NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004-2013.
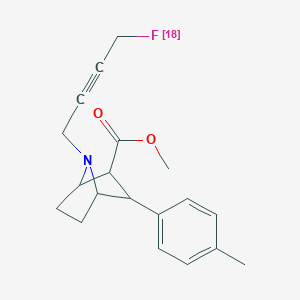
| Chemical name: | N-4-[18F]Fluorobut-2-yn-1-yl-2β-carbomethoxy-3β-phenyltropane |
|
| Abbreviated name: | [18F]PR04.MZ | |
| Synonym: | ||
| Agent category: | Compound | |
| Target: | Dopamine transporter (DAT) | |
| Target category: | Transporter | |
| Method of detection: | Positron emission tomography (PET) | |
| Source of signal: | 18F | |
| Activation: | No | |
| Studies: |
| Click on the above structure for additional information in PubChem. |
Background
[PubMed]
Dopamine, a neurotransmitter, plays an important role in the mediation of movement, cognition, and emotion. Parkinson’s disease (PD) is associated with a loss of dopamine-containing neurons in the striatum, resulting in a loss of dopamine transporter (DAT) in the presynaptic nerve terminals (1, 2). Reduction of DAT density is inversely correlated with the severity of motor dysfunction in PD patients. Several (-)-cocaine analogs were developed for the evaluation of DAT density in neurons of PD patients. Radiolabeled 2β-carboxymethoxy-3β-(4-iodophenyl)tropane (β-CIT) and N-(3-fluoropropyl)-2β-carbomethoxy-3β-(4-iodophenyl)nortropane (FP-CIT) have been used for brain imaging (3-6). Because of the short physical half-life of 11C-labeled analogs, equilibrium conditions are difficult to achieve in positron emission tomography (PET) measurements. [123I]β-CIT was studied in single-photon emission computed tomography (SPECT) and showed slow tracer uptake kinetics (7, 8). A tropane derivative, [11C]-(E)-N-(4-fluorobut-2-enyl)-2β-carbomethoxy-3β-(4'-tolyl)nortropane ([11C]LBT-999), was evaluated as a radioligand for studies of DAT with PET imaging (9-11). N-4-[18F]Fluorobut-2-yn-1-yl-2β-carbomethoxy-3β-phenyltropane ([18F]PR04.MZ) was developed through the use of a conformational restriction approach based on (-)-cocaine (12). PR04.MZ exhibited a 100-fold higher potency than (-)-cocaine in inhibition of human DAT and better selectivity over the human noradrenalin transporter (hNET) and human serotonin transporter (hSERT). [18F]PR04.MZ has been evaluated as a radioligand for studies of DAT with PET imaging.
Synthesis
[PubMed]
[18F]PR04.MZ was readily synthesized by standard 18F-fluorination of the mesylate precursor with [18F]KF/Kryptofix 2.2.2 in acetonitrile for a few min at 120°C (12). [18F]PR04.MZ was purified with high-performance liquid chromatography. Overall radiochemical yield (non-decay corrected) was 13 ± 3%, with a specific activity of 18–95 GBq/µmol (0.5–2.6 Ci/µmol) at the end of synthesis and a radiochemical purity of >98%. Total synthesis time was 50 min.
In Vitro studies: Testing in Cells and Tissues
[PubMed]
PR04.MZ binding affinity for hDAT, hSERT, and hNET was determined using stably transfected HEK293 cells (12). [3H]β-CFT, [3H]citalopram, and [3H]nisoxetine were used as radioligands, respectively. The 50% inhibition concentration values for hDAT, hSERT, and hNET were 1.9 ± 0.2 nM, 108.4 ± 1.3 nM, and 22.5 ± 0.8 nM, respectively. In vitro autoradiography studies with [18F]PR04.MZ in rat brain sections showed a high radioactivity level in the striatum, with low binding in the cortical regions and cerebellum. Binding in the brain was totally blocked in the presence of 1 µM β-CFT.
Animal Studies
Rodents
Riss et al. (12) performed ex vivo autoradiography studies in mouse brains (n = 3) after injection of 5 MBq (0.14 mCi) [18F]PR04.MZ at 35 min after injection. A high radioactivity level was observed in the striatum, with low binding in the cortical regions and cerebellum. The striatum/cerebellum ratio was 23.5. Binding in the striatum was totally blocked in the mice pretreated with 1.5 mg/kg β-CFT (n = 3 mice, 20 min before injection of [18F]PR04.MZ). Ex vivo biodistribution of [18F]PR04.MZ in normal rats (n = 3/group) was measured in the blood, brain, heart, liver, kidney, and bone at 5, 15, 30, and 60 min after injection. The initial standard uptake values (SUVs) at 5 min after injection were highest in the brain (7.0) and blood (7.3). The SUVs of both the brain and blood decreased to ~1.6 at 60 min. The liver (20.3), kidney (11.3), and heart (3.5) showed their highest SUVs at 15–30 min, with SUVs of 4.8, 3.8, and 1.2, respectively, at 60 min. The SUVs of bone were 0.2, 0.6, 1.5, and 1.5 at 5, 15, 30, and 60 min, respectively. [18F]PR04.MZ remained ~5% and ~94% intact in the plasma and brain, respectively, at 60 min.
Riss et al. (12) performed dynamic PR04.MZ PET brain scans in normal rats (n = 6). The peak striatal SUV of ~4 was reached at 20 min after injection with a striatum/cerebellum ratio of 7.3, which increased to 17.2 at 40 min. Injection of β-CFT (1.5 mg/kg) at 40 min after [18F]PR04.MZ reduced the striatum/cerebellum ratio to 5 at 20 min after β-CFT injection. β-CFT was able to displace [18F]PR04.MZ from binding to DAT.
References
- 1.
- Carbon M., Ghilardi M.F., Feigin A., Fukuda M., Silvestri G., Mentis M.J., Ghez C., Moeller J.R., Eidelberg D. Learning networks in health and Parkinson's disease: reproducibility and treatment effects. Hum Brain Mapp. 2003;19(3):197–211. [PMC free article: PMC6871830] [PubMed: 12811735]
- 2.
- Chesselet M.F., Delfs J.M. Basal ganglia and movement disorders: an update. Trends Neurosci. 1996;19(10):417–22. [PubMed: 8888518]
- 3.
- Abi-Dargham A., Gandelman M.S., DeErausquin G.A., Zea-Ponce Y., Zoghbi S.S., Baldwin R.M., Laruelle M., Charney D.S., Hoffer P.B., Neumeyer J.L., Innis R.B. SPECT imaging of dopamine transporters in human brain with iodine-123-fluoroalkyl analogs of beta-CIT. J Nucl Med. 1996;37(7):1129–33. [PubMed: 8965183]
- 4.
- Chaly T., Dhawan V., Kazumata K., Antonini A., Margouleff C., Dahl J.R., Belakhlef A., Margouleff D., Yee A., Wang S., Tamagnan G., Neumeyer J.L., Eidelberg D. Radiosynthesis of [18F] N-3-fluoropropyl-2-beta-carbomethoxy-3-beta-(4-iodophenyl) nortropane and the first human study with positron emission tomography. Nucl Med Biol. 1996;23(8):999–1004. [PubMed: 9004288]
- 5.
- Kazumata K., Dhawan V., Chaly T., Antonini A., Margouleff C., Belakhlef A., Neumeyer J., Eidelberg D. Dopamine transporter imaging with fluorine-18-FPCIT and PET. J Nucl Med. 1998;39(9):1521–30. [PubMed: 9744335]
- 6.
- Lundkvist C., Halldin C., Ginovart N., Swahn C.G., Farde L. [18F] beta-CIT-FP is superior to [11C] beta-CIT-FP for quantitation of the dopamine transporter. Nucl Med Biol. 1997;24(7):621–7. [PubMed: 9352532]
- 7.
- Ishikawa T., Dhawan V., Kazumata K., Chaly T., Mandel F., Neumeyer J., Margouleff C., Babchyck B., Zanzi I., Eidelberg D. Comparative nigrostriatal dopaminergic imaging with iodine-123-beta CIT-FP/SPECT and fluorine-18-FDOPA/PET. J Nucl Med. 1996;37(11):1760–5. [PubMed: 8917170]
- 8.
- Laruelle M., Wallace E., Seibyl J.P., Baldwin R.M., Zea-Ponce Y., Zoghbi S.S., Neumeyer J.L., Charney D.S., Hoffer P.B., Innis R.B. Graphical, kinetic, and equilibrium analyses of in vivo [123I] beta-CIT binding to dopamine transporters in healthy human subjects. J Cereb Blood Flow Metab. 1994;14(6):982–94. [PubMed: 7929662]
- 9.
- Chalon S., Hall H., Saba W., Garreau L., Dolle F., Halldin C., Emond P., Bottlaender M., Deloye J.B., Helfenbein J., Madelmont J.C., Bodard S., Mincheva Z., Besnard J.C., Guilloteau D. Pharmacological characterization of (E)-N-(4-fluorobut-2-enyl)-2beta-carbomethoxy-3beta-(4'-tolyl)nortropane (LBT-999) as a highly promising fluorinated ligand for the dopamine transporter. J Pharmacol Exp Ther. 2006;317(1):147–52. [PubMed: 16339913]
- 10.
- Dolle F., Emond P., Mavel S., Demphel S., Hinnen F., Mincheva Z., Saba W., Valette H., Chalon S., Halldin C., Helfenbein J., Legaillard J., Madelmont J.C., Deloye J.B., Bottlaender M., Guilloteau D. Synthesis, radiosynthesis and in vivo preliminary evaluation of [11C]LBT-999, a selective radioligand for the visualisation of the dopamine transporter with PET. Bioorg Med Chem. 2006;14(4):1115–25. [PubMed: 16219467]
- 11.
- Saba W., Valette H., Schollhorn-Peyronneau M.A., Coulon C., Ottaviani M., Chalon S., Dolle F., Emond P., Halldin C., Helfenbein J., Madelmont J.C., Deloye J.B., Guilloteau D., Bottlaender M. [11C]LBT-999: a suitable radioligand for investigation of extra-striatal dopamine transporter with PET. Synapse. 2007;61(1):17–23. [PubMed: 17068778]
- 12.
- Riss P.J., Debus F., Hummerich R., Schmidt U., Schloss P., Lueddens H., Roesch F. Ex vivo and in vivo evaluation of [18F]PR04.MZ in rodents: a selective dopamine transporter imaging agent. ChemMedChem. 2009;4(9):1480–7. [PubMed: 19588472]
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review N-4-Fluorobut-2-yn-1-yl-2β-carbo-[(11)C]methoxy-3β-phenyltropane.[Molecular Imaging and Contrast...]Review N-4-Fluorobut-2-yn-1-yl-2β-carbo-[(11)C]methoxy-3β-phenyltropane.Leung K. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review (1R,2S,3S,5S)-Methyl-8-{[(1S,2S)-2-([(18)F]fluoromethyl)cyclopropyl]methyl}-3-phenyl-8-azabicyclo[3.2.1]octane-2-carboxylate.[Molecular Imaging and Contrast...]Review (1R,2S,3S,5S)-Methyl-8-{[(1S,2S)-2-([(18)F]fluoromethyl)cyclopropyl]methyl}-3-phenyl-8-azabicyclo[3.2.1]octane-2-carboxylate.Leung K. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review 8-((E)-4-Fluoro-but-2-enyl)-3β-p-tolyl-8-aza-bicyclo[3.2.1]octane-2β-carboxylic acid [(11)C]methyl ester.[Molecular Imaging and Contrast...]Review 8-((E)-4-Fluoro-but-2-enyl)-3β-p-tolyl-8-aza-bicyclo[3.2.1]octane-2β-carboxylic acid [(11)C]methyl ester.Leung K. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review N-(3-[(18)F]Fluoropropyl)-2β-carbomethoxy-3β-(4-iodophenyl)nortropane.[Molecular Imaging and Contrast...]Review N-(3-[(18)F]Fluoropropyl)-2β-carbomethoxy-3β-(4-iodophenyl)nortropane.Leung K. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review 2β-Carbomethoxy-3β-(4-chlorophenyl)-8-(2-[(18)F]fluoroethyl)nortropane.[Molecular Imaging and Contrast...]Review 2β-Carbomethoxy-3β-(4-chlorophenyl)-8-(2-[(18)F]fluoroethyl)nortropane.Leung K. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- N-4-[18F]Fluorobut-2-yn-1-yl-2β-carbomethoxy-3β-phenyltropane - Molecular Imagin...N-4-[18F]Fluorobut-2-yn-1-yl-2β-carbomethoxy-3β-phenyltropane - Molecular Imaging and Contrast Agent Database (MICAD)
Your browsing activity is empty.
Activity recording is turned off.
See more...

 In vitro
In vitro