NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004-2013.
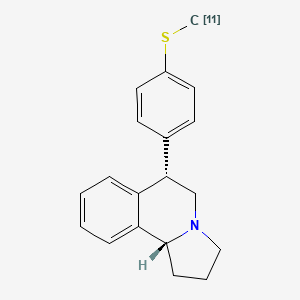
| Chemical name: | trans-(+)1,2,3,5,6,10b-Hexahydro-6-(4-([11C]methylthio)-phenyl)pyrrolo-(2,1-a)-isoquinoline |
|
| Abbreviated name: | [11C](+)McN5652; [11C]McN5652 | |
| Synonym: | ||
| Agent Category: | Compound | |
| Target: | Serotonin transporter (SERT); 5-HT | |
| Target Category: | Ligand binding | |
| Method of detection: | PET | |
| Source of signal: | 11C | |
| Activation: | No | |
| Studies: |
| Click on the above structure for additional information in PubChem. |
Background
[PubMed]
The neurotransmitter serotonin (5-HT) plays a major role in a variety of brain functions, such as appetite, sleep, and mood. Neuropsychiatric disorders, including major depression, schizophrenia, Alzheimer’s disease, and Parkinson’s disease (1-3), involve a dysfunction of the brain’s 5-HT system.
The serotonergic neurons – present in wide areas of the brain, including the raphe nuclei, hypothalamus, thalamus, and cerebral cortex – bear a protein called “serotonin transporter” (SERT) (4).The SERT, located on the cell bodies and terminals of 5-HT neurons, is a specific marker for the number and integrity of presynaptic terminals of serotonin-producing neurons. It regulates neurotransmission by removing released 5-HT from the extracellular space back into the presynaptic neuron. Commonly prescribed antidepressants are selective serotonin reuptake inhibitors, and their effects are obtained through interaction with (and inhibition of) the SERT (5). For that reason, in vivo imaging of the regional brain distribution of the SERT is an important tool for studying the 5-HT system and the treatment of neuropsychiatric disorders.
A variety of in vivo radioligands for positron emission tomography (PET) have been evaluated for imaging the SERT. trans-(+)1,2,3,5,6,10b-Hexahydro-6-(4-([11C]methylthio)-phenyl)pyrrolo-(2,1-a)-isoquinoline ([11C]McN5652) was the first successful and widely used agent (6, 7). However, it does have some limitations; for example, its kinetics in the brain are slow, and although adequate for regions with high SERT density, it often provides insufficient signal-to-noise differentials for imaging brain regions with intermediate to low SERT densities (e.g., limbic and neocortical regions) because of its high nonspecific binding. Over recent years, new PET radioligands have been synthesized and evaluated as SERT imaging agents and alternatives to [11C]McN5652 (e.g [11C]DASB, [11C]AFM, [11C]ADAM, [11C]HOMADAM).
[11C]McN5652 has two optical isomeric forms. The ability of its geometric trans (+), pharmacologically active enantiomer, [11C](+)McN5652, to block the SERT is at least two orders of magnitude higher than that of its inactive enantiomer, [11C](-)McN5652 (8, 9) (inhibition constant (Ki) ~ 0.7 nM for [11C](+)McN5652 versus 0.4 nM for [11C](-)McN5652) (also see the In Vitro Studies section). Brain uptake and retention of [11C](+)McN5652 correspond to the distribution of the SERT in rodents, non-human primates, and humans. Several methods have been used to quantify [11C](+)McN5652-specific binding, but the high level of nonspecific binding, the differences in nonspecific binding among regions, and the slow equilibration between specific and nonspecific binding populations have made such a quantification difficult (10).
Synthesis
[PubMed]
In 1995, Suehiro et al. (11) reported details of a synthetic procedure for [11C]McN5652 that addressed the instability issue of the thiol precursor used in previous established synthesis methods (12). This improved procedure involved the saponification of thioesters and enabled reaction of the precursor of McN5652 in a one-pot radiolabeling sequence. After hydrolysis of the thioester functionality (via tetrabutylammonium hydroxide for 10 min), the free thiol was reacted in situ with [11C]iodomethane (in dimethyl formamide, at 40-45 °C, for 1 min) without purification. The radiolabeled material produced was stable and could be used for multiple experiments.
The radiochemical yield obtained for [11C]McN5652 was ~26% (decay-corrected at end of bombardment), and the total synthesis time, as reported by Suehiro et al. (11), was about 18 min. The specific activity of the radiotracer produced was ~84,730 MBq/μmol (2,290 mCi/μmol).
Details of a synthetic method for [11C](+)McN5652 by S-methylation of the corresponding precursor with [11C]iodomethane using an automated process were reported by Sasaki et al. (13). In this method, the desmethyl precursor was obtained by demethylation of nonradioactive McN5652 and successive stabilization by the addition of dithiothreitol (DTT), a protecting agent for the SH group, immediately after reaction. This method led to a radiochemical purity of 98.6 ± 0.4% and specific activity for [11C](+)McN5652 of 181.3 ± 7.4 GBq/μmol (4,900 ± 200 mCi/μmol).
In Vitro Studies: Testing in Cells and Tissues
[PubMed]
In vitro inhibition studies performed by Shank et al. (9) showed that (+)McN5652 had the ability to prevent 5-HT uptake to a much greater extent than norepinephrine, whereas its potency for dopamine uptake was negligible. Its inhibition constant was found to be in the nanomolar range (Ki = 0.40 nM for synaptosomal 5-HT uptake), which was similar to those of paroxetine and sertraline, two high-affinity ligands for 5-HT. The optically inactive McN5652 enantiomer exhibited a much lower affinity (~150 lower) for the SERT than its optically active isomer (9).
Animal Studies
Rodents
[PubMed]
Mice studies performed by Suehiro et al. (7) showed that [11C](+)McN5652 had a higher uptake and longer retention in regions with high density of 5-HT uptake sites than the 11C-labeled racemic mixture and that [11C](-)McN5652 washed out rapidly. With the trans form [11C](+) enantiomer, the hypothalamus/cerebellum ratio reached 6 at 90 min. When mice received injections of the selective 5-HT uptake blocker paroxetine (5 mg/kg of tissue) before injections of [11C](+)McN5652, binding of [11C](+)McN5652 was inhibited by 45 to 73% in all regions except in the cerebellum. [11C](-)McN5652 showed no specific binding in any of the regions.
Other Non-Primate Mammals
[PubMed]
Brust et al. (14) performed comparative studies between [11C]McN5652 and its analog S-([18F]fluoromethyl)-(+)-McN5652 in 6-week-old female pigs. The animals received an infusion of the radiotracers (over 2 min into the jugular vein) at the following doses: 242 ± 94 GBq/μmol (6,540 ± 2,540 mCi/μmol) for [11C](+)McN5652 and 274 ± 110 GBq/μmol (7,405 ± 2,973 mCi/μmol) for S-([18F]fluoromethyl)McN5652. PET scans showed that the highest accumulation of both radiotracers was in the ventral midbrain, thalamus, olfactory lobe, and pons, consistent with the known densities of 5-HT transporters. In addition, the kinetics of S-([18F]fluoromethyl)-(+)-McN5652 appeared to be faster. A peak of activity in the midbrain was reached at ~12 min post injection, whereas the maximum radioactivity was reached at ~22 min post injection for [11C](+)McN5652. Strong inhibition of the specific binding was observed for both radiotracers after pretreatment with the selective 5-HT uptake inhibitor citalopram (5 mg/kg of tissue).
Non-Human Primates
[PubMed]
Szabo et al. (15) performed dynamic PET scans using [11C]McN5652 on 3 Papio anubis baboons. The animals first received injections of [11C](+)McN5652, followed by the pharmacologically inactive enantiomer [11C](-)McN5652 (specific activities at time of injection: 64,195 ± 21,645 MBq/μmol (1,735 ± 585 mCi/μmol) for the (+) enantiomer and 93,721 ± 42,661 MBq/μmol (2,533 ± 1,153 mCi/μmol) for the (-) enantiomer); two animals received [11C](+)McN5652 after pretreatment with the specific 5-HT uptake site inhibitor fluoxetine (5 mg/kg of tissue).
Results showed that the initial brain uptake into the brain was similar for both [11C](+)McN5652 and [11C](-)McN5652, but that at later times (45-120 min after injection), only [11C](+)McN5652 exhibited a distribution pattern characteristic for 5-HT uptake sites (prominent uptake in the midbrain, hypothalamus, striatum, and pons; medium uptake in the cerebral cortex; and minimal activity in the cerebellum). On the other hand, in studies with [11C](-)McN5652 and with [11C](+)McN5652 after 5-HT uptake site blockade with fluoxetine, the radiotracer uptakes were significantly lower and the distribution pattern was relatively even. The differences between [11C](+)McN5652 and [11C](-)McN5652 were calculated for the time interval 95-125 min post injection and used to estimate specific binding. Specific binding correlated well with the known density of 5-HT uptake sites (r = 0.95; P < 0.001) in the human brain, as determined in vitro by Laruelle and Maloteaux (16). There was also a high correlation (r = 0.81; P < 0.002) between specific binding obtained with [11C](+)McN5652 (or [11C](-)McN5652) and that obtained with blockade by fluoxetine. At 95-125 min post injection, the midbrain/cerebellum ratio obtained for [11C](+)McN5652 was 1.50 ± 0.38 without fluoxetine and 0.91 ± 0.16 with fluoxetine.
Other primate PET studies using both [11C](+)McN5652 and [11C](-)McN5652 were performed by Kakiuchi et al. (17). The aim of the studies was to investigate age-related changes in the SERT for living brains of conscious young (5.9 ± 1.8 years old) and aged (19.0 ± 3.3 years old) Macaca mulatta monkeys. Results showed that specific binding of the SERT was higher in the thalamus and striatum, with intermediate binding in the pons, hippocampus, cingulate gyrus, and cortical regions, and lower binding in the cerebellum (in both young and aged monkeys). Almost all regions – except the cerebellum – showed significant age-related decreases in specific binding of the SERT. When the SERT blocker fluvoxamine (1 mg/kg of tissue) was administered intravenously at 30 min after injection of the radiotracer, the specific binding of the SERT was displaced in both age groups.
Human Studies
[PubMed]
The first studies of the serotonin 5-HT transporter in the living human brain, using PET with [11C](+)McN5652, were reported by Szabo et al. (18). The experimental protocol involved injecting either [11C](+)McN5652 or [11C](-)McN5652 into three healthy subjects, and in two cases, injecting [11C](+)McN5652 after pretreatment with the 5-HT uptake site blocker fluoxetine. Results showed highest accumulation of [11C](+)McN5652, with a steady increase over 120 min after injection of the radiotracer. In contrast, with [11C](-)McN5652 and when [11C](+)McN5652 binding was inhibited with fluoxetine, radioactivity concentrations declined after reaching a peak at 20 to 30 min post injection.
Reivich et al. (19) used [11C](+)McN5652 as a PET agent to study alterations in the brain 5-HT system in patients with depression. In their experiments, four drug-free depressed patients and four healthy control subjects received intravenous injections containing 7-10 mCi of [11C](+)McN5652, and 18 sequential PET scans were obtained at increasing time periods. Distribution volume (DV) ratios of [11C](+)McN5652 (compared with the cerebellum) were calculated in selected regions of interest, using a two-compartment model (20). Results showed significantly larger DV ratios in the left frontal cortex (P = 0.013) and the right cingulate cortex (P = 0.043), compared with healthy controls. Mean region/cerebellum DV ratios were as follows: 1.941 (in patients) and 1.667 (in control subjects) for the right thalamus, 1.678 (patients) and 1.153 (controls) for the right pons, and 1.359 (patients) and 1.092 (controls) for the right cingulate.
PET studies comparing [11C](+)McN5652 and [11C]cyanoimipramine were performed by Takano et al. (21) in 15 healthy volunteers. [11C](+)McN5652 was injected at a dose of 179-759 MBq (4.8-20.5 mCi), and [11C]cyanoimipramine was injected at a dose of 360-721 MBq before PET scans were performed. The following mean thalamus/cerebellum, striatum/cerebellum, and frontal cortex/cerebellum ratios were obtained at 90 min post injection: 1.61 ± 0.15, 1.59 ± 0.10, and 1.08 ± 0.07 for [11C](+)McN5652 and 1.72 ± 0.18, 1.72 ± 0.18, and 1.17 ± 0.12 for [11C]cyanoimipramine. After pretreatment with 50 mg of clomipramine (injected dose, 370 MBq (100 mCi)), the DVs of [11C](+)McN5652 in the cerebellum and frontal cortex were not significantly changed, but those in the thalamus and striatum were dramatically decreased. The mean thalamus/cerebellum, striatum/cerebellum, and frontal cortex/cerebellum ratios for [11C](+)McN5652 obtained at 90 min post injection were 1.20 ± 0.06, 1.31 ± 0.05, and 0.98 ± 0.03, respectively.
Over recent years, a number of human PET studies using [11C](+)McN5652 have been performed to evaluate the neurotoxic effects of (±)3,4-methylenedioxymethamphetamine (MDMA; “ecstasy”) on human brain 5-HT system (22-24). In a study by McCann et al (23). using [11C](+)McN5652 and [11C]DASB, global reductions in SERT concentrations were found in MDMA users with both PET ligands. [11C](+)McN5652 has also been used in PET studies for quantifying the SERT in patients with bipolar disorder (21) and social phobia (25).
References
- 1.
- Owens MJ , Nemeroff CB . The serotonin transporter and depression Depress Anxiety 19988Suppl 15–12. [PubMed: 9809208]
- 2.
- Palmer AM , Francis PT , Benton JS , Sims NR , Mann DM , Neary D , Snowden JS , Bowen DM . Presynaptic serotonergic dysfunction in patients with Alzheimer's disease. J Neurochem. 1987;48(1):8–15. [PubMed: 2432177]
- 3.
- Roth BL , Xia Z . Molecular and cellular mechanisms for the polarized sorting of serotonin receptors: relevance for genesis and treatment of psychosis. Crit Rev Neurobiol. 2004;16(4):229–236. [PubMed: 15862107]
- 4.
- Laakso A , Hietala J . PET studies of brain monoamine transporters. Curr Pharm Des. 2000;6(16):1611–1623. [PubMed: 10974156]
- 5.
- White KJ , Walline CC , Barker EL . Serotonin transporters: implications for antidepressant drug development. Aaps J. 2005;7(2):E421–E433. [PMC free article: PMC2750979] [PubMed: 16353921]
- 6.
- Ichimiya T , Suhara T , Sudo Y , Okubo Y , Nakayama K , Nankai M , Inoue M , Yasuno F , Takano A , Maeda J , Shibuya H . Serotonin transporter binding in patients with mood disorders: a PET study with [11C](+)McN5652. Biol Psychiatry. 2002;51(9):715–722. [PubMed: 11983185]
- 7.
- Suehiro M , Scheffel U , Ravert HT , Dannals RF , Wagner HN . [11C](+)McN5652 as a radiotracer for imaging serotonin uptake sites with PET. Life Sci. 1993;53(11):883–892. [PubMed: 8366755]
- 8.
- Szabo Z , Scheffel U , Mathews WB , Ravert HT , Szabo K , Kraut M , Palmon S , Ricaurte GA , Dannals RF . Kinetic analysis of [11C]McN5652: a serotonin transporter radioligand. J Cereb Blood Flow Metab. 1999;19(9):967–981. [PMC free article: PMC2034412] [PubMed: 10478648]
- 9.
- Shank RP , Vaught JL , Pelley KA , Setler PE , McComsey DF , Maryanoff BE . McN-5652: a highly potent inhibitor of serotonin uptake. J Pharmacol Exp Ther. 1988;247(3):1032–1038. [PubMed: 2905001]
- 10.
- Ikoma Y , Suhara T , Toyama H , Ichimiya T , Takano A , Sudo Y , Inoue M , Yasuno F , Suzuki K . Quantitative analysis for estimating binding potential of the brain serotonin transporter with [11 C]McN5652. J Cereb Blood Flow Metab. 2002;22(4):490–501. [PubMed: 11919520]
- 11.
- Suehiro M , Musachio JL , Dannals RF , Mathews WB , Ravert HT , Scheffel U , Wagner HN . An improved method for the synthesis of radiolabeled McN5652 via thioester precursors. Nucl Med Biol. 1995;22(4):543–545. [PubMed: 7550033]
- 12.
- Suehiro M , Ravert HT , Dannals RF , Scheffel U , Wagner HN . Synthesis of a radiotracer for studying serotonin uptake sites with positron emission tomography: [11C]McN5652. J. Labelled Compds Radiopharm. 1992;31:841.
- 13.
- Sasaki M , Suhara T , Kubodera A , Suzuki K . [An improved automated synthesis and in vivo evaluation of PET radioligand for serotonin re-uptake sites: [11C]McN5652X] Kaku Igaku. 1996;33(12):1319–1327. [PubMed: 9023438]
- 14.
- Brust P , Zessin J , Kuwabara H , Pawelke B , Kretzschmar M , Hinz R , Bergman J , Eskola O , Solin O , Steinbach J , Johannsen B . Positron emission tomography imaging of the serotonin transporter in the pig brain using [11C](+)-McN5652 and S-([18F]fluoromethyl)-(+)-McN5652. Synapse. 2003;47(2):143–151. [PubMed: 12454952]
- 15.
- Szabo Z , Scheffel U , Suehiro M , Dannals RF , Kim SE , Ravert HT , Ricaurte GA , Wagner HN . Positron emission tomography of 5-HT transporter sites in the baboon brain with [11C]McN5652. J Cereb Blood Flow Metab. 1995;15(5):798–805. [PubMed: 7673372]
- 16.
- Laruelle M , Maloteaux JM . Regional distribution of serotonergic pre- and postsynaptic markers in human brain. Acta Psychiatr Scand Suppl. 1989;350:56–59. [PubMed: 2530792]
- 17.
- Kakiuchi T , Tsukada H , Fukumoto D , Nishiyama S . Effects of aging on serotonin transporter availability and its response to fluvoxamine in the living brain: PET study with [(11)C](+)McN5652 and [(11)C](-)McN5652 in conscious monkeys. Synapse. 2001;40(3):170–179. [PubMed: 11304754]
- 18.
- Szabo Z , Kao PF , Scheffel U , Suehiro M , Mathews WB , Ravert HT , Musachio JL , Marenco S , Kim SE , Ricaurte GA . et al. Positron emission tomography imaging of serotonin transporters in the human brain using [11C](+)McN5652. Synapse. 1995;20(1):37–43. [PubMed: 7624828]
- 19.
- Reivich M , Amsterdam JD , Brunswick DJ , Shiue CY . PET brain imaging with [11C](+)McN5652 shows increased serotonin transporter availability in major depression. J Affect Disord. 2004;82(2):321–327. [PubMed: 15488265]
- 20.
- Lammertsma AA , Bench CJ , Hume SP , Osman S , Gunn K , Brooks DJ , Frackowiak RS . Comparison of methods for analysis of clinical [11C]raclopride studies. J Cereb Blood Flow Metab. 1996;16(1):42–52. [PubMed: 8530554]
- 21.
- Takano A , Suhara T , Sudo Y , Inoue M , Hashimoto K , Zhang MR , Ichimiya T , Yasuno F , Suzuki K . Comparative evaluation of two serotonin transporter ligands in the human brain: [(11)C](+)McN5652 and [(11)C]cyanoimipramine. Eur J Nucl Med Mol Imaging. 2002;29(10):1289–1297. [PubMed: 12271409]
- 22.
- Thomasius R , Zapletalova P , Petersen K , Buchert R , Andresen B , Wartberg L , Nebeling B , Schmoldt A . Mood, cognition and serotonin transporter availability in current and former ecstasy (MDMA) users: the longitudinal perspective. J Psychopharmacol. 2006;20(2):211–225. [PubMed: 16510479]
- 23.
- McCann UD , Szabo Z , Seckin E , Rosenblatt P , Mathews WB , Ravert HT , Dannals RF , Ricaurte GA . Quantitative PET studies of the serotonin transporter in MDMA users and controls using [11C]McN5652 and [11C]DASB. Neuropsychopharmacology. 2005;30(9):1741–1750. [PMC free article: PMC2034411] [PubMed: 15841106]
- 24.
- Buchert R , Thomasius R , Petersen K , Wilke F , Obrocki J , Nebeling B , Wartberg L , Zapletalova P , Clausen M . Reversibility of ecstasy-induced reduction in serotonin transporter availability in polydrug ecstasy users. Eur J Nucl Med Mol Imaging. 2006;33(2):188–199. [PubMed: 16133393]
- 25.
- Kent JM , Coplan JD , Lombardo I , Hwang DR , Huang Y , Mawlawi O , Van Heertum RL , Slifstein M , Abi-Dargham A , Gorman JM , Laruelle M . Occupancy of brain serotonin transporters during treatment with paroxetine in patients with social phobia: a positron emission tomography study with 11C McN 5652. Psychopharmacology (Berl). 2002;164(4):341–348. [PubMed: 12457263]
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review 2-((2-Amino-4-chloro-5-[(18)F]fluorophenyl)thio)-N,N-dimethylbenzenmethanamine.[Molecular Imaging and Contrast...]Review 2-((2-Amino-4-chloro-5-[(18)F]fluorophenyl)thio)-N,N-dimethylbenzenmethanamine.The MICAD Research Team. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review [(11)C]2-(2-(Dimethylaminomethyl)phenylthio)-5-fluoromethylphenylamine.[Molecular Imaging and Contrast...]Review [(11)C]2-(2-(Dimethylaminomethyl)phenylthio)-5-fluoromethylphenylamine.The MICAD Research Team. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review [(11)C]2-(2-((Dimethylamino)methyl)phenylthio)-5-(2-fluoroethyl)phenylamine.[Molecular Imaging and Contrast...]Review [(11)C]2-(2-((Dimethylamino)methyl)phenylthio)-5-(2-fluoroethyl)phenylamine.The MICAD Research Team. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review [(11)C]N,N-Dimethyl-2-(2´-amino-4´-hydroxymethylphenylthio)benzylamine.[Molecular Imaging and Contrast...]Review [(11)C]N,N-Dimethyl-2-(2´-amino-4´-hydroxymethylphenylthio)benzylamine.The MICAD Research Team. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review [(11)C]2-2-(Dimethylaminomethyl)phenylthio)-5-fluorophenylamine.[Molecular Imaging and Contrast...]Review [(11)C]2-2-(Dimethylaminomethyl)phenylthio)-5-fluorophenylamine.The MICAD Research Team. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- trans-(+)-1,2,3,5,6,10b-Hexahydro-6-(4-([11C]methylthio)-phenyl)pyrrolo-(2,1-a)-...trans-(+)-1,2,3,5,6,10b-Hexahydro-6-(4-([11C]methylthio)-phenyl)pyrrolo-(2,1-a)-isoquinoline - Molecular Imaging and Contrast Agent Database (MICAD)
Your browsing activity is empty.
Activity recording is turned off.
See more...

 In vitro
In vitro