disasters to migrate, may introduce pathogens, parasites, and disease vectors into novel environments. The intersection of human, livestock, and wildlife movements and migration with climate change is discussed in greater detail later in this summary (see “Policy Implications”) and in Chapter 4. An even broader view of disease emergence, the “Convergence Model” (see Figure SA-4), places climate among other physical environmental factors in disease emergence that intersect with biological and socioeconomic factors, as well as with host (human) and microbe (IOM, 2003).

FIGURE SA-4
The Convergence Model. At the center of the model is a box representing the convergence of factors leading to the emergence of an infectious disease. The interior of the box is a gradient flowing from white to black; the white outer edges represent what (more...)
Observed Effects of Climate Variation on Infectious Disease Range and Transmission Dynamics
The many factors confounding the interrelationships between climate change and infectious disease emergence vastly complicate attempts to investigate causality. As Haines and coauthors note, “Empirical observation of the health consequences of recent climate change, followed by formulation, testing, and then modification of hypotheses would require long time-series (probably several decades) of careful monitoring” (Haines et al., 2006). To inform health policy in the immediate future, risk assessments will need to be developed from short-term observations of the effects of climate variation on infectious disease, taking into account the influence of confounding factors. Existing observations of these effects fall into two main categories: (1) climate-associated shifts in the geographical ranges of pathogens and vectors, and (2) studies of infectious disease transmission dynamics spanning relatively short periods of climatic variation.
Infectious Diseases in New Places
The following illustrative examples suggest that climate change has contributed to recent shifts in the geographic distribution of certain vector-borne diseases. In each case, additional factors may also contribute to the emergence and spread of these diseases.
- Bluetongue, a midge-borne viral disease of ruminant animals, emerged for the first time in northern Europe in 2006, during the hottest summer on record for that region and following nearly a decade of anomalously warm years. In the summer of 2007, the disease was reported in nine European countries, including the United Kingdom9 and Denmark, during a massive outbreak that affected tens of thousands of farms (Enserink, 2008; IOM, 2008; ProMed Mail, 2007a,b, 2008; see Figure SA-5).
- Ticks that carry viruses known to be associated with encephalitis have been found at increasingly higher latitudes in northern Europe. A recent study in Denmark reveals a marked shift in the distribution of the tick-borne encephalitis virus as predicted by climate change models (IOM, 2008; Skarphedinsson et al., 2005).
- A 2004 outbreak of Vibrio parahaemolyticus gastroenteritis, associated with human consumption of raw oysters taken from Alaskan waters, extended the northernmost documented source of shellfish carrying this pathogen by 1,000 km. Vibrio parahaemolyticus had not been found in oyster beds in this region before 2004 (McLaughlin et al., 2005).
- In South Africa, the spread of wheat stripe rust has accompanied changes in rainfall patterns (Garrett et al., 2006), while needle blight of pine trees caused by Dothistroma septosporum, formerly a concern only in the Southern Hemisphere, is causing massive defoliation and mortality in the forests of British Columbia following climate change-associated increases in summer precipitation (Woods et al., 2005).
- Malaria incidence in the highlands of East Africa has risen since the late 1970s. The specific influence of rising temperatures on disease incidence has been a subject of considerable debate. Recent analyses employing a dynamical model suggest that a significant warming trend in this region has amplified mosquito population dynamics so as to contribute, along with drug resistance and land-use patterns, to the increased incidence of malaria (Harrus and Baneth, 2005; IOM, 2008; Pascual et al., 2006).

FIGURE SA-5
Progression of bluetongue viruses emergence in Europe. SOURCE: Figure updated from Osburn (2008) and created by Rick Hayes, School of Veterinary Medicine, University of California, Davis.
Climate Variation and Infectious Disease Transmission
Several recent studies have examined the relationship between short-term climatic variation and the occurrence of infectious diseases, in particular the influence of the El Niño/Southern Oscillation (ENSO) on the transmission of such vector- and non-vector-borne diseases as malaria, dengue fever, cholera, Rift Valley fever (RVF), and hantavirus pulmonary syndrome (Anyamba et al., 2006; McMichael et al., 2006; see Figure SA-6). ENSO, the irregular cycling between warm (El Niño) and cool (La Niña) phases of surface water temperatures across the central and east-central equatorial Pacific, is a well-known source of climate variability (see Haines in Chapter 1 and Chretien in Chapter 2). ENSO-associated shifts in ocean surface temperatures influence temperature and precipitation patterns throughout the global tropics, simultaneously producing excessive rainfall in some areas and drought in others (Kovats et al., 2003).

FIGURE SA-6
Hot spots of potential elevated risk for disease outbreaks under El Niño conditions, 2006–2007. SOURCE: Anyamba et al. (2006).
Global climate change is expected to intensify ENSO-related climate variability (WHO et al., 2003), which in turn offers a means to study the effects of climate variability on infectious disease (see Haines in Chapter 1). In his workshop presentation, Jean-Paul Chretien, of the U.S. Department of Defense, described key examples of such research, which examined connections between ENSO-related weather extremes and two infectious diseases: RVF and chikungunya fever (see Chretien in Chapter 2).
El Niño and Rift Valley fever An acute mosquito-borne viral disease, RVF primarily affects livestock (e.g., cattle, buffalo, sheep, goats) but can also be transmitted to humans through direct contact with the tissue or blood of infected animals, as well as by mosquito bites. Outbreaks of RVF among animals can spread to humans. The largest reported human outbreak, which occurred in Kenya during 1997–1998, resulted in an estimated 89,000 infections and 478 deaths (CDC, 2007b). For decades, RVF outbreaks have been associated with periods of heavy rainfall, which occur during El Niño; this observation led researchers to develop an operational model for RVF risk based on vegetation density (a marker for rainfall) as measured by satellite (see Figure SA-7A; Linthicum et al., 1999).

FIGURE SA-7A
Using satellites to track Rift Valley fever. NOTE: Scientists have discovered that the combination of warmer-than-normal equatorial Pacific Ocean temperatures associated with El Niño and rising sea surface temperatures in the western equatorial (more...)
During the El Niño event of 2006–2007, above normal rainfall resulted in anomalous vegetation growth in East Africa, northern Australia, and parts of eastern China, and drought and diminished vegetation growth in southeastern Australia and northern South America. Above normal rainfall and anomalous vegetation growth in eastern Africa created ideal ecological conditions for the emergence of mosquito vectors of RVF, resulting in an outbreak of the disease in East Africa from December 2006 to May 2007 (see Figure SA-7B; A. Anyamba, personal communication, April 2008).10

FIGURE SA-7B
January 2007 combined global Normalized Difference Vegetation Index (NDVI) (depicted over land surfaces) and sea surface temperature (SST) (depicted over oceans) anomaly mosaic. NDVI and SST data are collected daily by several satellites in an ongoing (more...)
Throughout the autumn of 2006, this model identified high risk for RVF in the same area affected by the 1997 epidemic, leading the U.S. Army Medical Research Unit (USAMRU) in Kenya to intensify its surveillance of local mosquitoes. Positive results provided early warning of a pending epidemic, enabling the Kenyan government—in concert with international partners including the Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO)—to mount a timely and targeted response, Chretien said (see CDC, 2007b).
La Niña and chikungunya fever Another mosquito-borne viral disease, chikungunya fever, is rarely fatal, but can cause severe joint pain, prolonged disability, and complications including protracted fatigue and arthritis (CDC, 2007b). A string of outbreaks along the Kenyan coast in 2004 apparently spread to several western Indian Ocean islands and to India, resulting in the largest chikungunya fever epidemic on record (Chretien et al., 2007). Upon investigation, Chretien and coworkers discovered that at the time of the initial outbreaks in Kenya, a regional drought—corresponding to the La Niña phase of ENSO—had gripped the region. “There is some evidence that suggests that there may be a connection [between the drought and the chikungunya fever epidemic],” Chretien observed. “We know from the outbreak investigations in [Kenya], that domestic water wasn’t being changed as frequently as usual because of the drought, and it wasn’t being protected properly from the peridomestic mosquitoes that transmit chikungunya virus.” Also, he noted, previous experimental studies in Kenya found that warm conditions can accelerate viral development within the mosquito (Chretien et al., 2007).
In addition to ENSO-associated weather anomalies, other short-term variations in climate, including drought, temperature, and wind patterns, have also been linked with changes in infectious disease incidence and geographic range:
Drought and diarrheal disease While diarrheal disease is frequently associated with periods of heavy rainfall and flooding and the subsequent contamination of water supplies with fecal bacteria (NRC, 2001), Haines described findings from a recent review of cross-sectional studies from 36 low- and middle-income countries that correlate increased incidence of diarrhea in young children with decreased rainfall (Lloyd et al., 2007). Because the vast majority of freshwater is used for irrigation, rather than for personal consumption, the relationship between these variables is unclear. Haines noted that handwashing behavior has been shown to decline when freshwater is less available (Curtis and Cairncross, 2003).
Temperature and food poisoning Comparing data from 16 sites in industrialized countries, investigators examined the incidence of sporadic cases of food poisoning (rather than outbreaks, which tend to be triggered by specific contamination incidents) attributed to the bacterium Salmonella. They found that such cases rose in a linear relationship to the previous week’s temperature (Kovats et al., 2004). The lag in time suggests that temperature exerts this effect by accelerating bacterial replication in prepared food, Haines observed. Similar patterns of seasonal incidence also occur in cases of gastroenteritis caused by another bacterial agent Campylobacter (Kovats et al., 2005; Louis et al., 2005; Tam et al., 2006). However, unlike salmonellosis, seasonal patterns of Campylobacter infection in humans are not completely attributable to food-borne transmission of the pathogen, according to speaker Rita Colwell of the University of Maryland.
In a study conducted in England and Wales, Colwell and colleagues found that an increased incidence of Campylobacter gastroenteritis was associated with higher temperatures in districts supplied primarily with surface water, while those with the lowest incidence received mainly groundwater (Louis et al., 2005). The researchers therefore hypothesized that water ingested by poultry was the source of the seasonal increase in cases of human Campylobacter gastroenteritis and noted that surface water may be especially prone to contamination with the pathogen in the spring, when cattle and sheep give birth and are put out to pasture.
Wind-borne disease The annual arrival of dry, dust-laden winds—thought to render mucosal membranes vulnerable to infection—heralds the onset of epidemic meningococcal meningitis in West Africa (Sultan et al., 2005). There is some evidence that the geographical distribution of meningococcal meningitis in West Africa has expanded in the recent past, possibly as a result of changes in land use and climate (Molesworth et al., 2003; see Haines in Chapter 1).
Coccidioidomycosis—a fungal disease caused by inhaling the spores of Coccidioides immitis—along with meningococcal meningitis, can travel across continents in spore-laden desert dust clouds (Flynn et al., 1979; Garrison et al., 2003; NRC, 2001). The winds pick up these spores, along with dry, dusty soils, and transport them hundreds of miles (NRC, 2001; Schneider et al., 1997).
High winds and extreme weather have also been linked to the emergence, reemergence, and long-distance transport of vector-borne pathogens such as bluetongue and the citrus tristeza virus (IOM, 2003, 2008; NRC, 2001). Asian soybean rust, a pathogenic fungus, was apparently blown into the United States from South America by Hurricane Ivan in 2004 (Schneider et al., 2005). This nonnative plant pathogen has now become established in soybean-growing areas of the United States and Canada.
Synergies and Threshold Effects of Climate Change on Infectious Disease Emergence
In addition to the short-term observations of the effects of climate variation on the range and transmission of infectious disease described in the previous section, workshop participants considered the apparent near-term and long-range impacts of climate change on infectious diseases in several illustrative contexts: plant communities and crops; aquatic and marine environments; the Arctic; and central Asian ecosystems that have long served as incubators for plague epidemics. A common theme uniting these diverse accounts was the recognition that climate does not act gradually or entirely predictably upon ecosystems, but combines with other influences to produce threshold effects. Although typically expressed in terms of population dynamics (e.g., explosions, migrations, extinctions), such threshold effects also include the emergence of infectious diseases.
Plant Disease
According to speaker Karen Garrett of Kansas State University, climate change has the potential to produce huge—and largely unanticipated—impacts on agricultural and natural systems by altering patterns of plant infections. These effects include the direct consequences of crop diseases, such as declining food supplies; indirect effects on agricultural productivity, such as reduced soil formation (and thereby lower crop yields) resulting from more frequent tillage to remove infected plant residue; and health risks associated with increased pesticide usage. While efforts to understand these potential impacts typically focus on ecosystems, populations, and communities, Garrett and coworkers study plant responses to infectious disease at the molecular level, in order to understand and model genetic constraints for pathogen and plant adaptation to climate change (see Garrett in Chapter 2 for specific examples of these studies in various crop plants and plant communities).
Extending such observations to predict the repercussions of climate change on plant disease at the ecosystem level requires consideration of a broad range of influences on each member of the disease triad. Moreover, Garrett explained, any such perturbation may cross a threshold to an unexpectedly dramatic response. Many diseases, such as potato late blight, the disease that caused the Irish potato famine in the mid-nineteenth century, exhibit compound interest increases during a growing season, so that a slightly longer growing season can result in much higher regional inoculum loads. “The effects of climate change will be most important when there are thresholds and interactions that produce unanticipated large responses, and one of the most important effects might be that the systems will change more rapidly than in the past,” Garrett observed.
Considerable resource investments will be needed to improve our understanding of the various and interacting factors that influence plant disease, she said. These include long-term, large-scale records of pathogen and host distributions (currently lacking even for agriculturally-important diseases); models of regional processes that incorporate disease dynamics; data and models that describe the dispersal of pathogens and vectors; and integrated, multidisciplinary, international collaborative networks for data collection and synthesis.
Research is also needed to identify and improve the introduction of disease resistance genes, a proven and promising strategy for responding to changes in disease threats to crops. In the tropics, where climate change is viewed as a considerable threat to food security due to the likelihood of greater climate variability, and where resources for crop protection are limited, efforts to characterize genetic resources are especially important. The Consultative Group for International Agricultural Research (CGIAR, 2008) currently undertakes such efforts on a “shoestring budget,” Garrett reported.
Aquatic and Marine Environments
Two speakers at this workshop offered different perspectives on the direct and indirect influences of climate change in aquatic ecosystems. Leslie Dierauf of the U.S. Geological Survey (USGS) described the apparent impacts of climate and disease trends for a broad cross-section of aquatic and marine species and ecosystems, while Colwell discussed ecological and climatological factors that influence cholera, a water-borne infectious disease of considerable public health significance.
Aquatic and marine wildlife Marine life has suffered significant increases in the frequency and number of novel disease epidemics over the past few decades due to a variety of factors including, but not limited to, the disruption of ocean ecosystems by climate variability and warming water temperatures (Harvell et al., 1999). Much like their human counterparts in drought- or storm-stricken areas, marine mammals are being forced out of their home ranges by warming-induced population declines in plankton. As they follow their food to new territories, migrant marine mammals both encounter and introduce novel disease agents. Mass die-offs of certain species (e.g., seals, dolphins, porpoises) have occurred when these animals were exposed to morbilliviral diseases, such as distemper, for the first time during their annual migrations. Phocine distemper virus, identified as the cause of a die-off of harbor and gray seals in northern European coastal waters, is thought to have been transmitted to these species by harp seals that migrated to this region in response to overfishing-induced food shortages around their native Greenland in the late 1980s (Harvell et al., 1999).
The effects of climate variability on the health and disease of aquatic (freshwater-dwelling) and marine (ocean-dwelling) organisms are frequently exerted through the food web, as shown in Figure SA-8. In addition to these relationships, Dierauf emphasized that because aquatic and marine ecosystems are interconnected, infectious diseases of fish and wildlife may have the opportunity to move from freshwater sources to intertidal zones to marine environments, affecting species that may have not encountered these disease agents before. She also noted the particular vulnerability of coastal and intertidal zones to the effects of extreme weather, both directly as a result of damaging winds and water and indirectly though runoff from inland floods. On the U.S. Gulf Coast where, she said, “two hurricanes can turn an intertidal seagrass area into a mudflat,” a majority of such areas—which act as buffers between ocean and land, and between fresh and marine waters—have been lost in recent years.
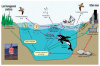
FIGURE SA-8
Interconnectedness of terrestrial, aquatic, and marine food webs. SOURCE: Figure courtesy of Mary Ruckelshaus, NOAA Fisheries.
Examples of emerging infectious diseases along the aquatic-marine continuum, and their potential links to climate change, are presented in Box SA-2. Noting the lack of evidence-based literature on the effects of climate change and wildlife health, Dierauf joined the chorus of workshop participants calling for greater investments in collaborative efforts to monitor, model, and research these connections.
Water-borne human disease The incidence and distribution of food- and water-borne diseases are shaped by numerous factors, including climate variation, water temperature, precipitation patterns, and/or water salinity. Extreme weather events, including heavy rainfall and flooding, are associated with outbreaks of several important water-borne diseases (NRC, 2001). These include cholera, an acute diarrheal illness caused by the bacterium Vibrio cholerae (CDC, 2005); cryptosporidiosis, one of the most common water-borne diseases in the United States, caused by microscopic parasites of the genus Cryptosporidium (CDC, 2007a); and giardiasis, another diarrheal illness common in the United States, which is caused by the single-celled parasite Giardia intestinalis (CDC, 2004). Soil washed into coastal waters by floods contains animal wastes (and, therefore, fecal bacteria) as well as other organic matter and nutrients that promote rapid growth, or “blooms,” of certain toxic algae species. These harmful algal blooms (also known as “red tides”) produce neurotoxins that can be transferred through the marine web—killing some marine animals along the way—to seafood-consuming humans (Woods Hole Oceanographic Institution, 2008).
A complex web of ecological relationships is involved in the incidence and prevalence of cholera, which Colwell estimated affects 100,000 people per year and kills 10,000 (see Colwell in Chapter 2). Over the course of three decades of study, she and coworkers have determined that this water-borne disease, although caused by a bacterium (V. cholerae), is actually transmitted by the plankton species with which it associates (Colwell, 2004). Vibrio cholerae is a natural inhabitant of aquatic environments of appropriate salinity, but remains quiescent except when temperatures rise above 15°C, and an influx of nutrients causes the plankton to bloom, increasing V. cholerae concentrations to levels capable of causing disease when water is consumed. This relationship is sufficiently robust to permit the use of remote sensing data—incorporating sea surface temperature, sea surface height, and chlorophyll levels (an indicator of phytoplankton bloom) observed in the Bay of Bengal—to predict the onset of cholera epidemics in the Ganges delta region of Bangladesh, known as the “home of cholera” due to its long history of epidemic disease.
Primarily confined to the Indian subcontinent, cholera was spread by the shipping trade from India to Europe and the Americas in the early nineteenth century (Colwell, 2004). Subsequent improvements in sanitation drastically reduced cholera incidence in the West, but the disease reemerged in Peru in 1991, after being absent from that country for nearly a century. Although initially attributed to contaminated ballast water from a foreign ship, cholera’s return to Peru was eventually linked to elevated sea surface temperature, coincident with El Niño (Lipp et al., 2003).
The Arctic
The physical effects of climate change are dramatically apparent in the Arctic, where temperatures have increased at nearly twice the global average over the past century, causing widespread melting of land and sea ice (see Figure SA-13; Borgerson, 2008; IPCC, 2007b). This trend is expected to continue and intensify, resulting in warmer winters, increased annual precipitation, more frequent extreme weather events, and—as the ice continues to melt—greater river discharge and increased sea height, according to workshop speaker Alan Parkinson, of the CDC’s Arctic Investigations Program in Anchorage, Alaska.

FIGURE SA-13
The Arctic ice cap, September 2001 (Top) and September 2007 (Bottom). SOURCE: NASA, as printed in Borgerson (2008).
These rapidly changing environmental conditions are ripe for infectious disease emergence on several fronts, Parkinson observed (see Chapter 2). Higher temperatures at these latitudes permit the survival and replication of cold-sensitive pathogens such as Vibrio parahaemolyticus, as previously noted (McLaughlin et al., 2005), or increase the prevalence of existing pathogens such as Clostridium botulinum (a particular concern for indigenous peoples, who traditionally preserve food by fermentation). Preliminary studies suggest that warmer ambient temperatures, which would be predicted to occur with climate change, may result in higher rates of food-borne botulism associated with the consumption of fermented seal meat (Leclair et al., 2004). In addition to fermentation, many Arctic residents store fish and meat by air-drying or by burying it on or near the permafrost; changes in climate may therefore result in higher rates of spoilage of food preserved by either method (see Parkinson in Chapter 2).
As temperatures increase, reservoir species for zoonotic diseases may survive winters in larger numbers, increase in population, or expand their geographic ranges. Beavers, common hosts for the water-borne protozoan Giardia intestinalis, are migrating northward in Alaska, into areas that have become more habitable due to changes in vegetation and habitat (Parkinson and Butler, 2005). Similarly, climate conditions that favor range expansion by foxes or rodents that carry alveolar echinococcosis—a lethal zoonotic infection caused by the larval stage of the tapeworm Echinococcus multilocularis—may increase the human incidence of this disease (Holt et al., 2005; Parkinson and Butler, 2005; Schweiger et al., 2007).
Climate change may enable mosquito-borne diseases such as the West Nile virus (WNV) to move into the Arctic by increasing vector survival and disease transmission rates, as well as by altering migration patterns of birds and other reservoir species. WNV has already reached Canadian provinces adjacent to Alaska at a latitude of 57ºN, and its mosquito vector, Aedes albopictus, is present in the state, Parkinson reported. Climate change is also projected to shift the range of the tick vector of Lyme disease northward (Ogden et al., 2005), but as with WNV, the consequences of such movements for human disease depend on a range of factors, including land use, human population density, and temperatures warm enough for pathogens to reach an infective dose in the vector.
Public health challenges Together with a catalog of health impacts attributable to climate change in the Arctic, Parkinson noted two indirect effects that appear especially favorable to infectious disease transmission: (1) damage to the sanitation infrastructure resulting from the melting of permafrost (upon which many Arctic communities are built) and from flooding, and (2) the opening of the Northwest Passage.
Inadequate housing and sanitation are already important determinants of infectious disease transmission in many Arctic regions, Parkinson observed. In a recent study conducted in western Alaska, Parkinson and coworkers found significantly higher rates of hospitalization for young children with pneumonia, influenza, and respiratory syncytial virus (RSV) and for people of all ages with outpatient Staphylococcus aureus infections and hospitalization for skin infections, in communities without in-house piped water service, compared to communities with in-house piped water service (Hennessey et al., 2008). This suggests that the loss of existing basic sanitation services, through climate change-related infrastructural damage, may raise infectious disease rates in Arctic populations. Furthermore, Parkinson noted, sewage leaking from pipes ruptured by melting permafrost contains water-borne pathogens such as Giardia, Cryptosporidium, and the hepatitis A virus.
A second potential route for infectious disease emergence in the Arctic is being cleared along with the sea ice. With the opening of the Northwest Passage—and perhaps, eventually, the Northeast Passage—more ships will take this shorter route as a sea lane alternative to the Panama Canal when crossing between the Atlantic and Pacific Oceans (see Figure SA-14). Increased maritime shipping in the Arctic is expected to bring many economic benefits to these north-ernmost communities, Parkinson noted, but it is also likely to expose the region’s inhabitants and ecosystems to invasive species of all kinds, including potentially pathogenic microbes and their vectors. In the face of these challenges, Parkinson echoed the suggestions of many other speakers at this workshop to enhance the surveillance and monitoring of climate-sensitive infectious diseases on a global basis and to establish international networks to share such information.

FIGURE SA-14
Arctic shipping shortcuts. SOURCE: Reprinted with permission from Foreign Affairs (Borgerson, 2008). Copyright 2008 by the Council on Foreign Relations, Inc.
Plague Dynamics
Throughout human history the various forms of plague, caused by the bacterium Yersinia pestis and transmitted by fleas among a wide range of hosts, have caused both endemic and epidemic disease. “Plague is a highly variable disease,” explained speaker Nils Christian Stenseth of the University of Oslo, Norway. “It is a complex system with complex temporal, seasonal, interannual dynamics.” In his contribution to Chapter 2, Stenseth describes these intricate relationships and his approach to modeling plague dynamics based on long-term monitoring of pathogen prevalence in central Asian rodent populations. These studies have led him to conclude that although relatively few cases of plague are currently reported, the disease poses a significant and imminent threat to human populations due, in part, to the influence of climate change.
Using longitudinal data collected over 50 years in Kazakhstan, a focal region for plague where cases are regularly reported, Stenseth and colleagues determined that Y. pestis prevalence increases dramatically in its primary host, the great gerbil (Rhombomys opimus), during warmer springs and wetter summers (Stenseth et al., 2006). Rodent populations also tend to increase under these conditions and, along with them, the possibility that plague will be transmitted to humans. These conditions apparently existed in central Asia during the onset of the Black Death in the fourteenth century, as well as in the years preceding a mid-nineteenth-century plague pandemic. As Earth’s climate warms, warmer springs and wet summers are expected to become increasingly common in the region, and also in North America.
Historical, Scientific, and Technological Approaches
Several workshop presentations described methods used to identify, measure, analyze, and predict the direct and indirect effects of climate change on infectious disease emergence. Each of the topics discussed below represents part of an interdisciplinary approach that, participants agreed, must continue to expand in order to pursue a common goal. As Colwell observed, understanding complex interactions between biological and physical environments paves the way for the development of predictive models and, thereby, for early and efficient responses to infectious disease threats.
Analysis of Historical Data
Historical analysis provides a perspective on climate and infectious disease far more sweeping than can be obtained from scientific monitoring. When speaker Rodolfo Acuña-Soto of the Universidad Nacional Autónoma de México and coworkers chronicled major epidemics (based on historical accounts) and climate conditions (as reflected in the width of tree rings) over the last millennium in the Valley of Mexico, they revealed an association between severe and prolonged droughts, catastrophic epidemics, and societal collapse (see Acuña-Soto et al. in Chapter 3).
Amid one such “megadrought” during the sixteenth century, hemorrhagic fever appears to have killed an estimated 80 percent of the indigenous people of the Valley of México; survivors mated primarily with Spanish colonists, repopulating the region with predominantly Mestizo offspring. Droughts also accompanied each of a series of 22 typhus epidemics that occurred between 1655 and 1915.
Drought is still a major problem for Mexico and is expected to continue to burden the country in the future, Acuña-Soto noted. In addition, contemporary increases in human connectivity and infectious disease emergence resemble the circumstances of past regional epidemics, such as those that followed the Spanish conquest of the region more than 400 years ago.
Wildlife Monitoring
Emerging infectious diseases of wildlife, such as those described in Box SA-2, arise from a disturbance in a delicate balance of host, pathogen, and environment. For this reason—and also because wild animals often serve as reservoir species for zoonotic threats to human health—they represent a critical target for infectious disease monitoring efforts of all sorts, including those that seek to track the influence of climate change, according to speaker William Karesh of the Wildlife Conservation Society (see Karesh in Chapter 3).
Wild animals also offer a number of advantages for disease monitoring programs, Karesh explained. Their comparatively short generation times reflect environmental changes more quickly than do humans; the great variety of wild species offers an equally wide range of life histories from which researchers can choose to model disease scenarios at different generational rates; and they provide sensitive sentinels for changes in the environments to which they are specifically adapted. Karesh observed that fish, bird, and marine mammal populations in South America declined dramatically during the El Niño event that occurred there in 1991–1992. In the case of Ebola hemorrhagic fever, Karesh observed that gorilla die-offs have preceded human outbreaks of Ebola virus by several weeks.
Highly pathogenic avian influenza can move between wild birds, domesticated poultry, and people, resulting in an increased risk of disease in cohabitated populations. Although wild birds cannot predict efficient human-to-human transfer of H5N1 avian influenza, the Global Avian Influenza Network for Surveillance (GAINS) gathers data in 23 developing countries—largely through the efforts of volunteers—on wild bird diseases; disseminates information to governments, international organizations, the private sector, and the general public; and helps to develop appropriate responses before outbreaks occur (GAINS, 2008).
Long-term monitoring of infectious diseases in wildlife also made possible the previously described model of plague dynamics and climate by Stenseth and coworkers (Stenseth et al., 2006). Under the Soviet regime, scientists began surveying rodent populations in Kazakhstan and testing them for plague in 1949; the practice continued through 1995, providing a wealth of data for statistical analysis. Such long-term studies are crucial to the prevention of human epidemics of plague and other zoonotic diseases that cannot be eradicated because they persist in a vast range of wildlife species, Stenseth said.
Remote Sensing
Satellite imagery is used to measure environmental variables over time, including land cover (a proxy for rainfall) and surface air temperature and humidity. Trends in these conditions, when compared with epidemiological data, reveal relationships between climate and infectious disease transmission and geographic distribution—for example, the previously discussed link between vegetation density and risk for epidemic RVF in humans (Linthicum et al., 1999).
In his workshop presentation, speaker Compton Tucker of the National Aeronautics and Space Administration (NASA) described the collection and analysis of remote sensing data and presented two examples of its use in examining links between climate and infectious disease (see Tucker in Chapter 3). The first involved a search for significant environmental factors common to sporadic outbreaks of Ebola hemorrhagic fever. Analyzing satellite data collected continuously since 1981, he and coworkers found an apparent “trigger event” that occurred prior to each outbreak: a period of drought, followed by a sudden return to very wet conditions (Pinzon et al., 2004). Today, satellite data from eastern equatorial Africa are screened routinely for this weather pattern; the results guide targeted testing for Ebola virus in local primates, which may provide an early warning of future outbreaks in humans.
Tucker and colleagues have also used satellite imagery to investigate an outbreak of RVF in Yemen, which seemed suspicious because of its proximity in location and time to a terrorist attack on a U.S. Navy ship, the USS Cole. Records of a satellite-derived index of photosynthetic capacity in local vegetation (another rainfall indicator) suggested that significant precipitation had fallen in the region prior to the outbreak, so the researchers concluded that it probably arose naturally.
Predictive Models
Several models for predicting the onset or prevalence of infectious diseases based on climatic indicators have been discussed in previous sections of this chapter (see also contributions to Chapter 2 by Chretien, Colwell, and Stenseth). Remote sensing of sea surface temperature and height, along with vegetation indices, are used to anticipate ENSO effects on a variety of diseases (Anyamba et al., 2006), to identify areas at risk for RVF outbreaks (Linthicum et al., 1999), and to provide early warning of epidemic cholera in Bangladesh (Gil et al., 2004; Speelmon et al., 2000). Stenseth suggested that statistical models capable of predicting past plague epidemics in central Asia (from tree-ring-derived measures of temperature and humidity) (Stenseth et al., 2006) could anticipate the influence of current climate conditions on population density and disease prevalence in rodent reservoirs of plague.
Climate-driven predictive models of mosquito-borne encephalitis transmission are also used by the State of California to estimate disease risk and inform public health interventions. Speaker William Reisen of the University of California, Davis, described the ongoing development of these models and their use in targeting surveillance to support integrated vector management (see Reisen and Barker in Chapter 3). The goal of these efforts, Reisen said, is to limit local population sizes of mosquitoes in order to prevent these vectors from amplifying West Nile and related viruses to levels that put humans at risk for infection and to do so as efficiently as possible.
Reisen and coworkers found that although regional mosquito abundance was positively correlated with antecedent (January–February) temperatures and with precipitation levels, and inversely correlated with summer temperatures, climate measures alone explained only a fraction of the variability in mosquito populations. They discovered that climate variation produced very different responses (in both mosquito population size and viral amplification) in different environments. “One model doesn’t fit all,” Reisen concluded. “These relationships are very complex and have to be developed for specific biomes.” Thus, the California Mosquito-borne Encephalitis Virus Surveillance and Response Plan currently incorporates measures of climate variation; however, the researchers are refining their models with the goal of using climate forecasts to provide earlier warning of transmission risk.
Challenges
As they explored the various routes by which climate variability and extreme weather events influence infectious disease emergence, workshop participants identified a range of challenges inherent to research on this topic. Many of these considerations were also discussed in Under the Weather (NRC, 2001), as noted in the Executive Summary of that report:
There are many substantial research challenges associated with studying linkages among climate, ecosystems, and infectious diseases. For instance, climate-related impacts must be understood in the context of numerous other forces that drive infectious disease dynamics, such as rapid evolution of drug- and pesticide-resistant pathogens, swift global dissemination of microbes and vectors through expanding transportation networks, and deterioration of public health programs in some regions. Also, the ecology and transmission dynamics of different infectious diseases vary widely from one context to the next, thus making it difficult to draw general conclusions or compare results from individual studies. Finally, the highly interdisciplinary nature of this issue necessitates sustained collaboration among disciplines that normally share few underlying scientific principles and research methods, and among scientists that may have little understanding of the capabilities and limitations of each other’s fields.
Consistent with these prior findings, workshop participants noted the following challenges intrinsic to the tasks of detecting, predicting, and mitigating infectious disease threats associated with climate change:
- Complexity of disease transmission patterns
- Global inequalities
- Varying space and time scales
- Establishing causation
- Lack of scientific certainty versus need for action
Complexity of Disease Transmission Patterns
Studies of influenza and dengue fever, as well as theoretical models, reveal that oscillations in disease incidence may occur even in the absence of seasonal changes in person-to-person transmissibility (see Burke in Chapter 1). Depending on parameters such as human birth rate, disease duration, and length of immunity, different epidemic viruses can display different intrinsic epidemic oscillatory frequencies. Burke observed that if such intrinsic epidemic frequency oscillations coincide with (resonate with) the annual seasonal changes in environmental conditions, then even very small annual environmentally-driven changes in transmissibility may, under some circumstances, drive very large seasonal changes in disease incidence (Dushoff et al., 2004). The impact of a given increment of change in climate upon the future transmission of a given disease cannot be determined without understanding the particular relationship between two oscillating patterns—the intrinsic incidence oscillation and the seasonally-driven oscillation. Resonance can raise the magnitude of seasonally epidemic disease. “Every effort should be made to isolate and thereby understand these component subsystems if we are to explain and predict epidemic patterns,” Burke concluded.
Global Inequalities
The effects of climate change are likely to be far greater in the tropics, where the majority of the world’s poorest people live, than in the wealthier temperate zones. As Haines observed, in the areas where links between climate and disease may best be studied, people are least able to investigate them. Similarly, a predictive model that highlights regions at higher risk for infectious disease emergence (see Figure SA-15) suggests that such “hot spots” are concentrated in equatorial developing countries, where opportunities for monitoring and research are severely limited (Jones et al., 2008). The model’s developers conclude that “[t]he global effort for [emerging infectious disease] surveillance and investigation is poorly allocated, with the majority of our scientific resources focused on places [such as North America, Europe, and Australia] from where the next important emerging pathogen is least likely to originate” (Jones et al., 2008). They argue, instead, that the resources for emerging infectious disease surveillance should target hot spots in tropical Africa, Latin America, and Asia, and populations at greatest risk for infection, in order to detect outbreaks of emergent diseases at the earliest possible stage.
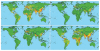
FIGURE SA-15
Global distribution of relative risk of an emerging infectious disease (EID) event. Maps depict predicted hot spots for EID events caused by (a) zoonotic pathogens from wildlife; (b) zoonotic pathogens from non-wildlife; (c) drug-resistant pathogens; (more...)
Varying Space and Time Scales
The influence of climate on infectious diseases is often highly dependent on local-scale parameters. It is sometimes impossible to extrapolate these relationships meaningfully to broader spatial scales; likewise, examples of seasonal or interannual climatic variability, such as ENSO, may not always provide a useful analog for the impacts of long-term trends in climate. Ecological responses on the timescale of an El Niño event, for example, may differ significantly from ecological responses and social adaptations that occur over the course of long-term climate change. Conversely, it is difficult to predict the influence of long-term climate change on regional patterns of climate variability, and even the effects of regional climate may be modified by landcover features.
Establishing Causation
In order to establish that a pattern of climatic variability or an extreme weather event caused a change in the transmission or geographic range of a particular infectious disease, several requirements must be met. For example, Haines observed, to infer a causal relationship between an El Niño cycle and a given health outcome, three elements are necessary: climate data, preferably local; a plausible biological relationship between a particular disease outcome and climate data; and a relatively long time series (e.g., decades) that can be analyzed statistically and adjusted for potentially confounding relationships.
Lack of Scientific Certainty Versus Need for Action
Because, as Haines observed, health lies at the end of a long chain of causality, several participants warned that by waiting to act on the potential adverse health impacts posed by climate change until their inevitability is scientifically confirmed, the world will lose the opportunity to prevent, and possibly to mitigate, these threats. “There is going to be a great deal of continuing debate about the precise magnitude and effects of climate change on health,” Haines conceded, “but I think in view of the potential for very major impacts, these uncertainties don’t justify inaction. We certainly need to adapt more effectively to a changing climate.” Indeed, as Haines noted, it would be unethical for scientists to observe the emergence of infectious diseases, whether or not this trend was caused by climate change, and not intervene.
Implications for Public Health Policy and Global Security
Several workshop discussions raised the urgent question of how to act on what is known—or even suspected—about the potential health consequences of climate change. At the same time, participants supported the continuation and expansion of research on the significance of climate to the health of organisms and ecosystems. The need for action, as well as for knowledge, underscored sessions devoted to scientific observations and technical approaches and was addressed directly in presentations that focused on issues of public health policy and of national and global security.
Research Framework
Haines described three basic tasks for researchers studying the potential health impacts of climate change: (1) to examine past associations between climate variability and health; (2) to determine climate’s role in present-day trends in disease transmission and geographic range; and (3) to create predictive models of future disease that account for a changing climate, as well as for other influential factors.
A fourth task was proposed by speaker Douglas MacPherson of McMaster University and Migration Health Consultants, Incorporated: to recognize and address the contribution of human behavior to global climate change and its further effects on infectious disease emergence. In his coauthored contribution to Chapter 4, MacPherson describes the complex, two-way association between climate change and human mobility—“a determinant of health that is directly linked to globalization of microbial disease threats and risks.”
Connecting Climate and Migration
The immense contribution of human mobility and migration to infectious disease emergence is often illustrated in terms of annual global statistics such as these presented by MacPherson:
- 802 million international arrivals (2006)
- 200 million permanent residents outside of their country of birth
- 50,000 smuggled and trafficked persons
- 15,000 unaccompanied or separated children
Human mobility also includes the movement of animals, plants, and microbes, as well as trade in goods that include living organisms and their byproducts—topics that will be taken up in a future workshop of the Forum on Microbial Threats in calendar year 2008.
Interactions between human mobility, climate change, and infectious disease dynamics take several different forms, MacPherson explained. Extreme weather events create opportunities for infectious disease outbreaks; these conditions may force people and animals (including disease vectors) to migrate to different ecosystems, where they may both encounter and introduce novel pathogens. Slowly evolving impacts of climate change (such as sea level elevation, reduced freshwater availability, or increased average temperatures) may force or attract human population movements. Patterns of temporary migration (e.g., travel), and thereby disease transmission, are also likely to shift with climate change.
Calling the policy implications of these complex associations “daunting,” MacPherson observed that in many cases, preventing the global spread of an infectious disease is not feasible. In his view, health officials should focus their energies on anticipating and mitigating problems that will doubtless arrive, rather than waiting to react.
Addressing Climate’s Contribution to the Global Disease Burden
Certain high-profile outbreaks in developed areas of the world—such as shellfish poisoning (Vibrio parahaemolyticus) in Alaska, WNV in the United States, and bluetongue in northern Europe—have raised attention regarding the potential effects of climate change on infectious disease emergence and spread. However, as speaker Diarmid Campbell-Lendrum of WHO noted, climate change is expected to exact its most profound toll on the health of the world’s poor, through increased rates of malaria, diarrheal diseases, and malnutrition. “We shouldn’t lose sight of the fact that these [common and preventable] killers are also highly sensitive to climatic conditions,” he cautioned.
Separating the effects of climate variability and change from the context of other determinants, or assessing the influence of climate versus other factors as a mutually exclusive “either/or” debate, is unproductive. Instead, considering climate as one important determinant of health risks, mediated by other contextual determinants, is more likely to lead to sound health policy, according to Campbell-Lendrum. This is the approach that WHO has taken since 1990 with its Programme on Climate Change, as evidenced in various projects described in Chapter 4. Although initially involved in the issue of climate change from the perspective of risk assessment, WHO has recently assumed an operational role, organizing activities that include advocating for climate change as a health security issue, generating evidence for action, and monitoring and evaluating the health effects of climate change.
The most effective available protective measures against the adverse health effects of climate change are basic public health interventions, Campbell-Lend-rum said. “If we did a better job of controlling dengue now, or malaria now,” he said, “we would have less to worry about from climate change.”
Considerations for National and Global Security
At its summit in March 2008, a paper presented to the European Union included a grim catalog of threats to international security posed by climate change: conflicts over water, energy, and other increasingly scarce resources; loss of infrastructure and territory; border disputes; environmentally-induced migration; and political tension at all levels of governance (European Commission, 2008). Acknowledging that climate change may pose new challenges for national security, the National Intelligence Council (NIC) of the U.S. government is in the process of preparing a National Intelligence Assessment (NIA) forecasting these potential impacts over the next two decades.
In his workshop presentation, Major General Richard Engel (U.S. Air Force, retired), NIC deputy national intelligence officer for science and technology, described work in progress on this NIA, which is intended to inform decision making at the highest levels of the U.S. government (see Chapter 4). The NIC has chosen to evaluate the potential impacts of climate change on four essential components of national power: geopolitical power, military power, economic power, and social cohesion. “When we talk about climate change impacting the United States, we talk about it impacting one of those four classical elements of national power,” Engel explained. Inherent uncertainties in predicting the course of climate change prompted the NIC to consider a “system vulnerability approach” for this assessment, which identifies existing internal vulnerability of states or regions of interest to U.S. security, then examines how the added stress of climate change could affect these states or regions (see Chapter 4). To date, the NIC has received considerable nongovernmental expert opinion on this issue, from which Engel crafted his remarks; however, the NIC and the intelligence community have yet to complete their own analysis and interpretation of these contributions.
In addition to this focused analysis of potential challenges, the NIC is contemplating the international political response to climate change. Engel noted that climate change has the potential to create geopolitical divisions, several of which have already been reported in the open press. “Developed countries want the developing countries to participate so they don’t bear the full burden [of the cost of addressing climate change], and the developing countries want the developed countries to pay for it,” he observed. Experts have reported to the NIC that a north-south division even exists within Europe, resulting from the varied effects of climate change along this axis. The differential effects of climate change in regions of Asia—where some areas may suffer droughts while others flood—may also prove a source of tension, particularly where water is concerned. The NIC’s expert consultants agree that actions taken by the United States will profoundly influence the fate of a global consensus on climate change.
Needs and Opportunities
Two days of workshop discussion bore out Burke’s claim, made during his keynote address, of the continuing relevance of the recommendations offered in Under the Weather (NRC, 2001), as summarized in Box SA-3.
Participants placed particular emphasis on the following considerations for studying the influence of climate change on infectious disease emergence:
- Developing a greater understanding of the interaction of climate with other major factors in disease emergence and resurgence, such as the globalization of travel and trade, population growth, urbanization, land-use patterns, and habitat destruction;
- Establishing long-term monitoring programs to simultaneously track climate and infectious disease dynamics, and optimizing instruments (many of which were designed for other purposes) for use in such programs;
- Devising metrics to relate changes in the physical environment to ecological and epidemiological trends and to evaluate potential adaptation and mitigation measures; and
- Continuing the development and refinement of predictive models of climate and infectious disease as the basis for early warning and public health response systems, and involving of stakeholders in the operation of such systems.
Many discussants urged that immediate action be taken to address the health effects of climate change—and indeed climate change itself—before irreversible harm is done to the Earth and its inhabitants. Several participants advocated the implementation of “win-win” adaptation strategies: improving access to clean water and sanitation; increasing the availability and uptake of immunization; and strengthening health systems. Many of the workshop’s participants believed that these actions could produce near-term health benefits and might also improve the world’s ability to withstand the potential stress of climate change.
While the scope of the workshop necessarily limited discussion of the larger issue of climate change itself and the potential to address it, this topic was the proverbial “elephant in the room.” Despite the apparent gravity of the threats posed by climate change, some were able to view it as an opportunity, and one participant has characterized the demand for clean energy as “an engine of growth for the twenty-first century” (Epstein, 2005, 2007). Furthermore, Epstein said, by taking the “right” approach to addressing the consequences of climate change, “we can get a strong public health sector, and that will be good for security, good for the economy, and we certainly hope that it will stabilize the climate.”
APPENDIX SA-1. A BRIEF HISTORY OF CLIMATE CHANGE
Long-Term Trends
As illustrated in Figure SA-16, human history spans several periods of climatic upheaval (WHO et al., 2003). However, the warmth of the last half-century is unusual; indeed, evidence suggests that the last time the polar regions remained significantly warmer than they are today—approximately 125,000 years ago—reductions in polar ice volume caused global sea levels to rise by 4 to 6 meters.

FIGURE SA-16
Variation in Earth’s average surface temperature over the past 20,000 years. SOURCE: Reprinted from WHO et al. (2003) with permission from the World Health Organization. Copyright 2003.
Recent Changes
Over the last century, global average temperatures and sea levels have risen significantly, while snow cover in the Northern Hemisphere has declined (see Figure SA-17; National Geographic Society, 2007). The total temperature increase from 1850–1899 to 2001–2005, estimated at 0.76°C (0.57°C to 0.95°C), occurred during a warming trend that appears to be gaining momentum. The rate of warming for the last 50 years was double that during the previous half-century, and 11 of the last 12 years (1995–2006) rank among the 12 warmest years in the instrumental record of global surface temperature (since 1850). Over the last 50 years, cold days, cold nights, and frost have become less frequent, while hot days, hot nights, and heat waves have become more frequent.
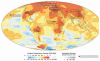
FIGURE SA-17
The Arctic is experiencing the fastest rate of warming as its reflective covering of ice and snow shrinks. In the midlatitudes, there are now fewer cold nights; heat waves are more common. The Indian Ocean and the western Pacific Ocean are warmer than (more...)
Measurements conducted since 1961 show that the average temperature of the global ocean has increased to depths of at least 3,000 meters and that oceans have absorbed more than 80 percent of the heat added to the climate system. Such warming causes seawater to expand, contributing to sea level rise, as have widespread decreases in glaciers and ice caps. Global average sea level rose at an average rate of 1.8 mm per year between 1961 and 2003 and at a rate of about 3.1 mm per year between 1993 and 2003. Current estimates indicate that sea levels rose 0.17 m over the course of the twentieth century (see Figure SA-18; IPCC, 2007).

FIGURE SA-18
Observed changes in (A) global average surface temperature; (B) global average sea level rise from tide gauge (blue) and satellite (red) data; and (C) Northern Hemisphere snow cover for March–April. All changes are relative to corresponding averages (more...)
Present Effects and Future Projections
A warmer global climate system accelerates the hydrological cycle, increasing the likelihood of extreme weather phenomena such as droughts, heavy precipitation, heat waves, hurricanes, typhoons, and cyclones (see Figure SA-19; National Geographic Society, 2007). More intense and longer droughts, which have been observed over wider areas since the 1970s and particularly in the tropics and subtropics, have been associated with higher global temperatures, but also with changes in wind patterns and decreases in snowpack and snow cover. Periods of heavy precipitation have occurred with greater frequency over most land areas in parallel with increases in atmospheric water vapor.

FIGURE SA-19
Drought is seizing more territory in the wake of mounting temperatures. Drying trends in the last 30 years are evident in the rain forests of Africa and South America and in already dry regions such as southern Europe and western North America. In wet (more...)
Over the next two decades, the Earth is expected to warm by an additional 0.2°C. Even if the concentrations of all greenhouse gases and aerosols (both of which cause the atmosphere to trap heat) could be kept the same levels as in 2000, warming would still be expected to proceed at about half the present rate. Continued greenhouse gas emissions at or above current rates are very likely to induce changes in the global climate system during the twenty-first century of even greater magnitude than has been observed during the late twentieth century.
REFERENCES
- Anderson PK, Cunningham AA, Patel NG, Morales FJ, Epstein PR, Daszak P. Emerging infectious diseases of plants: pathogen pollution, climate change and agrotechnology drivers. Trends in Ecology and Evolution. 2004;19(10):535–544. [PubMed: 16701319]
- Anyamba A, Chretien JP, Small J, Tucker CJ, Linthicum KJ. Developing global climate anomalies suggest potential disease risks for 2006–2007. International Journal of Health Geographics. 2006;5:60. [PMC free article: PMC1779293] [PubMed: 17194307]
- Barnett TP, Pierce DW, AchutaRao KM, Gleckler PJ, Santer BD, Gregory JM, Washington WM. Penetration of human-induced warming into the world’s oceans. Science. 2005;309(5732):284–287. [PubMed: 15933161]
- BBC News. The rise of bluetongue disease. BBC News. 2007. [accessed June 20, 2008]. http://news.bbc.co.uk/2/hi/uk_ news/7009288.stm.
- Borgerson SG. Arctic meltdown: the economic and security implications of global warming. Foreign Affairs. 2008;87(2):63–77.
- Calisher CH, Root JJ, Mills JN, Rowe JE, Reeder SA, Jentes ES, Wagoner K, Beaty BJ. Epizootiology of Sin Nombre and El Moro Canyon hantaviruses, southeastern Colorado, 1995–2000. Journal of Wildlife Diseases. 2005;41(1):1–11. [PubMed: 15827206]
- CDC (Centers for Disease Control and Prevention). Giardiasis fact sheet. 2004. [accessed March 17, 2008]. http://www.cdc. gov/ncidod/dpd/parasites/giardiasis/factsht_giardia.htm#child_diagnosed.
- CDC (Centers for Disease Control and Prevention). Cholera: frequently asked questions. 2005. [accessed March 17, 2008]. http://www
.cdc.gov/ncidod /dbmd/diseaseinfo/cholera_g.htm. - CDC (Centers for Disease Control and Prevention). Cryptosporidium infection fact sheet. 2007a. [accessed March 17, 2008]. http://www
.cdc.gov/ncidod /dpd/parasites/cryptosporidiosis /factsht _cryptosporidiosis.htm. - CDC (Centers for Disease Control and Prevention). Rift Valley fever outbreak—Kenya, November 2006-January 2007. Morbidity and Mortality Weekly Report. 2007b;56(4):73–76. [PubMed: 17268404]
- Center for Health and the Global Environment. Climate change futures: health, ecological and economic dimensions. Cambridge, MA: Harvard Medical School; 2005.
- CGIAR (Consultative Group for International Agricultural Research). 2008. [accessed March 16, 2008]. http://www
.cgiar.org/ - Chretien JP, Anyamba A, Bedno SA, Breiman RF, Sang R, Sergon K, Powers AM, Onyango CO, Small J, Tucker CJ, Linthicum KJ. Drought-associated chikungunya emergence along coastal East Africa. American Journal of Tropical Medicine and Hygiene. 2007;76(3):405–407. [PubMed: 17360859]
- Colwell RR. Infectious disease and environment: cholera as a paradigm for waterborne disease. International Microbiology. 2004;7(4):285–289. [PubMed: 15666250]
- Curtis V, Cairncross S. Effect of washing hands with soap on diarrhoea risk in the community: a systematic review. Lancet Infectious Diseases. 2003;3(5):275–281. [PubMed: 12726975]
- Daszak P, Cunningham AA, Hyatt AD. Emerging infectious diseases of wildlife—threats to biodiversity and human health. Science. 2000;287(5452):443–449. [PubMed: 10642539]
- Dushoff J, Plotkin JB, Levin SA, Earn DJ. Dynamical resonance can account for seasonality of influenza epidemics. Proceedings of the National Academy of Sciences. 2004;101(48):16915–16916. [PMC free article: PMC534740] [PubMed: 15557003]
- Enserink M. Animal disease: exotic disease of farm animals tests Europe’s responses. Science. 2008;319(5864):710–711. [PubMed: 18258867]
- Epstein PR. Climate change and human health. New England Journal of Medicine. 2005;353(14):1433–1436. [PubMed: 16207843]
- Epstein PR. Climate change: healthy solutions (Guest Editorial). Environmental Health Perspectives. 2007;115(4):A180. [PMC free article: PMC1852650] [PubMed: 17450189]
- Epstein PR, McCarthy JJ. Assessing climate stability. Bulletin of the American Meteorological Society. 2004;85(12):1863–1870.
- Epstein PR, Diaz HF, Elias S, Grabherr G, Graham NE, Martens WJM, Mosley-Thompson E, Susskind EJ. Biological and physical signs of climate change: focus on mosquito-borne disease. Bulletin of the American Meteorological Society. 1998;78:409–417.
- European Commission. Climate change and international security; Paper presented at European Union Summit; Brussels. 2008.
- Flynn NM, Hoeprich PD, Kawachi MM, Lee KK, Lawrence RM, Goldstein E, Jordan GW, Kundargi RS, Wong GA. New England Journal of Medicine. 1979;301(7):358–361. [PubMed: 460324]
- Frederick KD, Gleick PH. Water and global climate change: potential impacts on U.S. water resources. Arlington, VA: Pew Center on Global Climate Change; 1999.
- GAINS (Global Avian Influenza Network for Surveillance). Global avian influenza network for surveillance. 2008. [accessed June 20, 2008]. http://www
.gains.org/ - Garrett KA, Dendy SP, Frank EE, Rouse MN, Travers SE. Climate change effects on plant disease: genomes to ecosystems. Annual Review of Phytopathology. 2006;44:489–509. [PubMed: 16722808]
- Garrison VH, Shinn EA, Foreman WT, Griffin DW, Holmes CW, Kellogg CA, Majewski MS, Richardson LL, Ritchie KB, Smith GW. African and Asian dust: from desert soils to coral reefs. BioScience. 2003;53(5):469–480.
- Gil AI, Louis VR, Rivera IN, Lipp E, Huq A, Lanata CF, Taylor DN, Russek-Cohen E, Choopun N, Sack RB, Colwell RR. Occurrence and distribution of Vibrio cholerae in the coastal environment of Peru. Environmental Microbiology. 2004;6(7):699–706. [PubMed: 15186348]
- Gillett NP, Thompson DWJ. Simulation of recent Southern Hemisphere climate change. Science. 2003;302(5643):273–275. [PubMed: 14551433]
- Githeko AK, Lindsay SW, Confalonieri UE, Patz JA. Climate change and vector-borne diseases: a regional analysis. Bulletin of the World Health Organization. 2000;78(9):1136–1147. [PMC free article: PMC2560843] [PubMed: 11019462]
- Gleick PH. Water conflict chronology. Oakland, CA: The Pacific Institute; 2004.
- Groisman PY, Knight RW, Karl TR, Easterling DR, Sun B, Lawrimore JH. Contemporary changes of the hydrological cycle over the contiguous United States: trends derived from in situ observations. Journal of Hydrometeorology. 2004;5:64–85.
- Haines A, Patz JA. Health effects of climate change. Journal of the American Medical Association. 2004;291(1):99–103. [PubMed: 14709582]
- Haines A, Kovats RS, Campbell-Lendrum D, Corvalan C. Climate change and human health: impacts, vulnerability, and public health. Lancet. 2006;367(9528):2101–2109. [PubMed: 16798393]
- Harrus S, Baneth G. Drivers for the emergence and reemergence of vector-borne protozoal and bacterial diseases. International Journal of Parasitology. 2005;35(11–12):1309–1318. [PubMed: 16126213]
- Harvell CD, Kim K, Burkholder JM, Colwell RR, Epstein PR, Grimes DJ, Hofmann EE, Lipp EK, Osterhaus AD, Overstreet RM, Porter JW, Smith GW, Vasta GR. Emerging marine diseases—climate links and anthropogenic factors. Science. 1999;285(5433):1505–1510. [PubMed: 10498537]
- Harvell CD, Mitchell CE, Ward JR, Altizer S, Dobson AP, Ostfeld RS, Samuel MD. Climate warming and disease risks for terrestrial and marine biota. Science. 2002;296(5576):2158–2162. [PubMed: 12077394]
- Hennessy T, Ritter T, Holman RC, Bruden DL, Yorita KL, Bulkow L, Cheek JE, Singleton RJ, Smith J. Relationship between in-home water service and the risk of respiratory tract, skin, and gastrointestinal tract infections among Alaska Natives. American Journal of Public Health. 2008;98(5):1–8. [PMC free article: PMC2636427] [PubMed: 18382002]
- Holt DW, Hanns C, O’Hara T, Burek K, Frantz R. New distribution records of Echinococcus multilocularis in the brown lemming from Barrow, Alaska, USA. Journal of Wildlife Diseases. 2005;41(1):257–259. [PubMed: 15827234]
- Hoyos CD, Agudelo PA, Webster PJ, Curry JA. Deconvolution of the factors contributing to the increase in global hurricane intensity. Science. 2006;312(5770):94–97. [PubMed: 16543416]
- IOM (Institute of Medicine). Emerging infections: microbial threats to health in the United States. Washington, DC: National Academy Press; 1992. [PubMed: 25121245]
- IOM (Institute of Medicine). Microbial threats to health: emergence, detection, and response. Washington, DC: The National Academies Press; 2003. [PubMed: 25057653]
- IOM (Institute of Medicine). Vector-borne diseases: understanding the environmental, human health and ecological connections. Washington, DC: The National Academies Press; 2008. [PubMed: 21452451]
- IPCC (Intergovernmental Panel on Climate Change). Working Group I contribution to the third assessment report of the IPCC. New York: Cambridge University Press; 2001. Climate Change 2001: the scientific basis.
- IPCC (Intergovernmental Panel on Climate Change). Working Group II contribution to the fourth assessment report of the IPCC. Geneva, Switzerland: IPCC; 2007a. Climate change 2007: climate change impacts, adaptation and vulnerability.
- IPCC (Intergovernmental Panel on Climate Change). Working Group I contribution to the fourth assessment report of the IPCC. Geneva, Switzerland: IPCC; 2007b. Climate change 2007: the physical science basis.
- Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451(7181):990–993. [PMC free article: PMC5960580] [PubMed: 18288193]
- Karl TR, Trenberth KE. Modern global climate change. Science. 2003;302(5651):1719–1723. [PubMed: 14657489]
- Kovats RS, Haines A. Global climate change and health: recent findings and future steps. Canadian Medical Association Journal. 2005;172(4):501–502. [PMC free article: PMC548412] [PubMed: 15710942]
- Kovats RS, Bouma MJ, Hajat S, Worrall E, Haines A. El Niño and health. Lancet. 2003;362(9394):1481–1489. [PubMed: 14602445]
- Kovats RS, Edwards SJ, Hajat S, Armstrong BG, Ebi KL, Menne B. The effect of temperature on food poisoning: a time-series analysis of salmonellosis in ten European countries. Epidemiology and Infection. 2004;132(3):443–453. [PMC free article: PMC2870124] [PubMed: 15188714]
- Kovats RS, Edwards SJ, Charron D, Cowden J, D’Souza RM, Ebi KL, Gauci C, Gerner-Smidt P, Hajat S, Hales S, Hernandez Pezzi G, Kriz B, Kutsar K, McKeown P, Mellou K, Menne B, O’Brien S, van Pelt W, Schmid H. Climate variability and campylobacter infection: an international study. International Journal of Biometeorology. 2005;49(4):207–214. [PubMed: 15565278]
- Leclair D, Austin JW, Faber J, Cadieux B, Blanchfield B. Toxicity of aged seal meat challenged with Clostridium botulinum type E. Federal Food Safety and Nutrition Research Meeting; Ottawa, Ontario. October 4–5. 2004.
- Levin RB, Epstein PR, Ford TE, Harrington W, Olson E, Reichard EG. Drinking water challenges in the twenty-first century. Environmental Health Perspectives. 2002;110:43–52. [PMC free article: PMC1241146] [PubMed: 11834462]
- Levitus S, Antonov JI, Boyer T. Warming of the world ocean, 1955–2003. Geophysical Research Letters. 2005;32:LO2604.
- Linthicum KJ, Anyamba A, Tucker CJ, Kelley PW, Myers MF, Peters CJ. Climate and satellite indicators to forecast Rift Valley fever epidemics in Kenya. Science. 1999;285(5426):397–400. [PubMed: 10411500]
- Lipp EK, Rivera IN, Gil AI, Espeland EM, Choopun N, Louis VR, Russek-Cohen E, Huq A, Colwell RR. Direct detection of Vibrio cholerae and ctxA in Peruvian coastal water and plankton by PCR. Applied Environmental Microbiology. 2003;69(6):3676–3680. [PMC free article: PMC161524] [PubMed: 12788781]
- Lloyd SJ, Kovats RS, Armstrong BG. Global diarrhea morbidity, weather and climate. Climate Research. 2007;34:119–127.
- Louis VR, Gillespie IA, O’Brien SJ, Russek-Cohen E, Pearson AD, Colwell RR. Temperature-driven campylobacter seasonality in England and Wales. Applied Environmental Microbiology. 2005;71(1):85–92. [PMC free article: PMC544220] [PubMed: 15640174]
- McKie R, Revill J. The first case of bluetongue disease found at U.K. farm. Guardian. Sep 23, 2007. [accessed June 20, 2008]. http://www
.guardian.co .uk/uk/2007/sep/23/ruralaffairs .robinmckie. - McLaughlin JB, DePaola A, Bopp CA, Martinek KA, Napolilli NP, Allison CG, Murray SL, Thompson EC, Bird MM, Middaugh JP. Outbreak of Vibrio parahaemolyticus gastroenteritis associated with Alaskan oysters. New England Journal of Medicine. 2005;353(14):1463–1470. [PubMed: 16207848]
- McMichael AJ, Woodruff RE, Hales S. Climate change and human health: present and future risks. Lancet. 2006;367(9513):859–869. [PubMed: 16530580]
- Milly PCD, Dunne KA, Vecchia AV. Global pattern of trends in streamflow and water availability in a changing climate. Nature. 2005;438(7066):347–350. [PubMed: 16292308]
- Molesworth AM, Cuevas LE, Connor SJ, Morse AP, Thomson MC. Environmental risk and meningitis epidemics in Africa. Emerging Infectious Diseases. 2003;9(10):1287–1293. [PMC free article: PMC3033099] [PubMed: 14609465]
- NASA (National Aeronautics and Space Administration) Goddard Space Flight Center. Using satellites to track Rift Valley fever. 2000. [accessed April 16, 2008]. http://eospso
.gsfc.nasa .gov/ftp_docs/lithographs/RVF.pdf. - National Geographic Society. The warming Earth and precipitation fallout figures. National Geographic. 2007 October;(supplement)
- NRC (National Research Council). Under the weather: climate, ecosystems, and infectious disease. Washington, DC: National Academy Press; 2001. [PubMed: 25057537]
- Ogden NH, Maarouf A, Barker IK, Bigras-Poulin M, Lindsay LR, Morshed MG, O’Callaghan CJ, Ramay F, Waltner-Toews D, Charron DF. Climate change and the potential for range expansion by the Lyme disease vector Ixodes scapularis in Canada. International Journal for Parasitology. 2005;36(1):63–70. [PubMed: 16229849]
- Osburn BI.Institute of Medicine. Vector-borne diseases: understanding the environmental, human health, and ecological connections. Washington, DC: The National Academies Press; 2008. Vector-borne zoonotic diseases and their ecological and economic implications: bluetongue disease in Europe. [PubMed: 21452451]
- Parkinson AJ, Butler JC. Potential impacts of climate change on infectious diseases in the arctic. International Journal of Circumpolar Health. 2005;64(5):478–486. [PubMed: 16440610]
- Pascual M, Ahumada JA, Chaves LF, Rodo X, Bouma M. Malaria resurgence in the East African highlands: temperature trends revisited. Proceedings of the National Academy of Sciences. 2006;103(15):5829–5834. [PMC free article: PMC1416896] [PubMed: 16571662]
- Patz JA, Epstein PR, Burk TA, Balbus JM. Global climate change and emerging infectious diseases. Journal of the American Medical Association. 1996;275(3):217–223. [PubMed: 8604175]
- Patz JA, McGeehin MA, Bernard SM, Ebi KL, Epstein PR, Grambsch A, Gubler DJ, Reiter P, Romieu I, Rose JB, Samet JM, Trtanj J. The potential health impacts of climate variability and change for the United States: executive summary of the report of the health sector of the U.S. National Assessment. Environmental Health Perspectives. 2000;108(4):367–376. [PMC free article: PMC1638004] [PubMed: 10753097]
- Peters RL, Lovejoy TE. Global warming and biodiversity. New Haven, CT: Yale University Press; 1994.
- Pinzon JE, Wilson JM, Tucker CJ, Arthur R, Jahrling PB, Formenty P. Trigger events: enviroclimatic coupling of Ebola hemorrhagic fever outbreaks. American Journal of Tropical Medicine and Hygiene. 2004;71(5):664–674. [PubMed: 15569802]
- ProMed Mail. Assessment of the outbreak of bluetongue serotype 8 in Europe. July–December, 2008. 2007. [accessed February 29, 2008]. http://www
.promedmail.org. - Schneider E, Hajjeh RA, Spiegel RA, Jibson RW, Harp EL, Marshall GA, Gunn RA, McNeil MM, Pinner RW, Baron RC, Burger RC, Hutwagner LC, Crump C, Kaufman L, Reef SE, Feldman GM, Pappagianis D, Werner SB. A coccidioidomycosis outbreak following the Northridge, Calif, earthquake. Journal of the American Medical Association. 1997;277(11):904–908. [PubMed: 9062329]
- Schneider W, Hollier CA, Whitam HK, Palm ME, Mckemy JM, Hernandez J, Levy L, Devries-Paterson R. First report of soybean rust caused by Phakopsora pachyrhizi in the continental United States. Plant Disease. 2005;89:774. [PubMed: 30791253]
- Schweiger A, Ammann RW, Candinas D, Clavien PA, Eckert J, Gottstein B, Halkic N, Muellhaupt B, Prinz BM, Reichen J, Tarr PE, Torgerson PR, Deplazes P. Human alveolar echinococcosis after fox population increase, Switzerland. Emerging Infectious Diseases. 2007;13(6):878–882. [PMC free article: PMC2792858] [PubMed: 17553227]
- Skarphedinsson S, Jensen PM, Kristiansen K. Survey of tickborne infections in Denmark. Emerging Infectious Diseases. 2005;11(7):1055–1061. [PMC free article: PMC3371797] [PubMed: 16022780]
- Snieszko SF. The effects of environmental stress on outbreaks of infectious diseases of fishes. Journal of Fish Biology. 1974;6(2):197–208.
- Speelmon EC, Checkley W, Gilman RH, Patz J, Calderon M, Manga S. Cholera incidence and El Niño-related higher ambient temperature. Journal of the American Medical Association. 2000;283(23):3072–3074. [PubMed: 10865299]
- Stenseth NC, Samia NI, Viljugrein H, Kausrud KL, Begon M, Davis S, Leirs H, Dubyanskiy VM, Esper J, Ageyev VS, Klassovskiy NL, Pole SB, Chan KS. Plague dynamics are driven by climate variation. Proceedings of the National Academy of Sciences. 2006;103(35):13110–13115. [PMC free article: PMC1559761] [PubMed: 16924109]
- Sultan B, Labadi K, Guegan JF, Janicot S. Climate drives the meningitis epidemics onset in West Africa. PLoS Medicine. 2005;2(1):e6. [PMC free article: PMC545199] [PubMed: 15696216]
- Sutherst RW. Global change and human vulnerability to vector-borne diseases. Clinical Microbiology Reviews. 2004;17(1):136–173. [PMC free article: PMC321469] [PubMed: 14726459]
- Tam CC, Rodrigues LC, O’Brien SJ, Hajat S. Temperature dependence of reported Campylobacter infection in England, 1989–1999. Epidemiology and Infection. 2006;134(1):119–125. [PMC free article: PMC2870373] [PubMed: 16409658]
- Tanser FC, Sharp B, Le Sueur D. Potential effect of climate change on malaria transmission in Africa. Lancet. 2003;362:1792–1798. [PubMed: 14654317]
- UNDP (United Nations Development Programme). Human development report 2007/2008. Fighting climate change: human solidarity in a divided world. New York: United Nations Development Programme; 2007.
- UNHCR (Office of the United Nations High Commissioner for Refugees). UNHCR and international protection: a protection induction programme. Geneva, Switzerland: Office of the United Nations High Commissioner for Refugees; 2006.
- U.S. Climate Change Science Program and the Subcommittee on Global Change Research. Weather and climate extremes in a changing climate. In: Karl TR, Meehl GA, Miller CD, Hassol SJ, Waple AM, Murray WL, editors. Synthesis and Assessment Product 3.3. Washington, DC: U.S. Climate Change Science Program; 2008.
- van Lieshout M, Kovats RS, Livermore M, Martens P. Climate change and malaria: analysis of the SRES climate and socio-economic scenarios. Global Environmental Change. 2004;14:87–99.
- WHO (World Health Organization). Rift Valley fever. 2007. [accessed June 19, 2008]. http://www
.who.int/mediacentre /factsheets/fs207/en/ - WHO, World Meterological Organization, and United Nations Environment Programme. Climate change and human health—risks and responses. Geneva, Switzerland: World Health Organization; 2003.
- Woods A, Coates KD, Hamann A. Is an unprecedented dothistroma needle blight epidemic related to climate change? Bioscience. 2005;55(9):761–769.
- Woods Hole Oceanographic Institution. What are harmful algal blooms (HABs)? 2008. [accessed March 17, 2008]. http://www. whoi.edu/redtide/whathabs/whathabs.html.
- IPCC (Intergovernmental Panel on Climate Change). Working Group I contribution to the fourth assessment report of the IPCC. Geneva, Switzerland: IPCC; 2007. Climate change 2007: the physical science basis. Summary for policymakers; p. 17.
- National Geographic Society. Oct, 2007. [accessed June 11, 2008]. http://ngm.nationalgeographic.com/climate connections/climate-map.
- WHO (World Health Organization), World Meterological Organization, and United Nations Environment Programme. Climate change and human health—risks and responses. Geneva, Switzerland: World Health Organization; 2003.
Summary and Assessment References
Appendix SA-1 References
Footnotes
- 1
In a personal communication on June 11, 2008, Diarmid Campbell-Lendrum (WHO) stated: “Some benefits undoubtedly exist, for some populations. But I don’t know of any papers in the health literature, WHO, or otherwise that specifically focus on reviewing the benefits separate from the damages. These are usually referred to in reviews that look at the health effects overall. The health chapter of the IPCC refers to both harms and benefits, and I think this would be the best citation, and source for other studies.a In IPCC (2007a), Confalonieri et al. note that the most important benefits are likely to be reduced deaths in winter at high latitudes, increased food production in high latitudes (for moderate climate change), and disruption of transmission cycles of some infectious disease in some places (e.g., where it may become too hot or dry for malaria transmission in some locations).”
- 2
Emerging infectious diseases are caused by pathogens that (1) have increased in incidence, geographical, or host range; (2) have altered capabilities for pathogenesis; (3) have newly evolved; or (4) have been discovered or newly recognized (Anderson et al., 2004; Daszak et al., 2000; IOM, 1992).
- 3
Climate change in IPCC usage, and in this document as well, refers to any change in climate over time, whether due to natural variability or as a result of human activity.
- 4
North and South Poles.
- 5
In some cases, however, flooding may be associated with the destruction of vector breeding sites.
- 6
Findings indicative of climate instability include (1) increasing rates of change, (2) wider fluctuations from norms, and (3) the appearance of major outliers (several standard deviations from the norm; Epstein and McCarthy, 2004).
- 7
Plant and animal species first adapt to temperature changes by shifting their elevational ranges. A 1 km change in altitude is estimated to correspond to a geographic shift of 600 km north or south (Peters and Lovejoy, 1994). Highlands are considered sentinel regions for monitoring the biological response to global climate change.
- 8
While some vectors may already be present at higher altitudes, higher temperatures may shorten the extrinsic incubation period, allowing the vector to transmit disease.
- 9
It is believed that bluetongue was carried in a cloud of midges blown by warm winds across the English Channel from France, the Low Countries, or Germany, who, at the time, had similar outbreaks. The first case in the United Kingdom was discovered at a farm near Ipswich, Suffolk (BBC News, 2007; McKie and Revill, 2007).
- 10
Moisture is required for egg development. Flooding often occurs following periods of heavy precipitation, enabling full development of the larvae and an increase in the mosquito population, thus spreading the virus during their next bloodmeal (WHO, 2007).
- 11
The UNHCR collectively refers to people who have been forcibly uprooted from their homes as “persons of concern.” They include asylum-seekers, refugees, stateless persons, the internally displaced, and returnees (UNHCR, 2006).
The Forum’s role was limited to planning the workshop, and the workshop summary has been prepared by the workshop rapporteurs as a factual summary of what occurred at the workshop.
- Summary and Assessment - Global Climate Change and Extreme Weather EventsSummary and Assessment - Global Climate Change and Extreme Weather Events
Your browsing activity is empty.
Activity recording is turned off.
See more...