See "Essentials of Glycobiology, 4th Edition"
See the updated version of this chapter
Green plants constitute about half of the living matter on Earth and have a diversity ranging from simple green algae to flowering plants. Plants synthesize many of the same glycans that are found in animals and also produce a wide variety of unique glycans that have important roles during the life cycle of the plant. This chapter summarizes our current knowledge of plant glycobiology and shows some unique functions of glycans in these organisms.
BACKGROUND
Plants contain many glycans, especially in their cell walls. In fact, some of the earliest glycobiology studies were conducted on cellulose and other glycans of plant cell walls as well as on seed lectins, particularly those from leguminous plants. In the past several decades, however, most glycobiological research has focused on animal systems because of an increased interest in biomedicine and the availability of funding in that area. This biomedical emphasis is readily demonstrated by the high proportion of chapters of this book that are devoted to animal systems.
It is important to realize, however, that plant glycobiology has produced a number of findings of extreme interest to the biomedical community. The concept of oligosaccharide signaling in development originated in studies with plants (see Chapter 37), and a wide variety of plant lectins of different glycan specificities are currently used in biomedical research (see Chapter 26). Unique plant glycans provide major sources of food and fiber. Some plant species have easily manipulatable systems for genetic and molecular biology studies, including the expression of large amounts of glycoproteins.
Limited and unstable resources of oil have sparked a renewed interest in the use of plant-wall carbohydrates as sources of biomass for biofuel production. This may usher in a “golden age” for plant glycobiology, with rapid increases in our understanding of the function and synthesis of what appears to be the most structurally complex reservoir of carbohydrate structures in nature.
UNIQUE FEATURES OF THE PLANT SECRETORY PATHWAY
Most plants are not mobile and must grow in the environment in which their seeds are sown. They have evolved special defenses against predators such as insects, fungi, and other pathogens. Defense responses often involve the production of phenols and other toxic reagents, which are stored in the plant vacuoles. The vacuole is usually the largest organelle of mature plant cells and often accounts for most of the volume of the cell. This organelle is the plant equivalent of the animal lysosome and is a major component of the plant secretory system. In the seeds of many plant species, the vacuoles condense into compact organelles called protein bodies, which are packed with glycoproteins that are used as food upon germination.
Although glycoproteins enter the vacuole by way of the secretory system, plant cells do not use mannose-6-phosphate for targeting of proteins as is the case in mammalian cells (see Chapter 30), and the phosphotransferase employed by animal cells to direct proteins to lysosomes has not been found in plants. Several signals have been identified for vacuolar protein targeting, but none are glycan based.
The vacuole is also the site of synthesis and storage of a variety of polysaccharides, including fructans. These polysaccharides serve as reserve storage materials for energy production for the plants and may play a role in helping plants survive harsh environmental conditions and seed desiccation.
UNIQUE FEATURES OF PLANT N-GLYCANS
Most of the proteins that pass through the plant secretory system contain N-linked glycans of four main types: oligomannose, complex, hybrid, and paucimannose (Figure 22.1). No significant difference has been found between plants and animals in the initial stages of N-glycan synthesis, including the transfer of the dolichol oligosaccharide precursor and the control of protein folding in the endoplasmic reticulum (ER) (see Chapter 8). However, plants show some unique modifications of N-glycans upon passage through the Golgi.
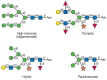
FIGURE 22.1
Types of N-glycans found in plants. Symbol Key:
As in vertebrates, the glycoproteins enter the plant Golgi with oligomannose-type N-glycans, and many of these glycans are trimmed in the cis-Golgi. As mentioned previously, in contrast to animal cells, no phosphorylation of mannose occurs in the plant cis-Golgi. After passage to the medial-Golgi and the addition of N-acetylglucosamine to the distal mannose of the core by N-acetylglucosamine transferase I (GnT-I) as in vertebrates, two specific plant modifications occur (Figure 22.2). The first modification is the addition of xylose in β1-2 linkage to the core β-mannose. This modification occurs in the medial-Golgi and is unique to plants. The second modification, which occurs in the trans-Golgi, is the addition of fucose in α1-3-linkage to the asparagine-linked N-acetylglucosamine residue. This core fucosylation has also been found in invertebrates. Both the xylotransferase and fucosyltransferase that catalyze the above reactions have been isolated, and substrate specificity studies have shown that they require at least one terminal N-acetylglucosamine residue. Each reaction is independent because neither enzyme requires the addition of the other sugar for activity. Following these additions, the glycan is trimmed by α-mannosidase II and a second N-acetylglucosamine is added to the trimmed chain by GnT-II. As in animal cells, some plant glycans do not undergo further mannose trimming and proceed through the Golgi as hybrid-type glycans (Figure 22.2).

FIGURE 22.2
Processing of N-glycans in the plant secretory system. Only those events that are unique to plants are shown in detail. Symbol Key:

The complex and hybrid-type glycans are further processed as they pass through the trans-Golgi. As in animals, these modifications include the addition of galactose and fucose (Figure 22.2). Although there is evidence for a gene resembling a sialyltransferase in plants, there are no conclusive data for the presence of sialic acid in plants. After leaving the Golgi, plant glycoproteins are either secreted from the cell or transported to the vacuoles. Many of the glycans found in the vacuoles are of the paucimannose type, suggesting that they are trimmed by vacuolar glycosidases.
Many of the glycosyltransferases that catalyze the processing of glycans in the plant Golgi have specificities similar to those of their animal homologs. cDNAs show homologies with the animal genes. The cDNAs of the unique core β1-2 xylosyltransferase and α1-3 fucosyltransferase have been sequenced. Core α1-3 fucosyltransferase is related to the Lewis fucosyltransferase family, whereas the β1-2 xylosyltransferase is unique and unrelated to other known glycosyltransferases.
These core modifications make plant N-glycans highly immunogenic and may also cause allergies to some plant products. Because plants are being used for mass production of animal glycoproteins, considerable attention is focused on preventing the addition of these residues to glycans to prevent an immune response.
PLANT GLYCOLIPIDS
The most abundant glycolipids in plants are the glycoglycerol lipids. The two main members of this class are mono- and digalactosyldiacylglycerol (Figure 22.3), which are present in all plants. Some species of plants also contain tri- and tetragalactosyldiacylglycerol. The synthesis of all of these galactolipids begins with the synthesis of diacylglycerol, which can occur in either the ER membrane or the chloroplast membrane. The former pathway produces mostly C16 fatty acids at the sn2 position and C18 fatty acids at the sn3 position, whereas the latter pathway produces C18 fatty acids at both positions. Each of these fatty acids is then desaturated to 16:3 or 18:3 acyl groups. Monogalactosyldiacylglycerol (MGDG) is synthesized by the transfer of galactose from UDP-galactose to diacylglycerol by an MGDG synthase. It may then be converted to digalactosyldiacylglycerol (DGDG) by the transfer of galactose from UDP-galactose to MGDG by a DGDG synthase. Both of these reactions occur primarily in the outer chloroplast membrane and the products are transported to the inner membrane and the thylakoid membranes of the chloroplasts. Small amounts of both galactolipids can also be found in the plasma membranes of the cells. The mechanism of galactolipid exchange among the membranes is not yet understood.
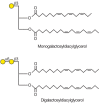
FIGURE 22.3
Most abundant plant galactolipids. Symbol Key:

The thylakoid membranes of chloroplasts are the site of photosynthesis, and it has long been suggested that the abundance of galactolipids in the thylakoids may play an important role in photosynthesis. Crystallization of the photosystem I complex of cyanobacteria and studies of a MGDG synthase mutant in Arabidopsis support this idea. Another glycerolipid synthesized from diacylglycerol is the sulfolipid, sulfoquinovosyldiacylglycerol. It is abundant in the thylakoid membrane, which suggests that it may also play a role in photosynthesis.
In addition to the above glycolipids, plant membranes also contain glycosphingolipids (see Chapter 10), which are found as components of the plasma membrane. Compared to animals, few plant glycosphingolipids have been characterized. Although such sugars as galactose and fucose are found in these glycans, no gangliosides have been identified.
Some plant glycoproteins have glycosylphosphatidylinositol-type (GPI) anchors (see Chapter 11). Only a few plant GPI anchors have been studied, and these contain a phosphoceramide core characteristic of yeast and Dictyostelium. A complete structure of the glycan of the GPI anchor of a pea arabinogalactan protein has been determined as -Manα1-2Manα1-6Manα1-4GlcNH2-inositol. A unique feature of this structure is that more than 50% of the anchors are further substituted at the mannose attached to the glucosamine unit (see Chapter 11) with β1-4 galactose.
PLANTS CONTAIN A LARGE VARIETY OF O-GLYCANS
The most abundant O-glycans in plants are the hydroxyproline-rich glycoproteins (HRGPs) found in the cell wall. These proteins have a high content of proline that is converted to hydroxyproline by prolyl hydroxylases in the ER. Hydroxyproline residues are O-glycosylated as they pass through the ER and Golgi apparatus. The degree and type of hydroxyproline glycosylation depend on the primary structure of each protein backbone and are dictated by the arrangement of hydroxyprolines in the sequence. The glycosylation of hydroxyproline is found in plants and Dictyostelium (see Chapter 17) and is initiated by the addition of an arabinose or galactose residue in plants. Contiguous hydroxyproline residues are arabinosylated, whereas clustered noncontiguous hydroxyproline residues are galactosylated. Plant proteins with these modifications are usually divided into three subclasses as discussed below. These proteins can also be O-glycosylated through serine residues and occasionally through threonine. Another feature of these cell wall proteins is that they can form intra- and intermolecular cross-linkages between tyrosines, a reaction that is catalyzed by specific peroxidases in the cell wall.
The first subclass of HRGPs are the extensins, which are characterized by a Ser(Hyp)4 repeat and contain about 50–60% (w/w) glycan, most of which consists of short chains of one to four arabinose residues bound to hydroxyproline. They also have a small number of single galactose residues α-linked to serine residues. The second HRGP subclass, termed the proline/hydroxyproline-rich glycoproteins, is primarily distinguished from the extensins by amino acid sequence. Not as much is known of their glycosylation patterns, but the extent of glycosylation is known to range from 3% to 70% (w/w). Both of these two subclasses of HRGPs are thought to play a structural role in the cell wall, and their expression is developmentally regulated. Wounding and fungal attack of plant tissues have been found to induce the synthesis of some of these proteins.
The third HRGP subclass is the arabinogalactans. They have a neutral to acidic peptide backbone and a glycan content of more than 90% (w/w) containing both galactosyl-O-serine and galactosyl-O-hydroxyproline linkages of 30–150 residues. Most of these chains consist of a β1-3 galactose backbone with extensive branches of β1-6-linked galactose residues. These chains are substituted with β1-3-linked and terminal arabinose and other residues. The arabinogalactans are secreted into the cell wall and can also be anchored to the plasma membrane. Proposed roles for these glycans include participation in signaling, development, cell expansion, cell proliferation, and somatic embryogenesis.
Another interesting group of hydroxyproline-rich, O-glycosylated glycoproteins are the lectins of the Solanaceous family of plants. This group includes the potato, tomato, and thorn-apple lectins, which contain two domains: a nonglycosylated carbohydrate-binding domain that is rich in cysteine and glycine and a highly glycosylated hydroxyproline-rich domain. The glycan chains in this latter domain are similar to those of the extensins and most contain three or four residues of arabinose β-linked to hydroxyproline and single galactose residues α-linked to serine. These lectins are found in the vacuoles of Solanaceous plants and they are thought to play a role in defense by binding to chitin present on the surface of pathogens.
In addition to unique types of O-glycans, plants also contain O-GalNAc in α-linkage to serine and threonine residues (see Chapter 9). O-GlcNAc occurs in cytoplasmic and nuclear proteins at serine and threonine residues where phosphorylation occurs (see Chapter 17).
THE GLYCAN-RICH CELL WALL HELPS CONTROL GROWTH OF PLANT CELLS
The cell wall is the extracellular matrix of the plant cell. It must be strong enough to support the plant and withstand the internal turgor pressure of the cell. Yet, it must also be able to extend during cell growth and participate in interactions with the environment. The cell wall is composed of a network of strong rod-like molecules tethered together by cross-linked glycans and embedded in a highly organized matrix of acidic polysaccharides, some of which partially resemble the proteoglycans of animal cells.
Plant cells use two types of cell walls to perform these functions, termed the primary and secondary walls. The primary wall is the first wall laid down in dividing and growing plant cells, and it is the terminal wall in many cells in the soft parts of the plant (e.g., surrounding palisade cells in leaves). The primary wall contains 80–90% polysaccharide and 10–20% protein. Cellulose, hemicellulose, and pectin are the main polysaccharide components in the primary wall (Figure 22.4). Secondary walls, which surround specialized cells that serve a structural role such as fiber cells and xylem cells in vascular bundles, generally have less pectin, more cellulose, and a different class of hemicellulose (β1-4 xylans) and are often rigidified by lignification.

FIGURE 22.4
Model of the primary cell wall (type I) found in most flowering plants (except grasses). Cellulose microfibrils are embedded in a hemicellulose (e.g., xyloglucan) and pectin matrix. The model depicts partial hydrogen bonding of xyloglucan to cellulose (more...)
Instead of the collagen fibrils used by animal cells to provide the tensile strength of the extracellular matrix, plant cells synthesize cellulose, a polysaccharide that consists of long, parallel, linear β1-4 glucan chains hydrogen-bonded into crystalline microfibrils containing about 36 polysaccharide chains. Cellulose is the most abundant biopolymer in nature. The synthesis of these chains occurs on the plasma membrane of the cell where cellulose synthase is assembled into rosette structures containing six subunits. Each of these subunits is thought to be composed of six cellulose synthases, each of which adds glucose onto the growing glucan chains using cytoplasmic UDP-glucose generated from sucrose by the reverse action of sucrose synthase. The cellulose fibers that are produced are parallel with one another (Figure 22.4) and are in alignment with the cytoskeleton underlying the membrane.
The cellulose fibers are coated and cross-linked with one another by glycans called hemicelluloses. Xyloglucan is the major hemicellulose in the type I primary cell walls of most higher plants (Figure 22.5). It is a polymer consisting of repetitive segments of four residues of a β1-4 glucan backbone substituted on the first three positions with α1-6 xylose. The xyloses at positions 2 and 3 can have galactose attached in a β1-2 linkage and a fucose is usually found in an α1-2 linkage to the galactose at C-2. Other, less abundant polysaccharides, such as the glucomannans and galactomannans, are also thought to help orient the cellulose fibrils. In grasses and other commelinoid monocots with type II walls, glucuronoarabinoxylan is the major hemicellulose, whereas during expansion in grasses β1-3/β1-4 mixed-linkage glucans are prevalent.
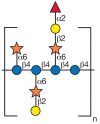
FIGURE 22.5
Repeating subunit found in xyloglucan. Symbol Key:

As cells increase in volume, there is a loosening of the hydrogen bonds that bind hemicelluloses to cellulose, and an enzymatic cleavage of some of the xyloglucan chains allows the internal osmotic pressure of the cell to push apart the cellulose microfibrils. This process is accompanied by a laying down of layers of new cellulose microfibrils with their associated hemicellulose polymers. In the cell plate of dividing cells or at times during growth, callose (a β1-3 glucan polymer) is found among the cellulose fibrils.
The cellulose-xyloglucan network is embedded in a matrix of complex acidic polysaccharides called pectins (Figure 22.6). Pectins are the most structurally complex plant-wall polysaccharides. They are somewhat comparable to the glycosaminoglycans found in the extracellular matrix of animal cells, but they are not sulfated and it is not clear which, if any, are attached to protein. A number of different pectins have been isolated. Homogalacturonans represent approximately 65% of pectin polysaccharides and they are linear chains of α1-4-linked galacturonic acid residues, which may be methyl-esterified on some of their carboxyl groups and acetylated at the C-2 or C-3 hydroxyls. Pectin methyl esterases in the cell wall can alter the extent of methylation of the pectin, allowing it to associate with other pectin molecules by calcium links between the acidic groups. The extent of methylation can therefore determine the porosity of the pectin matrix in the wall and the extent of cell-wall stiffening. The pectin rhamnogalacturonan-I (RG-I) has a repeating backbone of GalAα1-2Rhaα1-4. The galacturonic acid in the backbone may be O-acetylated at C-2 or C-3. Approximately 20–80% of the rhamnose residues are substituted at C-4 with linear or branched side chains of arabinose, galactose, or arabinogalactans. Another pectin, rhamnogalacturonan-II (RG-II), has a backbone of α1-4-linked galacturonic acid residues and is substituted with four highly complex and conserved side chains that contain 12 different sugars in more than 20 different linkages. A nonasaccharide and an octasaccharide chain are attached to C-2 of the backbone galacturonic acid residues, and two different disaccharide chains are attached to C-3 of the backbone making a highly branched structure. RG-II exists most often in plant cell walls as a borate diester formed between the apiose residue in two RG-II octasaccharide side chains of two distinct RG-II molecules. Because RG-II is embedded in a homogalacturonan chain, these RG-II dimers affect wall porosity and function. Mutations in RG-II structure that cause reduced RG-II dimer formation have dramatic effects on plant growth including dwarfism (see the next section).

FIGURE 22.6
Schematic structure of pectin showing the three main pectic polysaccharides: rhamnogalacturonan I (RG-I, left) and rhamnogalacturonan II (RG-II, right), at each side of a homogalacturonan (HG) chain. A region of substituted galacturonan, known as xylogalacturonan (more...)
Depending on the cell type and the stage of cell growth, there are many variations in wall fine structure and in the interactions between cellulose, hemicellulose, and pectin. It is expected that ongoing intensive studies will elucidate the role of wall glycans in cell expansion and tissue morphology. A number of cell wall proteins such as the HRGP classes of proteins discussed in the previous section are also immersed in the pectin matrix. The cell wall is thus emerging as a highly complex intricate network (Figure 22.4). It contains a wide variety of glycans that can interact with one another and with proteins to help adapt the wall to growth and mediate the interaction of the cell with its environment. The structure of this matrix is altered by the cell during cell division and development and in response to environmental stimuli by differential synthesis and modifications of the matrix components or by addition of new ones. Recent success in identifying genes that encode cell-wall polysaccharide biosynthetic enzymes, including many of those required for xyloglucan and cellulose synthesis and some of those required for pectin synthesis, is providing a framework for an increased understanding of how the plant wall is synthesized. Progress in this area will impact our ability to provide superior plant wall–based products. The complexity of the cell wall, including the crystalline nature of cellulose microfibrils and the interactions of hemicellulose, pectin, and lignin with cellulose, also makes the wall recalcitrant to deconstruction to the simple sugars needed for bioethanol production. An increased understanding of wall synthesis will also improve our ability to generate plants that can serve as better sources of biomass for bioethanol production.
PLANT MUTANTS ARE PROVIDING CLUES TO GLYCAN FUNCTION
In recent years, the production and characterization of plant mutants have provided clues to the function of plant glycans. Arabidopsis thaliana is a good model plant because of its relatively small genome, short life cycle, and ease of growth. A variety of banks of mutants are available for screening for glycosylation defects. An early mutant identified by this process was the cgl mutant, which was found by screening leaf extracts of chemically produced mutants with antisera against complex glycans. This mutant lacked GnT-I activity. It produced no complex glycans, but accumulated Man5GlcNAc2. This mutant had no apparent effect on the development and morphology of the plants. More recent studies on a mutant of a subunit of the oligosaccharyltransferase complex, dgll, demonstrated that reduced N-linked glycosylation leads to a variety of effects in plants, ranging from reduced cell elongation to embryonic lethality. These results show that N-glycosylation is essential for normal plant growth and development.
Another mutant Arabidopsis line, mgd1, was identified by screening transfer-inserted mutant lines for plants with defects in chloroplast biogenesis. The plants of this line were chlorotic (deficient in chlorophyl production) and had a change in chloroplast ultrastructure. They were found to contain only about 50% of normal levels of MGDG, thus helping to support the proposed role for this glycolipid in photosynthesis.
The mur1 mutant of Arabidopsis was identified by making hydrolysates of cell walls of chemically produced mutants and screening the alditol acetate derivatives by gas-liquid chromatography (see Chapter 47). These plants were found to be deficient in an isoform of GDP-mannose-4,6-dehydrase (see Chapter 4) and therefore they were deficient in fucose. As a result, reduced amounts of RG-II dimer were formed because L-galactose substituted for fucose in RG-II, and dwarf plants with fragile cell walls were created. These observations provide evidence for a crucial role for RG-II in plant growth.
GLYCANS AND GLYCOCONJUGATES SERVE AS SIGNALS TO REGULATE PLANT DEVELOPMENT AND INTERACTIONS WITH THE ENVIRONMENT
Glycan signals help regulate plant development, defense, and other interactions of plants with the environment. Early studies of plant–pathogen interactions showed that glycans arising from the degradation of the cell wall of either the plant or the pathogen can act as elicitors to trigger defense responses to the pathogen. These responses consist of the activation of a number of plant genes that lead to the cross-linking of cell wall polymers, the production of additional cell wall components (such as HRGPs), the production of glycanases (such as β1-3 glucanases and chitinases) that can degrade the pathogen cell wall, and the production of phytoalexins that kill the invading pathogen. The end result is that necrotic spots appear at the site of infection and limit the spread of the pathogen. The structures of some of the glycans that are released upon degradation of plant and pathogen cell walls and that can act as elicitors are shown in Figure 22.7. These glycans are quite small, containing 4–20 sugar residues. As mentioned below, a few plant receptors that recognize some of these signals have been identified, but little is known about the signaling mechanism.
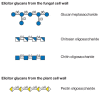
FIGURE 22.7
Glycans from fungal and plant cell walls that elicit plant defense responses. Symbol Key:

Xyloglucan fragments from plant cell walls act as signals to trigger the expansion of cell walls and inhibit auxin-induced growth in stems. The most potent fragment is a nonasaccharide. Partial hydrolysates of plant cell walls also affect plant growth and organogenesis. Oligosaccharides released from the pectic polysaccharides (including oligogalacturonides released from homogalacturonan) can alter plant organogenesis in vitro and stimulate plant defense responses. Changes in the concentrations of some plant hormones have been found to stimulate the production of the glycosidases involved in cell-wall degradation and these hormones may regulate this process.
Glycan signals also initiate the nitrogen-fixing Rhizobium–legume symbiosis. In response to flavonoids produced by the legume roots, Rhizobium bacteria are stimulated to produce increased amounts of a lipochitooligosaccharide called a Nod factor. The Nod factor consists of a short chitin backbone with a long-chain fatty acid at the nonreducing end. The Nod factor triggers the deformation of the root hairs and the differentiation of some of the cells in the root cortex to form a new organ called the nodule. The Rhizobia nestle in the curl of the root hairs and cause the plant to synthesize an infection thread, which they follow to take up residence in the nodule. Picomolar amounts of isolated Nod factor added to the roots can cause root hair deformation and nodule formation. Recognition between particular strains of Rhizobia and certain species of legumes is specific. This specificity is due to the fact that the different Rhizobia strains make different derivatives of Nod factor that vary in the substituents attached at different positions of the chitin backbone. A generic Nod factor structure is shown in Figure 37.4 (see Chapter 37 on glycan signals).
GLYCAN-BINDING PROTEINS IN PLANTS
Plant lectins were first discovered in the late 1800s and since then they have been found in every plant species studied. They are particularly abundant in the seeds of leguminous plants and can account for about 10% of the nitrogen in the total seed extract. The lectins were initially identified on the basis of their carbohydrate-binding activities by hemagglutination or precipitin techniques. More recently, they have been tentatively identified by screening for DNA homology.
In general, lectins within the same family of plants are very similar in structure, but they differ from the structures of lectins from other plant families. Chapters 26–29 discuss various types of plant lectins, but little is known about their natural function. Their abundance in seeds and in the bark of some trees led to hypotheses that they may be involved in pathogen recognition, and some lectins are clearly toxic to insects. An initial idea that lectins recognize glycan signals produced by Rhizobia for symbiosis is incorrect. In fact, none of the conventional lectins from plants have been shown to recognize any of the glycan signals discussed above. Searches in plants for ligands for these conventional lectins have so far been unsuccessful. Studies of the distribution and localization of lectins throughout the life cycles of plants show that some species contain more than one lectin encoded by separate genes with different spatial and temporal expression. The question of biological function for these highly characterized lectins remains open at this time.
Some of the conventional legume lectins have been found to have a separate hydrophobic binding site in addition to their carbohydrate site. This hydrophobic site binds to adenine and derivatives of the plant hormone cytokinin. A recent finding has shown that the seed lectin from the legume Dolichos biflorus is also a lipoxygenase. Putative receptors for glycans that play signaling roles in plants have recently been identified. Although these proteins are lectins by definition, they do not resemble the conventional lectins identified from these plants. Receptors for chitin oligosaccharides that act as elicitors have been identified in the plasma membranes of several plants, and one of them was recently isolated and cloned. A novel lectin was isolated from legume roots and found to bind with Nod factors produced by rhizobial symbionts of that plant; this lectin was also found to have nucleotide phosphorylase activity and was thus called an LNP (lectin nucleotide phosphorylase). In both cases, suppression of expression of the proteins inhibited the signaling response suggesting that these proteins indeed play roles in signal recognition (see Chapter 37).
FURTHER READING
- Carpita NC, Gibeaut DM. Structural models of primary cell walls in flowering plants: Consistency of molecular structure with the physical properties of the walls during growth. Plant J. 1993;3:1–30. [PubMed: 8401598]
- Lerouge P, Cabanes-Macheteau M, Rayon C, Fischette-Laine AC, Gomord V, Faye L. N-Glycoprotein biosynthesis in plants: Recent developments and future trends. Plant Mol Biol. 1998;38:31–48. [PubMed: 9738959]
- Sommer-Knudsen J, Bacic A, Clarke A. Hydroxyproline-rich plant glycoproteins. Phytochemistry. 1998;4:483–497.
- Gaspar Y, Johnson KL, McKenna JA, Bacic A, Schultz CI. The complex structures of arabinogalactan-proteins and the journey towards understanding function. Plant Mol Biol. 2001;47:161–176. [PubMed: 11554470]
- Kieliszewski MJ. The latest hype on Hyp-O-glycosylation codes. Phytochemistry. 2001;57:319–323. [PubMed: 11393510]
- Ridley BL, O’Neill MA, Mohnen D. Pectins: Structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry. 2001;57:929–967. [PubMed: 11423142]
- Dormann P, Benning C. Galactolipids rule in seed plants. Trends Plant Sci. 2002;7:112–118. [PubMed: 11906834]
- Reiter WD. Biosynthesis and properties of the plant cell wall. Curr Opin Plant Biol. 2002;5:536–542. [PubMed: 12393017]
- Wilson IBH. Glycosylation of proteins in plants and invertebrates. Curr Opin Struct Biol. 2002;12:569–577. [PubMed: 12464307]
- Ritsema T, Smeekens S. Fructans: Beneficial for plants and humans. Curr Opin Plant Biol. 2003;6:223–230. [PubMed: 12753971]
- Kelly AA, Dormann P. Green light for galactolipid trafficking. Curr Opin Plant Biol. 2004;7:262–269. [PubMed: 15134746]
- Lerouxel O, Cavalier DM, Liepman AH, Keegstra K. Biosynthesis of plant cell wall polysaccharides—A complex process. Curr Opin Plant Biol. 2006;9:621–630. [PubMed: 17011813]
- Himmel ME, Ding SY, Johnson DK, Adney WS, Nimlos MR, Brady JW, Foust TD. Biomass recalcitrance: Engineering plants and enzymes for biofuels production. Science. 2007;315:804–807. [PubMed: 17289988]
- Somerville C. Biofuels. Curr Biol. 2007;17:R115–119. [PubMed: 17307040]
Publication Details
Author Information and Affiliations
Authors
Marilynn E Etzler and Debra Mohnen.Copyright
Publisher
Cold Spring Harbor Laboratory Press, Cold Spring Harbor (NY)
NLM Citation
Etzler ME, Mohnen D. Viridiplantae. In: Varki A, Cummings RD, Esko JD, et al., editors. Essentials of Glycobiology. 2nd edition. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2009. Chapter 22.






