NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Kamkin A, Kiseleva I, editors. Mechanosensitivity in Cells and Tissues. Moscow: Academia; 2005.
This review covers aspects of the cardiac mechanotransduction field at different levels, and advocates the possibility that mechanoelectro-chemical transduction forms part of a network of mechanically linked crosstalk in heart – Mechanically Mediated Crosstalk (MMC). It assembles evidence and observations in the literature to promote this hypothesis. Mechanical components can provide the bond between interactions at molecular, cellular, and macro levels to enable the crosstalk. Stretch activated channels exist in heart, but stresses and strains can affect other membrane channels or receptors. A cellular mechanical change can thus promote several ionic or downstream changes. Cell signal cascades have been implicated and, mostly via intracellular Ca, can affect membrane electrophysiology. MMC could shape downstream signals to alter intracellular Ca. MMC also spans other regulatory systems and processes, such as the autonomic nervous system, and in addition, operates through to the whole heart as an integrative system. Finally, supporting the hypothesis, if elements of the normal crosstalk become deranged it contributes to cardiovascular disease and, potentially, lethal arrhythmia.
Introduction
This review advocates the possibility that mechanoelectro-chemical transduction forms part of a network of what this article will term Mechanically Mediated Crosstalk (MMC) in heart. In support of this hypothesis it addresses disparate inputs in the cardiac mechanotransduction field at different levels. Some licence is taken in characterizing "crosstalk" as being a mechanical component, which provides a common link between the various interactions at molecular, cellular, and macro levels. In searching the literature to sustain the hypothesis, we track crosstalk along a path from mechanosensor to the whole heart within an integrative system. It spans other regulatory systems and processes. Finally, if elements of the normal crosstalk experience derangement, and the hypothesis holds, it contributes to cardiovascular disease, and potentially lethal arrhythmia.
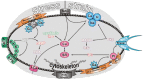
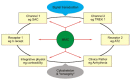
Virtually all the anatomical subdivisions of the heart, membrane through to intact heart including man, show mechanoelectric transduction/coupling/ feedback, and although the exploration of the crosstalk begins from the bottom up, it uses a clock directional reference system, particularly in fig. 2.
Mechanically mediated crosstalk (MMC) at the channel level
Passive, mechanically activated channels (Fig. 1 at 10 and 2 o'clock)
Stretch-activated channels (SAC) (Fig. 2 at 12 o'clock)
The regulatory integrative chain discussed in this article begins at the membrane with mechanogated, mechanosensitive channels. Since their definitive description, [38] stretch activated channels have been described in many tissues (for reviews see [15, 35, 75, 95] including heart [20]. Membrane stretch opens the channels to admit charge-carrying ions, which influences the membrane potential. Although the myocardium is actively contracting here, in the current context the resultant geometric shape changes are regarded as being passively transmitted.
Micropipette patch studies start with a residual membrane tension that may not be commensurate with that found in multicellular preparations, and multicellular preparations experience more tension than isolated cells (See also [7]). This questions the physiological validity of patch studies in the intact heart. However, characteristics of the channel electrophysiology have found equivalence in intact heart, where monophasic action potentials (MAPs) can be used to gain qualitative insights into cellular electrophysiology: for example isolated perfused heart of frog [61], and mammals [27, 34, 42]. Notably, there is a type of reversal potential, [61, 120] where a stretch early in the action potential produces a repolarising tendency, whereas if late it produces depolarisation. There is no change at the "reversal potential". There are other examples of the equivalence, in different guises, in intact heart in situ in experimental preparations [23] and in man [106]. SACs can thus explain load induced reductions in action potential duration in these examples. Another example is that SAC blockers in intact preparations are commonly used to block stretch induced electrophysiological changes. However, the results may have to be carefully interpreted. Streptomycin's effects as a blocker appear heterogeneous in intact atrium [4]. It looks so far as if SACs may well operate in intact heart.
Mechanically mediated crosstalk (MMC) and other channels
The hypothesis requires that the crosstalk applies to other channels apart from SACs. The ATP activated potassium (KATP) channel subunits (Kir6.1, Kir6.2, SUR1A, SUR1B, SUR2A, and SUR2B) [5] have been found in atria from neonatal rats. The KATP channel (Fig. 2 at 9 o'clock) is attached to the cytoskeleton, and it has been suggested to be mechanosensitive [111].
Several other channels appear to be mechanosensitive, including the L type Ca2+-channel [73] sodium channels [105] and the tandem pore potassium channels such as TREK-1 (Fig. 1 at 2 o'clock; Fig. 2 at 8 o'clock) [71], which appears not to involve the cytoskeleton - and IKAA [55], ion exchangers, (Fig. 2 at 1 o'clock) [84].
MMC and metabolism
In cultured chick ventricular myocytes, a stretch-activated ion channel [51] was identified as a high-conductance K+-selective channel blocked by gadolinium, tetraethylammonium, and charybdotoxin from the extracellular surface. It was identified as a Ca2+-activated K+ (KCa) channel type, also reversibly activated by ATP on the intracellular surface. That is, a stretch activated KCa,ATP channel.
The large (111 ± 3.0 pS) K+ channels, TREK-1: from the tandem pore family (Fig. 1 at 2 o'clock: Fig. 2 at 8 o'clock) which have been found in adult rat ventricular myocytes, are not only mechanosensitive, they are also activated by low intracellular pH, as well as intracellular ATP [107]. It does seem that there is a crosstalk with metabolism, albeit vestigial.
MMC and the neuro-humoral system
The autonomic nervous system, and the endocrine system are integrative regulatory systems, and they interact with each other. If the current hypothesis holds as a fully integrated system, the question arises as to whether MMC interplays with these other regulatory systems.
β Receptor (Fig. 2 at 11 o'clock)
Mechanically induced electrophysiological changes can be significantly modulated by β receptor agonism. This includes, first, the electrical restitution curve [45]. Load changes accentuate the supernormal period, and steepen the initial rising phase. β receptor agonism exacerbates these changes. Second, β receptor antagonism curtails mechanically induced arrhythmia [65]. Raised intraventricular pressure releases catecholamines from the ventricle [60] and this could promote mechanically induced arrhythmia. The β receptor effects would be via the cell signal chain involving β agonist/receptor, ATP, cAMP, and Ca channels. In support of this signal chain, catecholamine store depletion curtails mechanoarrhythmia whether the depletion is pharmacological [28] or by chronic sympathetic denervation in the intact preparation [29]. This notion has been further supported by a study in the intact preparation in which reserpine (also depletes catecholamines) and propranolol curtailed load-induced changes in electrical excitability and monophasic action potential duration [68].
Acetyl choline receptor (Fig. 2 at 11 o' clock)
G-protein regulated inward-rectifier potassium channels (GIRK) are part of a superfamily of inward-rectifier K+ channels. GIRK1 and 4 are found in atrium and sinoatrial node [72]. G-proteins and mechanical stretch (Fig 2 at 11 o' clock) modulates GIRK1-4 (also designated Kir3.1–4). Rabbit atrial muscarinic potassium channels are rapidly and reversibly inhibited by membrane stretch, possibly serving as part of the mechanoelectrical feedback interaction. Hypo-osmolar stress inactivates the heteromeric Kir3.4 channel expressed in Xenopus oocytes [49]. Stretch activates the cardiac G protein (GK)-gated, muscarinic K+ acetylcholine (KACh) channel if ACh is present and this appears to be independent of receptor/G protein, probably via a direct effect on the channel protein/lipid bilayer [86].
Active myofibrillar interaction, calcium and mechanoelectric transduction (Fig. 1 at 7 o'clock; Fig. 2 myofibrils – upper left quadrant)
Active myocardial contraction provides an intracellular generator of stress and strain. Stretched or isometric muscle has a short action potential duration compared with the shortening muscle [50, 64]. Moreover, isotonic (shortening) muscle, with the reduced force production, has the longer duration calcium transient. This is counter-intuitive: but a reasonable explanatory mechanism is related to force deactivation. Briefly, the reduced force with the shortening muscle reduces the affinity of troponin c for calcium [8, 64] (see also [121], and [10, 108]). This reduced calcium buffering allows intracellular calcium to rise in the face of a reduced force production. The raised calcium would influence transmembrane currents to prolong the action potential duration. For example via electrogenic Na/Ca exchange (Fig. 2 bi-directional arrow in 11 o'clock direction. These calcium aspects, also important in arrhythmogenesis (see below), have been reviewed [10] and includes mechanical influences on Sarcoplasmic Reticulum (SR) calcium cycling (see also [36, 66, 102, 103]).
Facilitators of MMC
Mechanically Mediated Crosstalk, if a tenable hypothesis, may well need systems to facilitate its integrative functions. If some of these could be identified, it could strengthen the tenant.
Calcium and cell signal cascades (Fig. 1 at 6 o'clock; Fig. 2 centre)
Mechano-chemical processes often interact with mechanoelectric and signalling processes, with calcium as an interlink. A mechanical perturbation can induce a Ca rise [10, 100]. However, mechanical stimulation releases a multitude of other second messengers [70, 97] represented in figure 2 ("cog" East of 3 o`clock – "kinase" at centre) that includes tyrosine kinase, [96] cAMP [43, 116], inositol trisphosphate [89] (Fig. 2 bottom quadrant, right), arachidonic acid [56, 57, 85]. It also activates mechanisms involving G protein [6, 59], and phospholipase C (Fig. 2 at 4 o'clock). PKC (Fig. 2, 3 o'clock) (bio)physically translocates from the cytosol, under the influence first of DAG and then of RACK in the membrane, to bring it to its target protein, allowing MAPk to switch on IEGs. More detailed individual mechano-chemo-sensitivity, including ionic currents carried by channels other than stretch-activated ones, has been reviewed [15].
Crosstalk could also be provided by AT2 and ET1 receptors, (Fig. 2 at 5 o'clock) - see also brief overview [63]) which would affect PLC via Gq (Fig. 2 at 4 o clock). This can alter intracellular Ca, first, via IP3 and SR Ca release, and second, through PLC to facilitate membrane translocation (DAG/RACK/PKC – Fig. 1 at 3 o'clock) to influence Na/H exchange, and so intracellular Ca via Na/Ca exchange (Fig. 2 at 1 o'clock).
Cytoskeleton, "tensegrity": A MMC vehicle? (Figs. 1 at 6 o'clock and 2 at 6 o'clock)
Mechanotransduction appears to involve, or to be modulated by, the intracellular cytoskeleton, and its insertions into the surrounding cell membrane via focal adherence complexes. From the electrophysiological aspect the cytoskeleton may be important because, physiologically and geometrically, parts of the cytoskeleton are associated with ion channel function [13, 14, 15, 90]. Many stimuli (Fig. 2) share intracellular signal pathways with mechano-electrochemical ones, and this can produce composite responses. Here the role of the intracellular cytoskeleton appears to be crucial. It can form the mechanical engineering and biochemical basis for intracellular and intercellular crosstalk. Any membrane channel associated with the cytoskeleton could provide a candidate for crosstalk. Stress-strain could be transmitted to the channel via the cytoskeleton to activate it, or the cytoskeleton could somehow shield the channel from the mechanical transmission of strain via other paths. The ATP activated potassium (KATP) channel, which is mechanosensitive, is attached to the cytoskeleton. Several ion channels are regionally fixed in the membrane [37], and their modulation by the cytoskeleton is also possible.
Under physiological conditions, the mechanical interconnecting system is pre-stressed, conferring mechanical balance and stability. This has been described as "tensegrity" [47], and there is thus a clear line of force communication from the extracellular matrix to intracellular cytoplasmic structures [114]. Mechanical stimuli can provoke changes in adhesions between the cell and the extracellular matrix via the fibronectin and collagen networks. In this way, mechanically distorting one cell can raise intracellular Ca2+ of its neighbouring cell [100].
The elaborate adhesion complex [40, 119] also contains focal adhesion kinase (Fig. 2 at 12 and 6 o'clock). This kinase can be the initiator or modulator of many downstream cell signals and kinases, including tyrosine kinase [87] and the renin angiotensin system (Fig. 2 at 5 o'clock) as reviewed by [97]. These are possible/probable mechanisms whereby chronic stretch can produce remodelling and hypertrophy.
MMC in the intact heart: Regulation of cardiac function
So far, the observations above, at the cellular and intracellular level, are in keeping with the presented MMC concept. If so, they should find expression at "higher" levels. Spatially, the integrative stress/strain system transmits to the whole heart. This would be by cell attachment through the adherence proteins to the extracellular matrix, which connects cells to each other. Forces at one end of a group of cells are thus transmitted throughout the organ, invoking tensegrity. This provides a clear mechanism by which a mechanically induced functional crosstalk can occur in the intact heart. Additionally, the heart is part of a hydrodynamic system. MMC in the intact heart could be via a mechanical, hydraulically mediated mechanism. That is, pressure volume changes in a system containing incompressible blood could provide the Mechanically Mediated Crosstalk. Systemic arterial pressure, and or venous volume changes can influence cardiac electrophysiology and function [15, 21, 24, 32, 33, 62, 99]. This would invoke the variety of cellular mechanisms covered above.
We know that stretch can raise heart rate by mechanisms other than neural reflexes. Sinoatrial stretch in isolated perfused hearts increases heart rate (Bainbridge, 1915; Blinks, 1956), probably by a mechano-electric mechanism [83] working through stretch activated channels and increasing the slope of the diastolic depolarisation. Thus, in addition to nervous reflex mechanisms, cellular mechanoelectric transduction provides integrative control mechanisms for raising heart rate in response to an increased venous return to the heart.
On a marginally different track, one of the major mechanosensitive regulatory mechanisms in heart is related to contractile function per se. Myocardial stretch increases force production. This has been well studied, including the pioneering work of Frank, Starling, Patterson in the intact ventricle. The prevailing dogma for the familiar Starling's Law of the Heart is a length/force sensitivity of the contractile proteins – perhaps related to calcium [1, 54]. A membrane mechanosensitivity probably also makes a contribution in the intact ventricle by mechanotransduction, possibly via stretch activated channels [28]. Studies at the membrane level corroborate this, showing that stretch can raise intracellular calcium by affecting mechanosensitive channels [10, 67, 100, 117], and so increase force.
MMC and pathophysiology (Fig. 1 at 5 o'clock)
As with other physiological integrative regulatory systems in heart, if MMC functions similarly, derangements in function should produce pathophysiological situations. Mechanoelectric feedback and its crosstalk, perhaps acting as a homeostatic feedback control system in the normal situation [63], is amplified in cardiac pathology [11, 46, 76, 91, 109, 115, 122]. The feedback is now a destabilising mechanism, because the crosstalk interactions have altered their interactive "gains". The tentative argument is that it is this derangement in crossstalk in arrhythmic death that provides the lethal expression of MMC.
Intracellular calcium changes have a pivotal role in crosstalk between intracellular signals and the generation of arrhythmia [25, 69, 78, 79]. Sustained [Ca]i increases promote [Ca]i oscillations [2] and electro-physiological oscillations are conducive to arrhythmia. Calcium is heavily involved in mechanoelectric feedback as alluded above and as reviewed by Calaghan and White [10]. Calcium changes could be pivotal in mechanically linked crosstalk during electrophysiological derangement in myocardial pathology.
MMC and mechanical components in arrhythmia
Mechanoelectric feedback/transduction is increasingly being highlighted as a possible cause of sudden cardiac arrhythmic death in man, and there are clear correlations with ventricular ejection fraction, which is a purely mechanical rather than electrophysiological predictor [19, 52, 77].
Many clinical correlates with lethal arrhythmia have their equivalent in mechanoelectric feedback in pathological mechanical changes, which produce electrical changes. At one extreme, this would be ischemically induced dyskinesia or infarction with localised stretch in the affected part of the ventricular wall. Mechanoelectric dispersion, and thus arrhythmogenic electrical dispersion, would be gross. At the other end, remodelling would produce less mechanoelectric dispersion, but, nonetheless a potentially grave one, perhaps because of its patchy nature.
MMC and the neuro-humoral component in arrhythmia
β Blockade (Fig. 1 at 9 o'clock; Fig. 2 at 11 o'clock)
One of the few therapeutic agents reducing mortality from sudden (arrhythmic) death in many subsets of patients and in related experimental situations are the β blockers. Study in this area is already voluminous, rising exponentially and a few recent reviews and studies are included here [12, 44, 53, 93, 98, 101, 110, 118]. The clinical situations covered include, infarction, hypertension, subaortic stenosis, cardiomyopathy, mitral valve prolapse, congestive cardiac failure. Moreover, depleting endogenous catecholamines by bretylium tosylate is one method for treating clinical arrhythmia [3, 16, 30, 58, 112, 113].
Although the mechanism of the therapeutic efficacy may well be due to a combination of direct anti-ischemic effects and preservation of vagal tone, all the above observations have elements of MMC. First, many of the clinical conditions associated with the autonomic nervous system and arrhythmia are associated with abnormal mechanical loads or wall motion. Secondly, the clinically related observations are in keeping with the mechanoelectric studies illustrated above. That is, electrophysiology can be modified by autonomic agonists/antagonists. Moreover, bretylium tosylate, analogous to its therapeutic effects in abating clinical arrhythmia, curtails stretch arrhythmia [28]. We need a note of caution, for the action of bretylium may be directly electrophysiological [80].
MMC and the Renin-Angiotensin system (Fig. 2 at 5 o'clock)
It is likely that the Renin Angiotensin system displays cross-talk with mechanotransduction, either by its action as a load-reducing peripheral vasodilator, or at the cell signal transduction level [31]). Either crosstalk mechanism could affect arrhythmia. Clinical trials have suggested that the reduction in sudden arrhythmic death observed with Angiotensin Converting Enzyme (ACE) inhibitors may be related to its load reducing actions on electrophysiology (see reviews in this area [24, 92, 94]. That is, by its action in reducing wall stress/strain [18]. However, cell signal crosstalk can also explain this effect, and this could be related to ACE inhibitor's slowing of cardiac remodelling. Cardiovascular drugs, such as the ACE inhibitors, and carvedilol (possessing β blocker activity); which interact with the remodelling of heart have proved their efficacy in term of cardiovascular mortality, antiarrhythmic action and prevention of sudden deaths.
MMC and hypokalaemia
Diuretic therapy in patients can reduce serum potassium, which can be arrhythmogenic [39, 88, 104], although interaction with other factors may be important [22]. Electrolyte depletion has been considered a risk factor [82]. Hypokalaemia can be related to experimental mechanotransduction [26, 32, 48]. For example, arrhythmia was generated in isolated heart, normally perfused, by increasing ventricular load. Perfusion with low potassium solutions increased the incidence/severity of this mechanically induced arrhythmia.
Longer term mechanoelectric changes
The foregoing perusal of observations supporting the existence of some sort of Mechanically Mediated Crosstalk focus mainly on the acute, short term. The question arises as to whether there are longer term manifestations.
MMC and ischemic preconditioning
Ischemic preconditioning is a consequence of a short period of ischaemia protecting the myocardium from the damage produced by a subsequent more prolonged period of ischaemia. Stretch also seems to induce preconditioning [81]. This may be related to activation of adenosine receptors, (KATP) channels, and/or PKC. In one study, 5 min ischaemia, and 5 minutes reperfusion induced ischemic preconditioning. However, a transient volume overload can also produce a "mechanical" preconditioning. The infarct size-reducing effect of stretch, with no ischemia, was prevented by the stretch channel blocker Gd3+, and Glibencamide. This means that activation of mechanosensitive ion channels may produce an analogue of ischemic preconditioning - probably by downstream activation of PKC, adenosine receptors, and/or KATP channels [41].
Remodelling and cardiac failure (Fig. 1 at 5 o'clock; Fig. 2 bottom right quadrant)
Chronic increase in load produces cascades leading to hypertrophy and remodelling. Intracellular calcium, cell signals and the cytoskeleton would be possible contenders in the cascades, which inevitably experience crosstalk (see for example, [9]), switching on immediate early genes.
Myocardial failure with a dilated, stretched heart is associated with sudden arrhythmic death. In keeping with the altered interactive gains in the pathological situation alluded to above, studies at the membrane level show, strikingly, that SACs are persistently activated in heart failure [17]. Moreover, mechanosensors are thought to be responsible for the downstream molecular changes [97] in remodelling ventricular structure and function in heart failure (Fig. 2, "Kinase" (e.g. MAPK, tyrosine kinase) to IEG). This remodelling involves a crosstalk between several cellular mechanisms, which may also be conducive to arrhythmia, as early onset genes are turned on in parallel with changes in electrophysiology [74]. It appears that in the longer term, load-induced remodelling in global myocardial failure can produce subtler but significant mechanical heterogeneity, crosstalk, and thus electrophysiological heterogeneity, which is arrhythmogenic.
Conclusion and perspectives
In summary, the existence of some type of Mechanically Mediated Crosstalk in heart has no shortage of observations supporting it as a hypothesis. Mechanosensitivity (mechanoelectric feedback or transduction) has several putative paths for crosstalk. The mechanically mediated crosstalk embraces a plethora of channels, exchangers, and cell "signalsomes". This is at a molecular, cellular, micro and macro level. This crosstalk can be spatio-temporal. It has the possibility of becoming deranged, this derangement contributing to mortality; but within MMC there is a deluge of potential therapeutic targets.
References
- 1.
- Allen DG, Kentish JC. The cellular basis of the length-tension relation in cardiac muscle. J Mol Cell Cariol. (1985);17:821–840. [PubMed: 3900426]
- 2.
- Allen DG, Eisner DA, Orchard CH. Characterization of oscillations of intracellular calcium concentration in ferret ventricular muscle. J Physiol. (1984);352:113–128. [PMC free article: PMC1193201] [PubMed: 6747885]
- 3.
- Anderson JL. Bretylium tosylate: profile of the only available class III antiarrhythmic agent. Clin Ther. (1985);7:205–224. [PubMed: 3886143]
- 4.
- Babuty D, Lab M. Heterogeneous changes of monophasic action potential induced by sustained stretch in atrium. J Cardiovasc Electrophysiol. (2001);12:323–329. [PubMed: 11291806]
- 5.
- Baron A, van Bever L, Monnier D, Roatt A, Baertschi AJ. A novel K(ATP) current in cultured neonatal rat atrial appendage cardiomyocytes. Circ Res. (1999);85:707–715. [PubMed: 10521244]
- 6.
- Basdra EK, Papavassiliou AG, Huber LA. Rab and rho GTPases are involved in specific response of periodontal ligament fibroblasts to mechanical stretching. Biochim Bio-phys Acta. (1995);1268:209–213. [PubMed: 7662710]
- 7.
- Brady AJ. Mechanical properties of isolated cardiac myocytes. Physiol Rev. (1991);71:413–428. [PubMed: 2006219]
- 8.
- Bremel RD, Weber A. Cooperation within actin filament in vertebrate skeletal muscle. Nat New Biol. (1972);238:97–101. [PubMed: 4261616]
- 9.
- Bustamante JO, Ruknudin A, Sachs F. Stretch-activated channels in heart cells: relevance to cardiac hypertrophy. J Cardiovasc Pharmacol. (1991);17(Suppl 2):S110–S113. [PubMed: 1715454]
- 10.
- Calaghan SC, White E. The role of calcium in the response of cardiac muscle to stretch. Prog Biophys Mol Biol. (1999);71:59–90. [PubMed: 10070212]
- 11.
- Calkins H, Maughan WL, Kass DA, Sagawa K, Levine JH. Electrophysiological effect of volume load in isolated canine hearts. Am J Physiol. (1989);256:1697–1706. [PubMed: 2735439]
- 12.
- Campbell RW. ACE inhibitors and arrhythmias. Heart. (1996);76:79–82. [PMC free article: PMC484493] [PubMed: 8977367]
- 13.
- Cantiello HF. Role of the actin cytoskeleton on epithelial Na+ channel regulation. Kidney Int. (1995);48:970–984. [PubMed: 8569107]
- 14.
- Cantiello HF, Stow JL, Prat AG, Ausiello DA. Actin filaments regulate epithelial Na+ channel activity. Am J Physiol. (1991);261:C882–C888. [PubMed: 1659214]
- 15.
- Cazorla O, Pascarel C, Brette F, Le Guennec JY (1999) Modulation of ion channels and membrane receptor activity by stretch in cardiomyocytes. Possible mechanisms for mechanosensitivity. Progr Bioph Mol Biol 71, pp. 29–58. Ref Type: Journal (Full). [PubMed: 10070211]
- 16.
- Chamberlain DA. Lignocaine and bretylium as adjuncts to electrical defibrillation. Resuscitation. (1991);22:153–157. [PubMed: 1661021]
- 17.
- Clemo HF, Stambler BS, Baumgarten CM. Swelling-activated chloride current is persistently activated in ventricular myocytes from dogs with tachycardia-induced congestive heart failure. Circ Res. (1999);84:157–165. [PubMed: 9933247]
- 18.
- Cohn JN, Archibald DG, Ziesche S, Franciosa JA, Harston WE, Tristani FE, Dunkman WB, Jacobs W, Francis GS, Flohr KH. Effect of vasodilator therapy on mortality in chronic congestive heart failure. Results of a Veterans Administration Cooperative Study. N Engl J Med. (1986);314:1547–1552. [PubMed: 3520315]
- 19.
- Copie X, Hnatkova K, Blankoff I, Staunton A, Camm AJ, Malik M. Risk of mortality after myocardial infarction: value of heart rate, its variability and left ventricular ejection fraction. Arch Mal Coeur Vaiss. (1996);89:865–871. [PubMed: 8869248]
- 20.
- Craelius W, Chen V, el Sherif N. Stretch activated ion channels in ventricular myocytes. Biosci Rep. (1988);8:407–414. [PubMed: 2852974]
- 21.
- Dalton GR, Jones JV, Evans SJ, Levi AJ. Wall stress-induced arrhythmias in the working rat heart as left ventricular hypertrophy regresses during captopril treatment. Cardiovasc Res. (1997);33:561–572. [PubMed: 9093526]
- 22.
- Dargie HJ, Cleland JG, Leckie BJ, Inglis CG, East BW, Ford I. Relation of arrhythmias and electrolyte abnormalities to survival in patients with severe chronic heart failure. Circ. (1987);75:IV98–I107. [PubMed: 3032475]
- 23.
- Dean JW, Lab MJ (1987) Effects of changes in afterload on the absolute refactory period of the pig ventricle. PACE 10, p. 987. Ref Type: Abstract.
- 24.
- Dean JW, Lab MJ. Arrhythmia in heart failure: role of mechanically induced changes in electrophysiology. Lancet. (1989);1:1309–1312. [PubMed: 2566835]
- 25.
- Di Diego JM, Antzelevitch C. High [Ca2+]o-induced electrical heterogeneity and extrasystolic activity in isolated canine ventricular epicardium. Phase 2 reentry. Circ. (1994);89:1839–1850. [PubMed: 7511994]
- 26.
- Dick DJ, Lab MJ (1995) Effect of manipulation of potassium concentration on stretch-induced arrhythmia in the isolated Langendorff rabbit heart. Journal of Physiology 487, p. 140P. Ref Type: Abstract.
- 27.
- Dick DJ, Harrison FG, O'Kane PD, Halliwell OT, Lab MJ. "Preconditioning" of mechanically induced premature ventricular beats in the isolated rabbit heart. Journal of Physiology. (1993);459:509P.
- 28.
- DickDJ, Lab MJ, Harrison FG, Green S, Gruber PC. A possible role of endogeneous catecholamines in stretch induced premature ventricular beats in the isolated rabbit heart. Journal of Physiology. (1994);479:133P.
- 29.
- Drake-Holland AJ, Noble MI, Lab MJ. Acute pressure overload cardiac arrhythmias are dependent on the presence of myocardial tissue catecholamines. Heart. (2001);85:576. [PMC free article: PMC1729734] [PubMed: 11303014]
- 30.
- Duff HJ, Roden DM, Yacobi A, Robertson D, Wang T, Maffucci RJ, Oates JA, Woosley RL. Bretylium: relations between plasma concentrations and pharmacologic actions in high-frequency ventricular arrhythmias. Am J Cardiol. (1985);55:395–401. [PubMed: 3969876]
- 31.
- Eriksson SV, Eneroth P, Kjekshus J, Offstad J, Swedberg K. Neuroendocrine activation in relation to left ventricular function in chronic severe congestive heart failure: a subgroup analysis from the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). Clin Cardiol. (1994);17:603–606. [PubMed: 7834934]
- 32.
- Evans SJ, Levi AJ, Lee JA, Jones JV. EMD 57033 enhances arrhythmias associated with increased wall- stress in the working rat heart. Clin Sci Colch. (1995);89:59–67. [PubMed: 7671569]
- 33.
- Franz MR. Mechano-electrical feedback in ventricular myocardium. Cardiovascular Res. (1996);32:15–24. [PubMed: 8776399]
- 34.
- Franz MR, Burkhoff D, Yue DT (1985) Mechano-electrical feedback in the intact isolated perfused canine heart. Circulation Supp III, p. 382. Ref Type: Abstract.
- 35.
- French AS. Mechanotransduction. Annual Review of Physiology. (1992);54:135–152. [PubMed: 1373277]
- 36.
- Gamble J, Taylor PB, Kenno KA. Myocardial stretch alters twitch characteristics and Ca2+ loading of sarcoplasmic reticulum in rat ventricular muscle. Cardiovasc Res. (1992);26:865–870. [PubMed: 1451163]
- 37.
- Gu Y, Gorelik J, Spohr HA, Shevchuk A, Lab MJ, Harding SE, Vodyanoy I, Klenerman D, Korchev YE. High-resolution scanning patch-clamp: new insights into cell function. FASEB J. (2002);16:748–750. [PubMed: 11923226]
- 38.
- Guharay F, Sachs F. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J Physiol. (1984);352:685–701. [PMC free article: PMC1193237] [PubMed: 6086918]
- 39.
- Gulker H, Haverkamp W, Hindricks G. Ion regulation disorders and cardiac arrhythmia. The relevance of sodium, potassium, calcium, and magnesium. Arzneimittelforschung. (1989);39:130–134. [PubMed: 2470384]
- 40.
- Gumbiner BM. Proteins associated with the cytoplasmic surface of adhesion molecules. Neuron. (1993);11:551–564. [PubMed: 8398146]
- 41.
- Gysembergh A, Margonari H, Loufoua J, Ovize A, Andre-Fouet X, Minaire Y, Ovize M. Stretch-induced protection shares a common mechanism with ischemic preconditioning in rabbit heart. Am J Physiol. (1998);274:H955–H964. [PubMed: 9530209]
- 42.
- Hansen DE, Craig CS, Hondeghem LM. Stretch-induced arrhythmias in the isolated canine ventricle: evidence for the importance of mechano-electrical feedback. Circ. (1990);81:1094–1105. [PubMed: 1689619]
- 43.
- He Y, Grinnell F. Role of phospholipase D in the cAMP signal transduction pathway activated during fibroblast contraction of collagen matrices. J Cell Biol. (1995);130:1197–1205. [PMC free article: PMC2120549] [PubMed: 7657704]
- 44.
- Hjalmarson A. Effects of beta blockade on sudden cardiac death during acute myocardial infarction and the postinfarction period. Am J Cardiol. (1997);80:35–39. [PubMed: 9375948]
- 45.
- Horner SM, Dick DJ, Murphy CF, Lab MJ. Cycle length dependence of the electrophysiological effects of increased load on the myocardium. Circulation. (1996);94:1131–1136. [PubMed: 8790056]
- 46.
- Horner SM, Lab MJ, Murphy CF, Dick DJ, Zhou B, Harrison FG. Mechanically induced changes in action potential duration and left ventricular segment length in acute regional ischaemia in the in situ porcine heart. Cardiovasc Res. (1994);28:528–534. [PubMed: 8181042]
- 47.
- Ingber DE. Tensegrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol. (1997);59:575–599. [PubMed: 9074778]
- 48.
- James MA, Jones JV. The paradoxical role of left ventricular hypertrophy in wall stress-related arrhythmia. Journal of Hypertension. (1992);10:167–172. [PubMed: 1313480]
- 49.
- Ji S, John SA, Lu Y, Weiss JN. Mechanosensitivity of the cardiac muscarinic potassium channel. A novel property conferred by Kir3.4 subunit. J Biol Chem. (1998);273:1324–1328. [PubMed: 9430664]
- 50.
- Kaufmann RL, Lab MJ, Hennekes R, Krause H. Feedback interaction of mechanical and electrical events in the isolated mammalian ventricular myocardium (cat papillary muscle). Pflugers Arch. (1971);324:100–123. [PubMed: 5102600]
- 51.
- Kawakubo T, Naruse K, Matsubara T, Hotta N, Sokabe M. Characterization of a newly found stretch-activated KCa, ATP channel in cultured chick ventricular myocytes. Am J Physiol. (1999);276:H1827–H1838. [PubMed: 10362660]
- 52.
- Kelly M, Thompson P, Quinlan M. Prognostic significace of left ventricular ejection fraction after acute myocardia infarction. Br Heart J. (1985);53:16–24. [PMC free article: PMC481715] [PubMed: 3966947]
- 53.
- Kennedy HL. Beta blockade, ventricular arrhythmias, and sudden cardiac death. Am J Cardiol. (1997);80:29J–34J. [PubMed: 9375947]
- 54.
- Kentish JC, Wrzosek A. Changes in force and cytosolic Ca concentration after length changes in isolated rat ventricular trabeculae. Journal of Physiology. (1998);506:431–444. [PMC free article: PMC2230716] [PubMed: 9490870]
- 55.
- Kim D. A mechanosensitive K+ channel in heart cells. Activation by arachidonic acid. J Gen Physiol. (1992);100:1021–1040. [PMC free article: PMC2229139] [PubMed: 1484283]
- 56.
- Kim D, Sladek CD, Aguado-Velasco C, Mathiasen JR. Arachidonic acid activation of a new family of K+ channels in cultured rat neuronal cells. J Physiol. (1995);484(Pt 3):643–660. [PMC free article: PMC1157950] [PubMed: 7623282]
- 57.
- Kirber MT, Ordway RW, Clapp LH, Walsh JV Jr,, Singer JJ. Both membrane stretch and fatty acids directly activate large conductance Ca2+-activated K+ channels in vascular smooth muscle cells. FEBS Lett. (1992);297:24–28. [PubMed: 1551431]
- 58.
- Kowey PR. An overview of antiarrhythmic drug management of electrical storm. Can J Cardiol. (1996);12(Suppl B):3B–8B. [PubMed: 8616726]
- 59.
- Kuchan MJ, Frangos JA. Shear stress regulates endothelin-1 release via protein kinase C and cGMP in cultured endothelial cells. Am J Physiol. (1993);264:H150–H156. [PubMed: 8381608]
- 60.
- La Farge CG, Monroe RG, Gamble WJ, Rosenthal A, Hammond RP. Left ventricular pressure and norepinephrine efflux from the dennervated heart. Am J Physiol. (1970);219:519–524. [PubMed: 5448085]
- 61.
- Lab MJ. Mechanically dependent changes in action potentials recorded from the intact frog ventricle. Circ Res. (1978);42:519–528. [PubMed: 630669]
- 62.
- Lab MJ. Mechanoelectric feedback (transduction) in heart: concepts and implications. Cardiovascular Res. (1996);32:3–14. [PubMed: 8776398]
- 63.
- Lab MJ. Mechanosensitivity as an integrative system in the heart: an audit. Prog Biophysics and Molec Biol. (1999);71:7–27. [PubMed: 10070210]
- 64.
- Lab MJ, Allen DG, Orchard CH. The effects of shortening on myoplasmic calcium concentration and on the action potential in mammalian ventricular muscle. Circ Res. (1984);55:825–829. [PubMed: 6499137]
- 65.
- Lab MJ, Dick D, Harrison FG (1992) Propranolol reduces stretch arrhythmia in isolated rabbit heart. Journal of Physiology 446, p. 539P. Ref Type: Abstract.
- 66.
- Lab MJ, Zhou BY, Spencer CI, Seed WA. Length dependent changes of mechanical restitution in isolated superfused guinea pig papiallry muscle. Journal of Physiology. (1993);459:506P.
- 67.
- Le Guennec JY, White E, Gannier F, Argibay JA, Garnier D. Stretch-induced increase in resting intracellular calcium concentration in single guinea-pig ventricular myocytes. Experimental Physiology. (1991);76:975–978. [PubMed: 1768419]
- 68.
- Lerman BB, Engelstein ED, Burkhoff D. Mechanoelectrical feedback: role of beta-adrenergic receptor activation in mediating load-dependent shortening of ventricular action potential and refractoriness. Circ. (2001);104:486–490. [PubMed: 11468214]
- 69.
- Levy MN, Wiseman MN. Electrophysiologic mechanisms for ventricular arrhythmias in left ventricular dysfunction: electrolytes, catecholamines and drugs. J Clin Pharmacol. (1991);31:1053–1060. [PubMed: 1753009]
- 70.
- Luna EJ, Hitt AL. Cytoskeleton--plasma membrane interactions. Science. (1992);258:955–964. [PubMed: 1439807]
- 71.
- Maingret F, Patel AJ, Lesage F, Lazdunski M, Honore E. Mechano- or acid stimulation, two interactive modes of activation of the TREK-1 potassium channel. J Biol Chem. (1999);274:26691–26696. [PubMed: 10480871]
- 72.
- Mark MD, Herlitze S. G-protein mediated gating of inward-rectifier K+ channels. Eur J Biochem. (2000);267:5830–5836. [PubMed: 10998041]
- 73.
- Matsuda N, Hagiwara N, Shoda M, Kasanuki H, Hosoda S. Enhancement of the L-type Ca2+ current by mechanical stimulation in single rabbit cardiac myocytes. Circ Res. (1996);78:650–659. [PubMed: 8635223]
- 74.
- Meghji P, Nazir SA, Dick DJ, Bailey ME, Johnson KJ, Lab MJ. Regional workload induced changes in electrophysiology and immediate early gene expression in intact in situ porcine heart. J Mol Cell Cardiol. (1997);29:3147–3155. [PubMed: 9405188]
- 75.
- Morris CE. Mechanosensitive ion channels. J Memb Biol. (1990);113:93–107. [PubMed: 1690807]
- 76.
- Murphy CF, Lab MJ. Ischaemia induced alternans in action potential duration. European Journal of Cardiology. (1994);15:580–581. [PubMed: 8070489]
- 77.
- Odemuyiwa O, Malik M, Farrell T, Bashir Y, Staunton A, Poloniecki J, Camm AJ. Multifactorial prediction of arrhythmic events after myocardial infarction. Combination of heart rate variability and left ventricular ejection fraction with other variables. Pace - Pacing and Clinical Electrophysiology. (1991);14:1986–1991. [PubMed: 1721212]
- 78.
- Opie LH. Calcium antagonists, ventricular arrhythmias, and sudden cardiac death: a major challenge for the future. J Cardiovasc Pharmacol. (1991);18(Suppl 10):S81–S86. [PubMed: 1725011]
- 79.
- Opie LH. Mechanisms whereby calcium channel antagonists may protect patients with coronary artery disease. Eur Heart J. (1997);18(Suppl A):A92–104. [PubMed: 9049544]
- 80.
- Orts A, Alcaraz C, Delaney KA, Goldfrank LR, Turndorf H, Puig MM. Bretylium tosylate and electrically induced cardiac arrhythmias during hypothermia in dogs. Am J Emerg Med. (1992);10:311–316. [PubMed: 1616517]
- 81.
- Ovize M, Kloner RA, Przyklenk K. Stretch preconditions canine myocardium. Am J Physiol. (1994);266:H137–H146. [PubMed: 8304494]
- 82.
- Packer M, Gottlieb SS, Blum MA. Immediate and long-term pathophysiologic mechanisms underlying the genesis of sudden cardiac death in patients with congestive heart failure. Am J Med. (1987);82:4–10. [PubMed: 2882674]
- 83.
- Pathak CL. Effect of stretch on formation and conduction of electrical impulses in the isolated sinoauricular chamber of the frog's heart. Am J Physiol. (1958);192:111–113. [PubMed: 13498160]
- 84.
- Perez NG, de Hurtado MC, Cingolani HE. Reverse mode of the Na+-Ca2+ exchange after myocardial stretch: underlying mechanism of the slow force response. Circ Res. (2001);88:376–382. [PubMed: 11230103]
- 85.
- Petrou S, Ordway RW, Kirber MT, Dopico AM, Hamilton JA, Walsh JV Jr,, Singer JJ. Direct effects of fatty acids and other charged lipids on ion channel activity in smooth muscle cells. Prostaglandins Leukot Essent Fatty Acids. (1995);52:173–178. [PubMed: 7784455]
- 86.
- Pleumsamran A, Kim D. Membrane stretch augments the cardiac muscarinic K+ channel activity. J Membr Biol. (1995);148:287–297. [PubMed: 8747560]
- 87.
- Plopper GE, McNamee HP, Dike LE, Bojanowski K, Ingber DE. Convergence of integrin and growth factor receptor signaling pathways within the focal adhesion complex. Mol Biol Cell. (1995);6:1349–1365. [PMC free article: PMC301292] [PubMed: 8573791]
- 88.
- Podrid PJ. Potassium and ventricular arrhythmias. Am J Cardiol. (1990);65:33E–44E. [PubMed: 2178376]
- 89.
- Prasad AR, Logan SA, Nerem RM, Schwartz CJ, Sprague EA. Flow-related responses of intracellular inositol phosphate levels in cultured aortic endothelial cells. Circ Res. (1993);72:827–836. [PubMed: 8443870]
- 90.
- Prat AG, Cantiello HF. Nuclear ion channel activity is regulated by actin filaments. Am J Physiol. (1996);270:C1532–C1543. [PubMed: 8967456]
- 91.
- Pye MP, Black M, Cobbe SM. Comparison of in vivo and in vitro haemodynamic function in experimental heart failure: use of echocardiography. Cardiovascular Res. (1996);31:873–881. [PubMed: 8759242]
- 92.
- Pye P, Cobbe SM. Mechanisms of ventricular arrhythmias in cardiac failure and hypertrophy. Cardiovascular Res. (1992);26:740–750. [PubMed: 1451147]
- 93.
- Reiter MJ, Reiffel JA. Importance of beta blockade in the therapy of serious ventricular arrhythmias. Am J Cardiol. (1998);82:9I–19I. [PubMed: 9737650]
- 94.
- Reiter MJ. Effects of mechano-electric feedback: potential arrhythmogenic influence in patients with congestive heart failure. Cardiovascular Res. (1996);32:44–51. [PubMed: 8776402]
- 95.
- Sackin H. Mechanosensitive channels. Annu Rev Physiol. (1995);57:333–353. [PubMed: 7539988]
- 96.
- Sadoshima J, Izumo S. Mechanical stretch rapidly activates multiple signal transduction pathways in cardiac myocytes: potential involvement of an autocrine /paracrine mechanism. EMBO J. (1993);12:1681–1692. [PMC free article: PMC413382] [PubMed: 8385610]
- 97.
- Sadoshima J, Izumo S. The cellular and molecular response of cardiac myocytes to mechanical stress. Annu Rev Physiol. (1997);59:551–571. [PubMed: 9074777]
- 98.
- Sager PT. Modulation of antiarrhythmic drug effects by beta-adrenergic sympathetic stimulation. Am J Cardiol. (1998);82:20I–30I. [PubMed: 9737651]
- 99.
- Sideris DA, Pappas S, Siongas K, Grekas G, Argyri Greka O, Koundouris E, Foussas S. Effect of preload and afterload on ventricular arrhythmogenesis. J Electrocardiol. (1995);28:147–152. [PubMed: 7616146]
- 100.
- Sigurdson W, Ruknudin A, Sachs F. Calcium imaging of mechanically induced fluxes in tissue-cultured chick heart: role of stretch-activated ion channels. Am J Physiol. (1992);262:1110–1115. [PubMed: 1373571]
- 101.
- Singh BN. Antiarrhythmic drugs: a reorientation in light of recent developments in the control of disorders of rhythm. Am J Cardiol. (1998);81:3D–13D. [PubMed: 9537217]
- 102.
- Slinker BK, Campbell KB. Previous beat contraction history alters mechanical restitution in the isolated left ventricle. Cardiovascular Res. (1994);28:535–541. [PubMed: 8181043]
- 103.
- Slinker BK. Cardiac cycle length modulates cardiovascular regulation that is dependent on previos beat contraction history. Circ Res. (1991);69:2–11. [PubMed: 2054933]
- 104.
- Stewart DE, Ikram H, Espiner EA, Nicholls MG. Arrhythmogenic potential of diuretic induced hypokalaemia in patients with mild hypertension and ischaemic heart disease. Br Heart J. (1985);54:290–297. [PMC free article: PMC481898] [PubMed: 4041299]
- 105.
- Tabarean IV, Juranka P, Morris CE. Membrane stretch affects gating modes of a skeletal muscle sodium channel. Biophys J. (1999);77:758–774. [PMC free article: PMC1300370] [PubMed: 10423424]
- 106.
- Taggart P, Sutton PMI, Treasure T, Lab MJ, Runnalls M, O'Brien W, Swanton RH, Emanuel RW (1988) Contraction excitation feedback in man. Br.Heart J. 59, p. 109. Ref Type: Abstract. [PubMed: 3370766]
- 107.
- Tan JH, Liu W, Saint DA. Trek-like potassium channels in rat cardiac ventricular myocytes are activated by intracellular ATP. J Membr Biol. (2002);185:201–207. [PubMed: 11891578]
- 108.
- Tavi P, Han C, Weckstrom M. Mechanisms of stretch-induced changes in [Ca2+]i in rat atrial myocytes: Role of increased troponin C affinity and stretch-activated ion channels. Circ Res. (1998);83:1165–1177. [PubMed: 9831710]
- 109.
- Tobler HG, Gornic CC, Anderson RW, Benditt DG. Electrophysiologic properties of the myocardial infarction border zone: Effects of transient aortic occlusion. Surgery. (1986);100:150–156. [PubMed: 2426817]
- 110.
- Van Gelder IC, Brugemann J, Crijns HJ. Current treatment recommendations in antiarrhythmic therapy. Drugs. (1998);55:331–346. [PubMed: 9530541]
- 111.
- Van Wagoner DR. Mechanosensitive gating of atrial ATP-sensitive potassium channels. Circ Res. (1993);72:973–983. [PubMed: 8477531]
- 112.
- Von Planta M, Chamberlain D. Drug treatment of arrhythmias during cardiopulmonary resuscitation. A statement for the Advanced Life Support Working Party of the European Resuscitation Council. Resuscitation. (1992);24:227–232. [PubMed: 1336883]
- 113.
- Waller DG. Treatment and prevention of ventricular fibrillation: are there better agents? Resuscitation. (1991);22:159–166. [PubMed: 1684244]
- 114.
- Wang N, Ingber DE. Probing transmembrane mechanical coupling and cytomechanics using magnetic twisting cytometry. Biochem Cell Biol. (1995);73:327–335. [PubMed: 8703406]
- 115.
- Wang Z, Taylor LK, Denney WD, Hansen DE. Initiation of ventricular extrasystoles by myocardial stretch in chronically dilated and failing canine left ventricle. Cir. (1994);90:2022–2031. [PubMed: 7522991]
- 116.
- Watson PA, Haneda T, Morgan HE. Effect of higher aortic pressure on ribosome formation and cAMP content in rat heart. Am J Physiol. (1989);256:C1257–C1261. [PubMed: 2544096]
- 117.
- White E, Le Guennec JY, Nigretto JM, Gannier F, Argibay JA, Garnier D. The effects of increasing cell length on auxotonic contractions; membrane potential and intracellular calcium transients in single guinea-pig ventricular myocytes. Exp Physiol. (1993);78:65–78. [PubMed: 8448013]
- 118.
- Wiesfeld AC, Crijns HJ, Tuininga YS, Lie KI. Beta adrenergic blockade in the treatment of sustained ventricular tachycardia or ventricular fibrillation. Pacing Clin Electrophysiol. (1996);19:1026–1035. [PubMed: 8823828]
- 119.
- Yamada KM, Geiger B. Molecular interactions in cell adhesion complexes. Curr Opin Cell Biol. (1997);9:76–85. [PubMed: 9013677]
- 120.
- Zabel M, Koller BS, Sachs F, Franz MR. Stretch-induced voltage changes in the isolated beating heart: importance of the timing of stretch and implications for stretch-activated ion channels. Cardiovascular Res. (1996);32:120–130. [PubMed: 8776409]
- 121.
- Zhang Y, Ter Keurs HE. Effects of gadolinium on twitch force and triggered propagated contractions in rat cardiac trabeculae. Cardiovasc Res. (1996);32:180–188. [PubMed: 8776415]
- 122.
- Zhou BY, Harrison FG, Dick DJ, Horner SM, Murphy CF, Lab MJ. Ventricular arrhythmogenesis is enhanced by mechanelectric feedback in regional ischaemic heart of the anaesthetised pig. Journal of Physiology. (1993);473:184P.
- Introduction
- Mechanically mediated crosstalk (MMC) at the channel level
- Active myofibrillar interaction, calcium and mechanoelectric transduction (Fig. 1 at 7 o'clock; Fig. 2 myofibrils – upper left quadrant)
- Facilitators of MMC
- MMC in the intact heart: Regulation of cardiac function
- MMC and pathophysiology (Fig. 1 at 5 o'clock)
- Longer term mechanoelectric changes
- Conclusion and perspectives
- References
- Review Mechanosensitive-mediated interaction, integration, and cardiac control.[Ann N Y Acad Sci. 2006]Review Mechanosensitive-mediated interaction, integration, and cardiac control.Lab MJ. Ann N Y Acad Sci. 2006 Oct; 1080:282-300.
- Review Mechanosensitivity as an integrative system in heart: an audit.[Prog Biophys Mol Biol. 1999]Review Mechanosensitivity as an integrative system in heart: an audit.Lab MJ. Prog Biophys Mol Biol. 1999; 71(1):7-27.
- Review Engineering Aspects of Olfaction.[Neuromorphic Olfaction. 2013]Review Engineering Aspects of Olfaction.Persaud KC. Neuromorphic Olfaction. 2013
- Pharmacological investigation of the bioluminescence signaling pathway of the dinoflagellate Lingulodinium polyedrum: evidence for the role of stretch-activated ion channels.[J Phycol. 2013]Pharmacological investigation of the bioluminescence signaling pathway of the dinoflagellate Lingulodinium polyedrum: evidence for the role of stretch-activated ion channels.Jin K, Klima JC, Deane G, Dale Stokes M, Latz MI. J Phycol. 2013 Aug; 49(4):733-45. Epub 2013 Jun 21.
- Membrane stretch and cytoplasmic Ca2+ independently modulate stretch-activated BK channel activity.[J Biomech. 2010]Membrane stretch and cytoplasmic Ca2+ independently modulate stretch-activated BK channel activity.Zhao HC, Agula H, Zhang W, Wang F, Sokabe M, Li LM. J Biomech. 2010 Nov 16; 43(15):3015-9. Epub 2010 Jul 29.
- Mechanically Mediated Crosstalk in Heart - Mechanosensitivity in Cells and Tissu...Mechanically Mediated Crosstalk in Heart - Mechanosensitivity in Cells and Tissues
Your browsing activity is empty.
Activity recording is turned off.
See more...
 .
.