NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Chattopadhyay A, editor. Serotonin Receptors in Neurobiology. Boca Raton (FL): CRC Press/Taylor & Francis; 2007.
Abstract
This chapter explores emerging roles for calmodulin as a regulator of 5-HT receptor function and describes recent work suggesting that calmodulin may be a so-called RIP (receptor-interacting protein) that binds to and modifies the functions of G protein-coupled 5-HT receptors.
INTRODUCTION
5-HT Receptors
5-hydroxytryptamine (5-HT, serotonin) is a monoamine that serves as a neurotransmitter, mitogen and hormone. 5-HT influences cells in the brain, nervous system and peripheral tissues by binding to, and activating, a diverse array of cell surface receptors. 5-HT receptors have been divided into seven families based on their molecular structures, pharmacology, and signal transduction linkages. One of the families of 5-HT receptors (5-HT3) is comprised of ligand-gated ion channels (termed 5-HT3A–5-HT3E), whereas six of the families (5-HT1, 5-HT2, 5-HT4, 5-HT5, 5-HT6, 5-HT7) are comprised of heptahelical receptors that signal primarily through coupling to heterotrimeric guanine nucleotide binding and regulatory proteins (G proteins). The G protein-coupled 5-HT receptor families have been characterized by pharmacological properties, amino acid sequences, gene organization, and second messenger coupling pathways. These receptors are integral membrane proteins with seven putative hydrophobic transmembrane domains connected by three intracellular loops (termed iL1–iL3) and three extracellular loops (termed eL1–eL3). The amino terminus of each receptor is oriented toward the extracellular space, whereas the carboxyl terminus (CT) is oriented toward the cytoplasm. These receptors possess conserved or common sites for posttranslational modifications. The extracellular domains are typically glycosylated and possess cysteine residues that may participate in disulfide bonds, which provide structural constraints on the conformation of the receptors. The intracellular domains variably contain phosphorylation sites for a wide array of kinases, palmitoylation sites in the CT for lipid anchoring, and various potential sites for protein–protein interactions.
Recent work has demonstrated an unanticipated diversity of signals linked to the various G protein-coupled receptors (GPCRs), including 5-HT receptors. The work also has led to a growing awareness that GPCR signaling diversity can be modulated by diverse regulatory proteins, many of which are likely to bind directly to the receptor. Those proteins that bind directly to specific motifs on GPCRs are termed receptor-interacting proteins (RIPs). The topic of this chapter focuses on the interaction of Ca2+–calmodulin (CaM), which is potential 5-HT receptor RIP, with G protein-coupled 5-HT receptors.
G Protein-Coupled Receptor-Interacting Proteins
A growing body of work suggests that GPCRs can bind to a variety of proteins. This should not be entirely surprising in that, very early on, it was recognized that GPCRs could bind to G proteins. Subsequently, molecular methods allowed the identification of other proteins (arrestins, protein kinase A, protein kinase C (PKC), and G protein-coupled receptor kinases) that bind to GPCRs and modify their functions and interactions with G proteins (48,63,64). Within the last several years, novel protein–protein interactions with GPCRs have been reported, including physical interactions with transmembrane proteins such as other GPCRs (47,76), and RAMPs (receptor associated modifying proteins) (17,19,58).
The most striking and well-characterized examples of GPCR RIPs are the RAMPs. The human calcitonin receptor like receptor (hCRLR) has drastically altered the ligand-binding properties depending upon the RAMP with which it is expressed. It becomes a functional calcitonin gene-related peptide receptor when cotransported with human RAMP1 to the cell surface, whereas it becomes a functional adrenomedullin receptor when cotransported with RAMP2 (53). Another excellent example is provided by the angiotensin II AT1A receptor, which has been shown to physically interact with ARAP1 (AT1 receptor associated protein 1), thereby promoting recycling of the receptor to the plasma membrane, enhancing its signaling (37). The importance of this relationship was highlighted by the recent finding that transgenic mice overexpressing ARAP1 develop hypertension and renal hypertrophy (36). Although the interaction of RAMPS with the hCRLR, and ARAP1 with the AT1A receptor, are quite remarkable, other GPCRs have more recently been demonstrated in a preliminary manner to interact with nonmembrane-spanning proteins such as 14-3-3 proteins (18,26,62,65,90), CaM (16,94), PDZ (PSD-95 discs-large ZO-1) proteins (8,75), and cytoskeletal elements (75,83,86). Most of these reports are preliminary, and the functional consequences of the interactions of GPCRs with these putative RIPs have not been completely elucidated. Indeed, unequivocal verification that these proteins actually interact with GPCRs in intact cells has generally not been completed.
For example, cytoskeletal elements (microtubules and spinophilin) have been shown to physically bind to peptides derived from the α2-adrenergic receptor (75,82,86). The dopamine D2 receptor i3L binds spinophilin (but not to its related protein, neurabin) in yeast two-hybrid and fusion protein interaction assays, although the functional significance of this interaction remains undefined. Spinophilin is expressed ubiquitously and contains multiple protein interaction domains (81). The α2-adrenergic receptor i3L was shown to interact with amino acids 169–255 of spinophilin, in a region between its F-actin-binding and phosphatase 1 regulatory domains. Because the interaction between the α2-adrenergic receptor and spinophilin was increased by agonist treatment, it would seem to serve a dynamic function (24). The same group showed that the disruption of microtubules with colchicine or nocodazole could alter the apical steady state distribution and delivery of A1-adenosine receptors and increase the binding capacity of α2B-adrenergic receptors expressed in MDCK-II cells (31). The authors suggested that the cytoskeleton and G proteins interact with distinct domains of the i3L of the α2B-adrenergic receptor and that the cytoskeletal effects may be mediated, independent of G proteins. These studies show that abundantly expressed cellular proteins, such as components of the actin cytoskeleton (or CaM), might play important roles in GPCR function, possibly independent of G proteins.
At least five well-characterized examples of GPCR RIPs involve 5-HT receptors. These include the interactions of caveolin-1 and PSD-95 with the 5-HT2A receptor, MUPP1 (multi-PD2 domain protein 1) with the 5-HT2C receptor, and EBP50 (ezrin/radixin/moesin-binding phosphoprotein 50) and SNX27 (new sorting nexin) with the 5-HT4a receptor. MUPP1 interacts with a PDZ domain of the 5-HT2B receptor. PDZ recognition motifs are contained in many signaling proteins, and these domains mediate key protein–protein interactions. For GPCRs, the best characterized example of a functionally significant GPCR interaction with a PDZ protein is the β2-adrenergic receptor, which interacts with NHERF (Na+/H+ exchanger regulatory factor)/EBP50 (32) via agonist-dependent binding of the first PDZ domain of NHERF to the CT of the β2-adrenergic receptor, resulting in regulation of the type 3 sodium-proton exchanger, NHE-3 (33). PDZ interactions of the β2-adrenergic receptor with NHERF have also been shown to control recycling of internalized receptors through an endocytic sorting pathway that leads to lysosomal degradation (34).
In yeast two-hybrid screens, 5-HT2 receptors interact with a multivalent PDZ protein called MUPP1. Coimmunoprecipitations and mutagenesis studies have shown that an SXV sequence at the extreme CT of the 5-HT2C receptor selectively interacts with the 10th PDZ domain of MUPP1 (23). This interaction is functionally important in that it induces a conformational change in MUPP1 (23), attenuates receptor phosphorylation on serine 453 (S453), and attenuates desensitization (35).
Bhatnagar and colleagues surprisingly demonstrated that caveolin-1 binds to 5-HT2A receptors in rat synaptic membranes, C6 glioma cells, and transfected HEK-293 cells (14). They demonstrated that this interaction is significant in that caveolin-1 (but not caveloin-2) facilitated increases in intracellular Ca2+ through increased coupling of the receptor with Gqα. This specificity of the interaction between caveolin-1 and 5-HT2A receptor was further supported by experiments showing that caveolin-1 knockdown greatly diminished both 5-HT2A and P2Y purinergic receptor signaling without altering PAR-1 (protease activated receptor-1 thrombin receptor) signaling. The same group also demonstrated that interaction of PSD-95 with the 5-HT2A receptor increases coupling of the receptor to Gqα/11 and blunts receptor internalization without altering its desensitization (9,95,96).
5-HT2 receptors are not the only 5-HT receptors that have been shown to interact functionally with RIPs. The subcellular localization of the 5-HT4a receptor is regulated by its binding to two RIPs. EBP50 also called NHERF) and SNX27a (a new sorting nexin, also called Mrt1a) have been shown to interact directly with the 5-HT4a receptor and to modify its subcellular localization. SNX27a redirects the 5-HT4a receptor into early endosomes, whereas EBP50/NHERF recruits the receptor into ezrin-containing microvilli (41). In the remainder of this chapter, we will describe findings that support the idea that CaM is an important 5-HT receptor RIP.
Calmodulin
CaM is a member of the superfamily of EF-hand proteins. CaM has four EF-hand motifs, each of which is composed of two α-helices connected by a 12-amino acid loop. When intracellular Ca2+ levels rise to the low micromolar range, all four EF-hands bind Ca2+, inducing a conformational change that results in binding to various target proteins (4,5,27,97). In CaM’s non Ca2+-bound state, the α-helices of the EF hands are aligned in a nearly parallel fashion, which is termed the closed conformation, whereas in its Ca2+-bound state, the EF hands are oriented in a nearly perpendicular fashion termed the open conformation. We liken this to two outstretched arms with hands ready to grasp a target. CaM is shaped like a barbell, and when it binds to a target α-helical peptide, it folds over the peptide (27,97), assuming a globular configuration. The crystal structure of CaM and other biochemical evidence have allowed for the construction of algorithms to predict potential CaM-binding proteins (97). Those algorithms identify α-helical peptides that have groupings of hydrophobic amino acids interspersed with positively charged amino acids. In helical wheel diagrams, the positively charged amino acids are often located on the opposite “side” of the peptide from the hydrophobic amino acids. Interestingly, many GPCRs (including 5-HT receptors) possess similar motifs that could serve as CaM-binding domains.
CaM is classically activated by increases in intracellular Ca2+, resulting in conformational changes in CaM and activation of target proteins. However, other mechanisms of regulating CaM are possible, although poorly understood. Primary among the alternate mechanisms of activating CaM is phosphorylation of CaM on serine–threonine or tyrosine residues. CaM can be phosphorylated by receptor and nonreceptor tyrosine kinases and serine–threonine kinases (25,30,38,68,77,80). Casein kinase II phosphorylates CaM in vitro on threonine and serine residues (T79, S81, S101, and T117) (78), whereas Ca2+-calmodulin-dependent protein kinase IV phosphorylates CaM primarily on T44 (40). In contrast, the EGF (epidermal growth factor) receptor phosphorylates Y99 of bovine brain CaM (10,28) with a stoichiometry of 1:1 (11), and the insulin receptor phosphorylates Y99 and Y138 of CaM in CHO-IR (Chinese hamster ovary-insulin receptor) cells (42,79). Nonreceptor tyrosine kinases Src (30) and Jak2 also phosphorylate CaM in response to a number of stimuli (31,32,49,59), but the identity of the tyrosine residues that are phosphorylated by these kinases have not been established. In any case, because nearly all of the G protein-coupled 5-HT receptors either increase intracellular Ca2+ or activate tyrosine kinases, any number of 5-HT receptors could reasonably be expected to activate CaM.
Role of CaM in 5-HT Receptor Signaling
There is a small but growing literature suggesting that CaM and CaM-dependent enzymes and effectors play key roles in signaling pathways initiated by various G protein-coupled 5-HT receptors. In that regard, several vascular and neuronal effects of 5-HT have been attributed to CaM. For example, an undefined 5-HT receptor subtype induces contractions in human umbilical artery that are mediated via CaM (54); an undefined 5-HT receptor subtype mediates contractions of bovine middle cerebral artery through CaM and CaM-dependent myosin light chain kinase (60); 5-HT evokes outward currents in dissociated rat hippocampal pyramidal neurons, and increased membrane conductance are sensitive to CaM inhibitors (93). CaM dysfunction has been suggested to be involved in the mechanism of enhanced intracellular Ca2+ response to 5-HT in bipolar disorder (89).
Gi/o-coupled 5-HT1A receptors exert tonic inhibition of AMPA receptors in excitatory hippocampal neurons through Ca2+-calmodulin-dependent protein kinase II (CaMK-II) (57,84,85). Similarly, 5-HT1A receptors inhibit the NR2B subunit involved in NMDA receptor-mediated ionic and synaptic currents in prefrontal cortex pyramidal neurons, and the NMDA receptor through CaMK-II (21,99). The 5-HT1A receptor transfected into CHO cells manifests CaM-dependent internalization and Erk (extracellular receptor kinase) activation (29).
CaM is involved in various functions of the Gq/11-coupled 5-HT2 receptors, including their desensitization (43,71). 5-HT2A (but not 5-HT2C) receptor desensitization requires CaM and CaMK-II (13). For example, 5-HT2A receptors regulate cyclic AMP accumulation in embryonic cortex A1A1 neuronal cells by protein kinase C-dependent and CaM-dependent mechanisms (12). 5-HT2A-mediated BDNF release in C6 glioma cells is mediated by CaMK-II (55). 5-HT2A receptors in PC12 cells activate extracellular signal-regulated kinase and tyrosine phosphorylation of a number of proteins by a CaM and tyrosine kinase-dependent pathway (69). 5-HT2A receptors in failing human cardiac ventricles mediate activation of Ca2+-calmodulin-dependent myosin light chain kinase (70). 5-HT2A receptors in mesangial cells increase COX-2 (cyclooxygenase 2) expression via CaM and CaMK-II (34,88). 5-HT2B and 5-HT2C receptors also couple to CaM-dependent signals. 5-HT2B receptors significantly potentiate NMDA-induced depolarizations in frog spinal cord motoneurones via calmodulin but not CaMK-II (39). 5-HT2A/2B receptors cause cardiac hypertrophy via calcineurin, which is a CaM-dependent phosphatase (20). 5-HT2C receptors expressed in A9 cells evoke Ca2+-dependent outward currents, which rapidly desensitize. This desensitization involves CaM (15).
Gs-coupled 5-HT7 receptors, but not 5-HT6 receptors, activate CaM-sensitive adenylyl cyclases, AC1 and AC8 through mobilization of Ca2+ (7). Thus, it is clear that a number of 5-HT receptors couple to diverse signaling pathways through Gi/o, Gq/11, and Gs. However, it is not at all clear whether the CaM-dependent effects are due to increased intracellular Ca2+, phosphorylation of CaM, or direct binding to the G protein-coupled 5-HT receptors. The notion that CaM can interact with 5-HT receptors is explored in the section titled “Interaction of CaM with GPCRs,” and in the subsections of “5-HT Receptors and Calmodulin.”
Interaction of CaM with GPCRs
CaM has been demonstrated to bind to peptides derived from at least six GPCRs, including glutamate mGlu7 receptor (58), μ-opioid OP3 receptor (21), dopamine D2 receptor (22), 5-HT1A receptor (91), 5-HT2A receptor (92), and angiotensin AT1A receptor (65). CaM is a ubiquitous Ca2+ sensor that can regulate numerous enzymes involved in signaling of GPCRs. Although the best characterized interactions between GPCRs and CaM occur downstream of the receptor, Sadee’s group detected a CaM-binding motif in i3L of the OP3 receptor. They showed that peptides derived from the OP3 receptor i3L strongly bound to CaM and could diminish binding between CaM and immuno-purified OP3 receptors. Agonist stimulation of the OP3 receptor resulted in release of CaM from the plasma membrane. CaM also had some effects on receptor functions in that CaM reduced basal and agonist-stimulated 35S-labeled guanosine 5′-3-O-(thio)triphosphate [35S] GTPβS incorporation (a measure of G protein activity). Overexpression of CaM or antisense to CaM inversely affected basal and agonist-induced G protein activity. They interpreted the results to mean that CaM competes with G proteins for binding to opioid receptors and that CaM could serve as an independent second messenger that is released from the membrane upon receptor stimulation (21).
CaM has been proposed to fulfill two major functions by binding to GPCRs, including blunting the G protein coupling and blunting the receptor phosphorylation. Bofill-Cardona et al. used a variety of methods to demonstrate that a peptide fragment of the dopamine D2 receptors binds to CaM, and that CaM suppresses D2 receptor activation of G proteins. Moreover, the effects of CaM were relatively specific as there were no effects on A1 adenosine and Mel1A melatonin receptors (22). For the mGlu7 receptor, there appears to be a complex interaction between serine–threonine phosphorylation of the CT of the receptor, binding of G protein β γ subunits, and CaM (58–61). Thus, there is tantalizing evidence that CaM can directly bind to, and regulate, various GPCRs. The evidence for direct interaction of CaM with 5-HT1A and 5-HT2A receptors is described in the sections titled “5-HT1a Receptor” and “5-HT2 Receptors,” respectively.
5-HT RECEPTORS AND CALMODULIN
5-HT1A Receptor
Because the 5-HT1A receptor has been linked to CaM in a few preliminary reports (21,29,57,84,85,99), it is a logical candidate to study the possible interaction between the receptor and CaM. The 5-HT1A receptor i3L contains a number of sequences that could potentially interact with RIPs. In that regard, we identified two putative CaM-binding domains in the proximal and distal juxtamembrane regions of the i3L of the 5-HT1A receptor, using a computer search algorithm (http://calcium.uhnres.utoronto.ca/ctdb/pub_pages/general/index.htm), for which scoring is based on evaluation criteria including hydropathy, α-helical propensity, residue charge, helical class, residue weight, and hydrophobic residue content (98). As a general rule, CaM-binding regions are characterized by the presence of several hydrophobic residues interspersed with several positively charged residues, often forming amphipathic α-helices. CaM-binding regions described to date have been divided into several motifs based on the distance between key hydrophobic residues. The putative proximal i3L CaM-binding region of the 5-HT1A receptor (Y215GRIFRAARFRIRKTVKKVEKTG237) was identified as a 1–12 motif, with key hydrophobic residues at positions 1 and 12. The more proximal sequence is also a putative G protein contact site. The more distal sequence (A330KRKMALARERKTVKTLGIIMG352) conforms to a standard CaM-recognition motif of hydrophobic residues at positions 1, 8, and 14 interspersed with positively charged amino acids. This distal peptide is in an intriguing location, as it overlaps with a G protein-contact site and a PKC phosphorylation site in the 5-HT1A receptor (45,46,50,51,72). The predicted locations of the two CaM-binding regions of the 5-HT1A receptor (as well as other 5-HT receptors) are depicted in Figure 4.1.

FIGURE 4.1
Schematic illustration of putative CaM-binding domains of various G protein-coupled 5-HT receptors. The upper face of each receptor represents the extracellular domains, and the lower face represents the intracellular domains. The grey bars represent (more...)
In order to illustrate the amphipathic, α-helical nature of the two putative CaM-binding regions from the 5-HT1A receptor, we modeled them with an α-helical wheel algorithm (http://marqusee9.berkeley.edu/kael/helical.htm), which showed that each peptide is predicted to form an α-helix with hydrophobic amino acids (λ) and charged amino acids (+) located on opposite sides of each helix (Figure 4.2), typical of the amphipathic nature of CaM-binding sites.
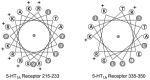
FIGURE 4.2
Helical wheel projections of putative CaM-binding domains of the 5-HT1A receptor. Projections were made using a computer algorithm (http://marqusee9.berkeley.edu/kael/helical.htm). Each circle represents an amino acid residue. Grey circles indicate hydrophobic (more...)
We used a number of methods (coimmunoprecipitation, gel-shift analysis, surface plasmon resonance spectroscopy [SPRS], slot blot, dansyl chloride spectroscopy, and bioluminescence resonance energy transfer [BRET]) to demonstrate that the 5-HT1A receptor interacts with CaM in vitro and in live cells and to calculate the affinities of the two sites for CaM (91). The apparent affinity of the proximal i3L putative CaM site was 87 ± 23 nM, and the apparent affinity of the distal i3L site was 1.70 ± 0.16 μM. Regarding the functional effects of the CaM sites, we have discovered that CaM-binding and phosphorylation of the 5-HT1A receptor i3L peptides by (PKC) or protein kinase C are mutually antagonistic processes in vitro, suggesting a possible role for CaM in the regulation of 5-HT1A receptor phosphorylation and desensitization (91). Furthermore, we have found that binding of CaM to the 5-HT1A receptor decreases coupling to Gi/o G proteins as assayed by GTPgS binding to crude membrane preparations (Turner et al., unpublished data).
These data support the idea that the 5-HT1A receptor contains high- and moderate-affinity CaM-binding regions that regulate G protein coupling, receptor phosphorylation, and (perhaps) desensitization.
The idea that CaM interferes with receptor phosphorylation is not limited to 5-HT receptors. Indeed, G protein-coupled receptor kinase (GRK)-mediated phosphorylation of rhodposin is inhibited by CaM (66). Additionally, phosphorylation of a conserved serine residue in the CT of group III metabotropic glutamate receptors by PKC inhibits CaMbinding (1). Thus, there seems to be a mutually antagonistic relationship between phosphorylation and CaM binding for GPCRs and for other signaling proteins. For example, phosphorylation of GRK2 (G protein-coupled receptor kinase 2) by PKC abolishes the ability of CaM to inhibit GRK2 (44), and phosphorylation of the MARCKS proteins (myristoylated alanine rich C-kinase substrate) inhibits CaM binding (35,52). However, this effect is not universal in that phosphorylation of a calcineurin peptide by CaMK-II does not significantly alter the binding of CaM when compared to the nonphosphorylated peptide (22).
Why would phosphorylation and CaM binding be mutually antagonistic? Although the structural basis for this antagonism is not known, it should be intuitive that phosphorylation, by adding a negative charge to a target protein, could disrupt the CaM recognition motif that relies on positive charges. Phosphorylation might also induce conformational changes in the target protein, perhaps by disrupting electrostatic tethering of the positively charged face of the peptide to acidic (negatively charged) phospholipids that are resident on the inner leaflet of the plasma membrane. The inner leaflet of mammalian plasma membranes typically contains between 15–30% of acidic lipids, primarily phosphatidylserine. There is increasing awareness that peptides can interact with plasma membrane lipids through positive charge domains and, especially, with negatively charged lipids in the inner leaflet of the plasma membrane (87). Indeed, electrostatic interactions between positive charges in the transmembrane domains of the S4 family of voltage-sensitive ion channels and negative charges on the inner plasma membrane induce a conformational change in the channels that results in pore opening and closing (56). The electrostatic interaction of positive charge domains with negatively charged lipids would be expected to occur selectively on the plasma membrane, rather than to other internal membranes, in that an inner leaflet of the plasma membrane has far more electrostatic charge than other cellular membranes (24,61).
Conversely, CaM could attenuate phosphorylation by competing with or blocking the access of various kinases to phosphorylation sites within or near to the CaM-binding domains. Alternatively, CaM could mask the positive charges in its target-binding domains, thereby disrupting the electrostatic interaction of the CaM-binding domains with the plasma membrane, resulting in conformational changes that are less favorable to phosphorylation.
Other 5-HT1 Receptors
Virtually nothing is known regarding the interaction of CaM with other 5-HT1 receptors, although a computer algorithm predicts that each 5-HT1 receptor might have several CaM sites (http://calcium.uhnres.utoronto.ca/ctdb/pub_pages/general/index.htm) (98). The 5-HT1B receptor has three potential CaM-binding domains, including a moderate- to high-likelihood site in the proximal juxtamembrane region of the i3L (L227YGRIYVEARSRILK241), an extended high-likelihood site in the distal juxtamembrane region of the i3L (N288QVKVRVSDALLEKKKLMAARERKATKTLGIILG321), and a moderate-likelihood site in the i1L (N67AFVIATVYRTRK79) (Figure 4.2).
The 5-HT1D receptor has three potential CaM-binding domains, including a moderate-likelihood site in the proximal juxtamembrane region of the i3L (L212LIILYGRIYRAARNRI228), an extended high-likelihood site in the distal juxtamembrane region of the I3L (N275HVKIKLADSALERKRISAARERKATKILGIIL307), and a moderate-likelihood site in the i2L K148RRTAGHAATMIAIV162).
The 5-HT1E receptor has three potential CaM-binding domains, including a moderate- to high-likelihood site in the i2L (a123itnaieyarkrtakraalmiltv146), and two moderate-likelihood sites in the proximal and distal juxtamembrane regions of the i3L (y203riyhaakslyqkr216 and s282strerkaarilglilg298).
The 5-HT1F receptor has two potential CaM-binding domains, including a high-likelihood site in the proximal and distal juxtamembrane regions of i3L (l198ilyykiyraaktlyhkrqas218 and i283sgtrerkaattlglilg300). Figure 9.2 clearly shows that all of the putative 5-HT-receptor CaM-binding domains reside in juxtamembrane regions that are also thought to be critical for G protein coupling, supporting the idea that CaM-binding to 5-HT receptors either shields the receptor from G proteins and/or induces conformational changes that could alter G protein coupling.
5-HT2 Receptors
5-HT2A receptors in various neuronal and peripheral cells mediate numerous effects through the intermediate actions of CaM (12,23,31,33,43,69,71,88) or CaM-dependent enzymes such as CaMK-II (3,13,55) and myosin light chain kinase (70). We therefore assessed the sequence of the human 5-HT2A receptor for possible CaM-binding sites. As a receptor that couples to Gq/11-type G proteins, the 5-HT2A receptor is capable of stimulating phosphoinositide turnover and subsequent increases in intracellular Ca2+ (73). Accordingly, we postulated that the 5-HT2A receptor might exert some of its Ca2+-sensitive intracellular effects by directly interacting with CaM. A search of the amino acid sequence using a computer algorithm (http://calcium.uhnres.utoronto.ca/ctdb/pub_pages/general/index.htm) (98) revealed two novel potential CaM-binding motifs, located in the i2L (S184RFNSRTKAFLKIIAVWTI202) and the juxtamembrane region of the CT (P367LVY TLFNKTYRSAFSRYIQ396) of the 5-HT2A receptor. Both sequences contain consensus phosphorylation sites and are potentially important for G protein coupling, suggesting that interaction of CaM with those sites could play roles in regulating receptor function. The CaM-binding region in the i2L of the 5-HT2A receptor conforms to a 1-8-14 motif, which is characterized by hydrophobic residues at positions 1, 8, and 14. The putative CaM-binding domain in the CT of the 5-HT2A receptor conforms to a 1-10 motif, with critical hydrophobic residues separated by 8 amino acids.
In order to illustrate the amphipathic nature of the putative CaM-binding sequences of the 5-HT2A receptor, we modeled them with an α-helical wheel algorithm (http://marqusee9.berkeley.edu/kael/helical.htm). Figure 4.3 shows that both putative CaM-binding domains of the 5-HT2A receptor contain clusters of positively charged amino acids on one side of the α-helix, with mostly hydrophobic amino acids concentrated on the opposite side.

FIGURE 4.3
Helical wheel projections of putative CaM-binding domains of the 5-HT2A receptor. Projections were made using a computer algorithm (http://marqusee9.berkeley.edu/kael/helical.htm). Each circle represents an amino acid residue. Grey circles indicate hydrophobic (more...)
We used a variety of techniques (coimmunoprecipitation, gel-shift analysis, slot blot, dansyl chloride spectroscopy, and BRET) to demonstrate that the 5-HT2A receptor interacts with CaM in vitro and in live cells, and to calculate the affinities of each of the two sites for CaM. Peptides from each of the sites bound to CaM in a Ca2+-dependent fashion, with the i2L peptide binding with an apparent higher affinity than that of the CT peptide, based on mobility shifting of CaM in a nondenaturing gel shift assay. We used fluorescence emission spectral analyses of dansyl-CaM to calculate the apparent KD values of 65 ± 30 nM for the i2L peptide and 168 ± 38 nM for the CT peptide (92).
Because the putative CT CaM-binding domain overlaps with a putative PKC site, we tested whether CaM and PKC exerted mutually antagonistic effects on this peptide sequence. We demonstrated that the CT peptide was readily phosphorylated by PKC in vitro and that CaM-binding and phosphorylation of this peptide were antagonistic. These results suggest that there is a potential role for CaM in the regulation of 5-HT2A receptor phosphorylation and desensitization (similar to what was shown for the 5-HT1A receptor in the section titled “5-HT1a Receptor”). We also tested whether CaM had an effect on G protein coupling of the 5-HT2A receptor by measuring [35S]GTPγS binding in the presence and absence of 5-HT. Those experiments showed that CaM decreases 5-HT2A receptor-mediated [35S]GTPγS binding to NIH-3T3 cell membranes, supporting a possible role for CaM in modulating receptor-G protein coupling. The i2L peptide (but not the CT peptide) was able to stimulate [35S]GTPγS binding, and this effect was blocked by CaM, suggesting that the i2L is critical for G protein activation. In contrast, the CT peptide (but not the i2L peptide) was a strong substrate for PKC-induced phosphorylation. These results strongly support two regulatory roles for CaM in 5-HT2A receptor function. First, CaM appears to blunt receptor coupling to G proteins through interaction with the i2L. Second, CaM blunts phosphorylation (and possibly desensitization) of the CT peptide of the 5-HT2A receptor (92). Thus, the 5-HT2A receptor contains two high-affinity-CaM-binding domains that appear to play important roles in both the initiation and the termination of receptor signaling.
The evidence for important roles of CaM in regulating other 5-HT2 receptors is less clear than for the 5-HT2A receptors. 5-HT2B receptors potentiate NMDA-induced depolarizations in frog spinal cord motor neurons through CaM, but not through elevations of intracellular Ca2+ or activation of CaMK-II (39). The human 5-HT2B receptor has one high-likelihood CaM-binding domain, (L382FNKTFRDAFGRYITCNYRATKSVKTLR409), which is located at the juxtamembrane region of the CT of the receptor.
There are two reports showing that calcineurin, which is a CaM-dependent phosphatase, inhibits the desensitization of the 5-HT2C receptor (2,15). The 5-HT2C receptor has a high-likelihood CaM-binding domain (V367YTLFNKIYRRAFSNYLRCNYK388) at the juxtamembrane region of the CT of the receptor, as well as two moderate-likelihood CaM-binding domains in the i2L (F165NSRTKAIMKIAIVW179) and distal i3L (A302INNERKASKVLGIVF317). Thus, it is possible that all three of the major subtypes of 5-HT2 receptors might be regulated through direct interactions with CaM, although that possibility has not been rigorously examined for the 5-HT2B and 5-HT2C receptors.
Other 5-HT Receptors (5-HT4, 5-HT5, 5-HT6, 5-HT7)
We were unable to find references mentioning the coupling of 5-HT4, 5-HT5, or 5-HT6 receptors to CaM. However, each of those receptors has potential CaM-binding domains. The human 5-HT4A receptor has two putative CaM-binding domains, including a high-likelihood site in iL1 (V44CWDRQLRKIKTNYFIVSLA63), and a moderate-likelihood site in the proximal juxtamembrane region of the CT (N308PFLYAFLNKSFRRAFLI325). The human 5-HT5a receptor has a moderate-likelihood site in the proximal juxtamembrane region of i3L (N206PFLYAFLNKSFRRAFLI224) and a high-likelihood site at the distal juxtamembrane region of i3L (D252SRRLATKHSRKALKASLTLGIL273). The human 5-HT6 receptor has two putative CaM-binding sites in the juxtamembrane regions of the i3L, including a potential moderate-likelihood site in the proximal i3L (D259SRRLATKHSRKALKASLTLGIL275) and a high-likelihood site in the distal i3L (H312ERKNISIFK REQKAATTLG IIVGA335).
In contrast to the human 5-HT4, 5-HT5, and 5-HT6 receptors, 5-HT7a receptors have been functionally linked to CaM in that they have been shown to activate CaM-sensitive adenylyl cyclases, AC1 and AC8, whereas the 5-HT6 receptor do not activate them (7). In their study, Baker and colleagues showed that the 5-HT6 receptor behaved in a typical manner for Gs-coupled receptors in that it stimulated AC5, a Gs-sensitive adenylyl cyclase, but not AC1 or AC8. AC1 and AC8 typically are not activated by Gs-coupled receptors in vivo. In contrast, the 5-HT7a receptor stimulated AC1 and AC8 by increasing intracellular Ca2+ (7).
The 5-HT7a receptor has three potential CaM-binding domains, two of which are in the juxtamembrane regions of i3L, including a proximal moderate-likelihood site (Y259YQIYKAARKSAAKHKF275) and a distal high-likelihood site (R313KNISIFK REQKAAT-TLG IIVGA335). The 5-HT7a receptor also has an unusual CaM-binding motif in the proximal juxtamembrane region of the CT (F381IYAFFNRDLRTTYRS397). This site contains a sequence that is termed an ilimaquinone or IQ motif, which is a consensus site for Ca 2+-independent CaM binding (74). The IQ motif is widely distributed in a variety of CaM-binding proteins, often in association with classical Ca2+-dependent binding motifs. IQ motif-containing proteins possess a dazzling array of biological functions including signal transduction, phosphorylation, cytoskeletal regulation, trafficking, cell cycle control, and polarized growth (74). The presence of the IQ motif in the 5-HT7a receptor is intriguing in that IQ motifs play critical roles in neuronal growth and plasticity, and in assembling neuronal signal transduction complexes (6).
The presence of the IQ motif in the human 5-HT7a receptor is also intriguing at the molecular level. One group has shown that the presence of the IQ motif can influence the binding of CaM to classical Ca2+-dependent CaM-binding motifs. They showed that the IQ motif in an abundant neuronal protein, PEP-19, accelerates the slow association and dissociation of Ca2+ from the C-domain of free CaM 40–50-fold, and also increases the rate of dissociation of Ca2+ from CaM when CaM is bound to CaMK-II. Thus, the IQ motif could regulate the dynamics of Ca2+-binding to both free CaM and CaM bound to target proteins. The authors of this study pointed out that this relationship is critical in that the binding of Ca2+ to the C-domain of CaM is a rate-limiting step for CaM-dependent enzyme activation (67). Scenarios in which the 5-HT7a receptor IQ motif enhances the dynamic interaction of CaM with the two putative juxtamembrane CaM-binding domains in the i3L of the 5-HT7a receptor, or with CaM target enzymes colocalized within a signaling complex with the 5-HT7a receptor, could be easily envisioned. Indeed, we speculate that the IQ motif could facilitate CaM-binding to GPCRs that heterodimerize with the 5-HT7a receptor. Those possibilities would be interesting to pursue through further experimentation.
CONCLUSIONS
There is a growing awareness of the potential importance of the interplay between G protein-coupled 5-HT receptors, CaM, and CaM-dependent enzymes in critical functions of neuronal and nonneuronal cells. The fact that all known G protein-coupled 5-HT receptors possess putative CaM-binding motifs, as described in this chapter using a computer algorithm (98), raises the possibility that some of the CaM-dependent effects of 5-HT receptors could be mediated by, or facilitated by, direct binding of CaM to the receptors of interest. Indeed, both 5-HT1A and 5-HT2A receptors possess bona fide CaM-binding domains that appear to regulate G protein coupling and receptor phosphorylation (91,92). Thus, CaM is a potentially important 5-HT receptor RIP. These insights open up new avenues of investigation for all G protein-coupled 5-HT receptors. In particular, it will be important to develop mutant 5-HT receptors that maintain normal G protein coupling, but which have impaired CaM-binding in order to fully elucidate the roles of direct binding of CaM in 5-HT receptor signal transduction, trafficking, and desensitization.
Acknowledgments
The authors gratefully acknowledge support from the Medical and Research Services of the Department of Veterans Affairs (Merit Awards and a REAP award to JRR and MNG, and a Veterans Integrated Service Network-7 Career Development Award to AKG), the National Institutes of Health (DK52448 and GM63909 to JRR, DK59950 to AKG, and GM08716 to JHT), the American Heart Association Mid-Atlantic Affiliate (a predoctoral fellowship to JHT), and a joint endowment sponsored by the Medical University of South Carolina and Dialysis Clinics, Incorporated (to JRR). The majority of the work by the authors described herein was performed in VA space leased from the Medical University of South Carolina. Shared equipment grants from the Department of Veterans Affairs and the National Institutes of Health (S10 RR13005) made portions of this work possible.
REFERENCES
- 1.
- Airas JM, Betz H, El Far O. PKC phosphorylation of a conserved serine residue in the C-terminus of group III metabotropic glutamate receptors inhibits calmodulin binding. FEBS Lett. 2001;494:60. [PubMed: 11297735]
- 2.
- Aiyar J, Grissmer S, Chandy KG. Full-length and truncated Kv1.3 K+ channels are modulated by 5-HT1c receptor activation and independently by PKC. Am J Physiol. 1993;265:C1571. [PubMed: 7506490]
- 3.
- Arvanov VL, Liang X, Russo A, Wang RY. LSD and DOB: interaction with 5-HT2A receptors to inhibit NMDA receptor-mediated transmission in the rat prefrontal cortex. Eur J Neurosci. 1999;11:3064. [PubMed: 10510170]
- 4.
- Babu YS, Bugg CE, Cook WJ. Structure of calmodulin refined at 2.2 Å resolution. J Mol Biol. 1988;204:191. [PubMed: 3145979]
- 5.
- Babu YS, Sack JS, Greenhough TJ, Bugg CE, Means AR, Cook WJ. Three-dimensional structure of calmodulin. Nature. 1985;315:37. [PubMed: 3990807]
- 6.
- Bahler M, Rhoads A. Calmodulin signaling via the IQ motif. FEBS Lett. 2002;513:107. [PubMed: 11911888]
- 7.
- Baker LP, Nielsen M, Impey S, Metcalf MA, Poser SW, Chan G, Obrietan K, Hamblin MW, Storm DR. Stimulation of type 1 and type 8 Ca2+/calmodulin-sensitive adenylyl cyclases by the Gs-coupled 5-hydroxytryptamine subtype 5-HT7A receptor. J Biol Chem. 1998;273:17469. [PubMed: 9651336]
- 8.
- Becamel C, Figge A, Poliak S, Dumuis A, Peles E, Bockaert J, Lubbert H, Ullmer C. Interaction of serotonin 5-hydroxytryptamine type 2C receptors with PDZ10 of the multi-PDZ domain protein MUPP1. J Biol Chem. 2001;276:12974. [PubMed: 11150294]
- 9.
- Becamel C, Gavarini S, Chanrion B, Alonso G, Galeotti N, Dumuis A, Bockaert J, Marin P. The serotonin 5-HT2A and 5-HT2C receptors interact with specific sets of PDZ proteins. J Biol Chem. 2004;279:20257. [PubMed: 14988405]
- 10.
- Benaim G, Cervino V, Villalobo A. Comparative phosphorylation of calmodulin from trypanosomatids and bovine brain by calmodulin-binding protein kinases. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1998;120:57. [PubMed: 9827017]
- 11.
- Benguria A, Hernandez-Perera O, Martinez-Pastor MT, Sacks DB, Villalobo A. Phosphorylation of calmodulin by the epidermal-growth-factor-receptor tyrosine kinase. Eur J Biochem. 1994;224:909. [PubMed: 7925415]
- 12.
- Berg KA, Clarke WP, Chen Y, Ebersole BJ, McKay RDG, Maayani S. 5-Hydroxytryptamine type 2A receptors regulate cyclic AMP accumulation in a neuronal cell line by protein kinase C-dependent and calcium/calmodulin-dependent mechanisms. Mol Pharmacol. 1994;45:826. [PubMed: 8190100]
- 13.
- Berg KA, Stout BD, Maayani S, Clarke WP. Differences in rapid desensitization of 5-hydroxytryptamine2A and 5-hydroxytryptamine2C receptor-mediated phospholipase C activation. J Pharmacol Exp Ther. 2001;299:593. [PubMed: 11602671]
- 14.
- Bhatnagar A, Sheffler DJ, Kroeze WK, Compton-Toth BA, Roth BL. Caveolin-1 interacts with 5-HT2A serotonin receptors and profoundly modulates the signaling of selected Gaq-coupled protein receptors. J Biol Chem. 2004;279:34614. [PubMed: 15190056]
- 15.
- Boddeke HW, Hoffman BJ, Palacios JM, Hoyer D. Calcineurin inhibits desensitization of cloned rat 5-HT1C receptors. Naunyn Schmiedebergs Arch Pharmacol. 1993;348:221. [PubMed: 7694158]
- 16.
- Bofill-Cardona E, Kudlacek O, Yang Q, Ahorn H, Freissmuth M, Nanoff C. Binding of calmodulin to the D2-dopamine receptor reduces receptor signaling by arresting the G protein activation switch. J Biol Chem. 2000;275:32672. [PubMed: 10926927]
- 17.
- Bomberger JM, Spielman WS, Hall CS, Weinman EJ, Parameswaran N. Receptor activity-modifying protein (RAMP) isoform-specific regulation of adrenomedullin receptor trafficking by NHERF-1. J Biol Chem. 2005;280:23926. [PubMed: 15805108]
- 18.
- Brock C, Boudier L, Maurel D, Blahos J, Pin JP. Assembly-dependent surface targeting of the heterodimeric GABAB Receptor is controlled by COPI but not 14-3-3. Mol Biol Cell. 2005;16:5572. [PMC free article: PMC1289403] [PubMed: 16176975]
- 19.
- Buhlmann N, Leuthauser K, Muff R, Fischer JA, Born W. A receptor activity modifying protein (RAMP)2-dependent adrenomedullin receptor is a calcitonin gene-related peptide receptor when coexpressed with human RAMP1. Endocrinology. 1999;140:2883. [PubMed: 10342881]
- 20.
- Bush E, Fielitz J, Melvin L, Martinez-Arnold M, McKinsey TA, Plichta R, Olson EN. A small molecular activator of cardiac hypertrophy uncovered in a chemical screen for modifiers of the calcineurin signaling pathway. Proc Natl Acad Sci USA. 2004;101:2870. [PMC free article: PMC365712] [PubMed: 14976250]
- 21.
- Cai X, Gu Z, Zhong P, Ren Y, Yan Z. Serotonin 5-HT1A receptors regulate AMPA receptor channels through inhibiting Ca2+/calmodulin-dependent kinase II in prefrontal cortical pyramidal neurons. J Biol Chem. 2002;277:36553. [PubMed: 12149253]
- 22.
- Calalb MB, Kincaid RL, Soderling TR. Phosphorylation of calcineurin, effect on calmodulin binding. Biochem Biophys Res Commun. 1990;172:551. [PubMed: 2173570]
- 23.
- Chen H, Li H, Chuang DM. Role of second messengers in agonist up-regulation of 5-HT2A receptor binding sites in cerebellar granule neurons: involvement of calcium influx and a calmodulin-dependent pathway. J Pharmacol Exp Ther. 1995;275:674. [PubMed: 7473154]
- 24.
- Cho W, Stahelin RV. Membrane-protein interactions in cell signaling and membrane trafficking. Annu Rev Biophys Biomol Struct. 2005;34:119. [PubMed: 15869386]
- 25.
- Corti C, Leclerc-L’Hostis E, Quadroni M, Schmid H, Durussel I, Cox J, Dainese Hatt P, James P, Carafoli E. Tyrosine phosphorylation modulates the interaction of calmodulin with its target proteins. Eur J Biochem. 1999;262:790. [PubMed: 10411641]
- 26.
- Couve A, Kittler JT, Uren JM, Calver AR, Pangalos MN, Walsh FS, Moss SJ. Association of GABAB receptors and members of the 14-3-3 family of signaling proteins. Mol Cell Neurosci. 2001;17:317. [PubMed: 11178869]
- 27.
- Crivici A, Ikura M. Molecular and structural basis of target recognition by calmodulin. Annu Rev Biophys Biomol Struct. 1995;24:85. [PubMed: 7663132]
- 28.
- De Frutos T, Martin-Nieto J, Villalobo A. Phosphorylation of calmodulin by permeabilized fibroblasts overexpressing the human epidermal growth factor receptor. Biol Chem. 1997;378:31. [PubMed: 9049062]
- 29.
- Della Rocca GJ, Mukhin YV, Garnovskaya MN, Daaka Y, Clark GJ, Luttrell LM, Lefkowitz RJ, Raymond JR. Serotonin 5-HT1A receptor-mediated Erk activation requires calcium/calmodulin-dependent receptor endocytosis. J Biol Chem. 1999;274:4749. [PubMed: 9988712]
- 30.
- Fukami Y, Nakamura T, Nakayama A, Kanehisa T. Phosphorylation of tyrosine residues of calmodulin in Rous sarcoma virus-transformed cells. Proc Natl Acad Sci USA. 1986;83:4190. [PMC free article: PMC323697] [PubMed: 2424020]
- 31.
- Garnovskaya MN, Mukhin YV, Turner JH, Vlasova T, Ullian ME, Raymond JR. Mitogen-induced activation of Na+/H+ exchange in vascular smooth muscle cells involves janus kinase 2 and Ca2+/calmodulin. Biochemistry. 2003;42:7178. [PubMed: 12795614]
- 32.
- Garnovskaya MN, Mukhin YV, Vlasova TM, Raymond JR. Hypertonicity activates Na+/H+ exchange through Janus kinase 2 and calmodulin. J Biol Chem. 2003;278:16908. [PubMed: 12626508]
- 33.
- Garnovskaya MN, Nebigil CG, Arthur JM, Spurney RF, Raymond JR. 5-Hydroxytryptamine2A receptors expressed in rat renal mesangial cells inhibit cyclic AMP accumulation. Mol Pharmacol. 1995;48:230. [PubMed: 7651356]
- 34.
- Goppelt-Struebe M, Hahn A, Stroebel M, Reiser CO. Independent regulation of cyclo-oxygenase 2 expression by p42/44 mitogen-activated protein kinases and Ca2+/calmodulin-dependent kinase. Biochem J. 1999;339(Pt. 2):329. [PMC free article: PMC1220161] [PubMed: 10191263]
- 35.
- Graff JM, Young TM, Johnson JD, Blackshear PJ. Phosphorylation-regulated calmodulin binding to a prominent cellular substrate for protein kinase C. J Biol Chem. 1989;264:21818. [PubMed: 2557340]
- 36.
- Guo DF, Chenier I, Lavoie JL, Chan JS, Hamet P, Tremblay J, Chen XM, Wang DH, Inagami T. Development of hypertension and kidney hypertrophy in transgenic mice overexpressing ARAP1 gene in the kidney. Hypertension. 2006;48:453. [PubMed: 16801480]
- 37.
- Guo DF, Chenier I, Tardif V, Orlov SN, Inagami T. Type 1 angiotensin II receptor-associated protein ARAP1 binds and recycles the receptor to the plasma membrane. Biochem Biophys Res Commun. 2003;310:1254. [PubMed: 14559250]
- 38.
- Heppel LA, Newton DL, Klee CB, Draetta GF. The phosphorylation of calmodulin and calmodulin fragments by kinase fractions from bovine brain. Biochim Biophys Acta. 1988;972:69. [PubMed: 2846073]
- 39.
- Holohean AM, Hackman JC. Mechanisms intrinsic to 5-HT2B receptor-induced potentiation of NMDA receptor responses in frog motoneurones. Br J Pharmacol. 2004;143:351. [PMC free article: PMC1575347] [PubMed: 15339859]
- 40.
- Ishida A, Kameshita I, Okuno S, Kitani T, Fujisawa H. Phosphorylation of calmodulin by Ca2+/calmodulin-dependent protein kinase IV. Arch Biochem Biophys. 2002;407:72. [PubMed: 12392717]
- 41.
- Joubert L, Hanson B, Barthet G, Sebben M, Claeysen S, Hong W, Marin P, Dumuis A, Bockaert J. New sorting nexin (SNX27) and NHERF specifically interact with the 5-HT4a receptor splice variant: roles in receptor targeting. J Cell Sci. 2004;117:5367. [PubMed: 15466885]
- 42.
- Joyal JL, Crimmins DL, Thoma RS, Sacks DB. Identification of insulin-stimulated phosphorylation sites on calmodulin. Biochemistry. 1996;35:6267. [PubMed: 8639568]
- 43.
- Kagaya A, Mikuni M, Muraoka S, Saitoh K, Ogawa T, Shinno H, Yamawaki S, Takahashi K. Homologous desensitization of serotonin 5-HT2 receptor-stimulated intracellular calcium mobilization in C6BU-1 glioma cells via a mechanism involving a calmodulin pathway. J Neurochem. 1993;61:1050. [PubMed: 8360672]
- 44.
- Krasel C, Dammeier S, Winstel R, Brockmann J, Mischak H, Lohse MJ. Phosphorylation of GRK2 by protein kinase C abolishes its inhibition by calmodulin. J Biol Chem. 1911;27:6. 2001. [PubMed: 11042191]
- 45.
- Kushwaha N, Albert PR. Coupling of 5-HT1A autoreceptors to inhibition of mitogen-activated protein kinase activation via G beta gamma subunit signaling. Eur J Neurosci. 2005;21:721. [PubMed: 15733090]
- 46.
- Kushwaha N, Harwood SC, Wilson AM, Berger M, Tecott LH, Roth BL, Albert PR. Molecular determinants in the second intracellular loop of the 5-hydroxytryptamine-1A receptor for G-protein coupling. Mol Pharmacol. 2006;69:1518–1526. [PubMed: 16410407]
- 47.
- Lee SP, Xie Z, Varghese G, Nguyen T, O’Dowd BF, George SR. Oligomerization of dopamine and serotonin receptors. Neuropsychopharmacology. 2000;23:S32. [PubMed: 11008065]
- 48.
- Lefkowitz RJ, Pitcher J, Krueger K, Daaka Y. Mechanisms of beta-adrenergic receptor desensitization and resensitization. Adv Pharmacol. 1998;42:416. [PubMed: 9327928]
- 49.
- Lefler D, Mukhin YV, Pettus T, Leeb-Lundberg LMF, Garnovskaya MN, Raymond JR. Jak2 and Ca2+/calmodulin are key intermediates for bradykinin B2 receptor-mediated activation of Na+/H+ exchange in KNRK and CHO cells. Assay Drug Dev Technol. 2003;1:281. [PubMed: 15090193]
- 50.
- Lembo PM, Albert PR. Multiple phosphorylation sites are required for pathway-selective uncoupling of the 5-hydroxytryptamine1A receptor by protein kinase C. Mol Pharmacol. 1995;48:1024. [PubMed: 8848001]
- 51.
- Lembo PM, Ghahremani MH, Morris SJ, Albert PR. A conserved threonine residue in the second intracellular loop of the 5-hydroxytryptamine 1A receptor directs signaling specificity. Mol Pharmacol. 1997;52:164. [PubMed: 9224826]
- 52.
- McIlroy BK, Walters JD, Blackshear PJ, Johnson JD. Phosphorylation-dependent binding of a synthetic MARCKS peptide to calmodulin. J Biol Chem. 1991;266:4959. [PubMed: 2002042]
- 53.
- McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG, Foord SM. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333. [PubMed: 9620797]
- 54.
- Medeiros YS, Calixto JB. Influence of calcium entry blockers and calmodulin inhibitors on 5-hydroxytryptamine-, potassium- and calcium-induced contractions in human umbilical artery in-vitro. J Pharm Pharmacol. 1991;43:411. [PubMed: 1681054]
- 55.
- Meller R, Babity JM, Grahame-Smith DG. 5-HT2A receptor activation leads to increased BDNF mRNA expression in C6 glioma cells. Neuromol Med. 2002;1:197. [PubMed: 12095161]
- 56.
- Miller C. Biophysics: Lonely voltage sensor seeks protons for permeation. Science. 2006;312:534. [PubMed: 16645081]
- 57.
- Moyano S, Del Rio J, Frechilla D. Role of hippocampal CaMKII in serotonin 5-HT1A receptor-mediated learning deficit in rats. Neuropsychopharmacology. 2004;29:2216. [PubMed: 15199370]
- 58.
- Muff R, Buhlmann N, Fischer JA, Born W. An amylin receptor is revealed following co-transfection of a calcitonin receptor with receptor activity modifying proteins-1 or -3. Endocrinology. 1999;140:2924. [PubMed: 10342886]
- 59.
- Mukhin YV, Garnovskaya MN, Collinsworth G, Bell JL, Tholanikunnel BG, Pettus T, Jaffa AA, Fitzgibbon W, Ploth DW, Raymond JR, Garnovskaya MN. Bradykinin B2 receptors activate Na+/H+ exchange in mIMCD-3 cells via Janus kinase 2 and Ca2+/calmodulin. J Biol Chem. 2001;276:17339. [PubMed: 11278760]
- 60.
- Nishikawa Y, Doi M, Koji T, Watanabe M, Kimura S, Kawasaki S, Ogawa A, Sasaki K. The role of rho and rho-dependent kinase in serotonin-induced contraction observed in bovine middle cerebral artery. Tohoku J Exp Med. 2003;201:239. [PubMed: 14690016]
- 61.
- Okeley NM, Gelb MH. A designed probe for acidic phospholipids reveals the unique enriched anionic character of the cytosolic face of the mammalian plasma membrane. J Biol Chem. 2004;279:21833. [PubMed: 15007075]
- 62.
- Olayioye MA, Guthridge MA, Stomski FC, Lopez AF, Visvader JE, Lindeman GJ. Threonine 391 phosphorylation of the human prolactin receptor mediates a novel interaction with 14-3-3 proteins. J Biol Chem. 2003;278:32929. [PubMed: 12819209]
- 63.
- Pitcher JA, Freedman NJ, Lefkowitz RJ. G protein-coupled receptor kinases. Annu Rev Biochem. 1998;67:653. [PubMed: 9759500]
- 64.
- Premont RT, Inglese J, Lefkowitz RJ. Protein kinases that phosphorylate activated G protein-coupled receptors. FASAB J. 1995;9:175. [PubMed: 7781920]
- 65.
- Prezeau L, Richman JG, Edwards SW, Limbird LE. The zeta isoform of 14-3-3 proteins interacts with the third intracellular loop of different a2-adrenergic receptor subtypes. J Biol Chem. 1999;274:13462. [PubMed: 10224112]
- 66.
- Pronin AN, Satpaev DK, Slepak VZ, Benovic JL. Regulation of G protein-coupled receptor kinases by calmodulin and localization of the calmodulin binding domain. J Biol Chem. 1997;272:18273. [PubMed: 9218466]
- 67.
- Putkey JA, Kleerekoper Q, Gaertner TA, Waxham MN. A new role for IQ motif proteins in regulating calmodulin function. J Biol Chem. 2003;278:49667. [PubMed: 14551202]
- 68.
- Quadroni M, James P, Carafoli E. Isolation of phosphorylated calmodulin from rat liver and identification of the in vivo phosphorylation sites. J Biol Chem. 1994;269:16116. [PubMed: 8206911]
- 69.
- Quinn JC, Johnson-Farley NN, Yoon JY, Cowen DS. Activation of extracellular-regulated kinase by 5-hydroxytryptamine2A receptors in PC12 cells is protein kinase C-independent and requires calmodulin and tyrosine kinases. J Pharmacol Exp Ther. 2002;303:746. [PubMed: 12388661]
- 70.
- Qvigstad E, Sjaastad I, Brattelid T, Nunn C, Swift F, Birkeland JA, Krobert KA, Andersen GO, Sejersted OM, Osnes JB, Levy FO, Skomedal T. Dual serotonergic regulation of ventricular contractile force through 5-HT2A and 5-HT4 receptors induced in the acute failing heart. Circ Res. 2005;97:268. [PubMed: 16002744]
- 71.
- Rahman S, Neuman RS. Multiple mechanisms of serotonin 5-HT2 receptor desensitization. Eur J Pharmacol. 1993;238:173. [PubMed: 8405090]
- 72.
- Raymond JR. Protein kinase C induces phosphorylation and desensitization of the human 5-HT1A receptor. J Biol Chem. 1991;266:14747. [PubMed: 1860872]
- 73.
- Raymond JR, Mukhin YV, Gelasco A, Turner J, Collinsworth G, Gettys TW, Grewal JS, Garnovskaya MN. Multiplicity of mechanisms of serotonin receptor signal transduction. Pharmacol Ther. 2001;92:179. [PubMed: 11916537]
- 74.
- Rhoads AR, Friedberg F. Sequence motifs for calmodulin recognition. FASEB J. 1997;11:331. [PubMed: 9141499]
- 75.
- Richman JG, Brady AE, Wang Q, Hensel JL, Colbran RJ, Limbird LE. Agonist-regulated Interaction between a2-adrenergic receptors and spinophilin. J BiolChem. 2001;276:15003. [PubMed: 11154706]
- 76.
- Rios CD, Jordan BA, Gomes I, Devi LA. G-protein-coupled receptor dimerization: modulation of receptor function. Pharmacol Ther. 2001;92:71. [PubMed: 11916530]
- 77.
- Sacks DB, Davis HW, Crimmins DL, McDonald JM. Insulin-stimulated phosphorylation of calmodulin. Biochem J. 1992;286(Pt 1):211. [PMC free article: PMC1133041] [PubMed: 1520270]
- 78.
- Sacks DB, Davis HW, Williams JP, Sheehan EL, Garcia JG, McDonald JM. Phosphorylation by casein kinase II alters the biological activity of calmodulin. Biochem J. 1992;283(Pt 1):21. [PMC free article: PMC1130984] [PubMed: 1314563]
- 79.
- Sacks DB, Fujita-Yamaguchi Y, Gale RD, McDonald JM. Tyrosine-specific phosphorylation of calmodulin by the insulin receptor kinase purified from human placenta. Biochem J. 1989;263:803. [PMC free article: PMC1133502] [PubMed: 2480780]
- 80.
- Sacks DB, Mazus B, Joyal JL. The activity of calmodulin is altered by phosphorylation: modulation of calmodulin function by the site of phosphate incorporation. Biochem J. 1995;312(Pt. 1):197. [PMC free article: PMC1136245] [PubMed: 7492313]
- 81.
- Satoh A, Nakanishi H, Obaishi H, Wada M, Takahashi K, Satoh K, Hirao K, Nishioka H, Hata Y, Mizoguchi A, Takai Y. Neurabin-II/spinophilin. An actin filament-binding protein with one PDZ domain localized at cadherin-based cell-cell adhesion sites. J Biol Chem. 1998;273:3470. [PubMed: 9452470]
- 82.
- Saunders C, Limbird LE. Disruption of microtubules reveals two independent apical targeting mechanisms for G-protein-coupled receptors in polarized renal epithelial cells. J Biol Chem. 1997;272:19035. [PubMed: 9228087]
- 83.
- Saunders C, Limbird LE. Microtubule-dependent regulation of a2B adrenergic receptors in polarized MDCKII cells requires the third intracellular loop but not G protein coupling. Mol Pharmacol. 2000;57:44. [PubMed: 10617677]
- 84.
- Schiapparelli L, Del Rio J, Frechilla D. Serotonin 5-HT receptor blockade enhances Ca2+/calmodulin-dependent protein kinase II function and membrane expression of AMPA receptor subunits in the rat hippocampus: implications for memory formation. J Neurochem. 2005;94:884. [PubMed: 16092936]
- 85.
- Schiapparelli L, Simon AM, Del Rio J, Frechilla D. Opposing effects of AMPA and 5-HT1A receptor blockade on passive avoidance and object recognition performance: correlation with AMPA receptor subunit expression in rat hippocampus. Neuropharmacology. 2006;50:897. [PubMed: 16620883]
- 86.
- Smith FD, Oxford GS, Milgram SL. Association of the D2 dopamine receptor third cytoplasmic loop with spinophilin, a protein phosphatase-1-interacting protein. J Biol Chem. 1999;274:19894. [PubMed: 10391935]
- 87.
- Streb JW, Miano JM. Cross-species sequence analysis reveals multiple charged residue-rich domains that regulate nuclear/cytoplasmic partitioning and membrane localization of a kinase anchoring protein 12 (SSeCKS/Gravin). J Biol Chem. 2005;280:28007. [PubMed: 15923193]
- 88.
- Stroebel M, Goppelt-Struebe M. Signal transduction pathways responsible for serotonin-mediated prostaglandin G/H synthase expression in rat mesangial cells. J Biol Chem. 1994;269:22952. [PubMed: 8083194]
- 89.
- Suzuki K. The mechanism of enhanced platelet intracellular calcium mobilization stimulated by serotonin — in the pathophysiology of mood disorders. Hokkaido Igaku Zasshi. 2001;76:277. [PubMed: 11593752]
- 90.
- Tazawa H, Takahashi S, Zilliacus J. Interaction of the parathyroid hormone receptor with the 14-3-3 protein. Biochim Biophys Acta. 2003;1620:32. [PubMed: 12595070]
- 91.
- Turner JH, Gelasco AK, Raymond JR. Calmodulin interacts with the third intracellular loop of the serotonin 5-hydroxytryptamine1A receptor at two distinct sites: putative role in receptor phosphorylation by protein kinase C. J Biol Chem. 2004;279:17027. [PubMed: 14752100]
- 92.
- Turner JH, Raymond JR. Interaction of calmodulin with the serotonin 5-hydroxytryptamine2A receptor. A putative regulator of G protein coupling and receptor phosphorylation by protein kinase C. J Biol Chem. 2005;280:30741. [PubMed: 15970592]
- 93.
- Uneyama H, Ueno S, Akaike N. Serotonin-operated potassium current in CA1 neurons dissociated from rat hippocampus. J Neurophysiol. 1993;69:1044. [PubMed: 8492147]
- 94.
- Wang D, Sadee W, Quillan JM. Calmodulin binding to G protein-coupling domain of opioid receptors. J Biol Chem. 1999;274:22081. [PubMed: 10419536]
- 95.
- Xia Z, Gray JA, Compton-Toth BA, Roth BL. A direct interaction of PSD-95 with 5-HT2A serotonin receptors regulates receptor trafficking and signal trans-duction. J Biol Chem. 1901;278:2. 2003. [PubMed: 12682061]
- 96.
- Xia Z, Hufeisen SJ, Gray JA, Roth BL. The PDZ-binding domain is essential for the dendritic targeting of 5-HT2A serotonin receptors in cortical pyramidal neurons in vitro. Neuroscience. 2003;122:907. [PubMed: 14643760]
- 97.
- Yap KL, Ames JB, Swindells MB, Ikura M. Diversity of conformational states and changes within the EF-hand protein superfamily. Proteins. 1999;37:499. [PubMed: 10591109]
- 98.
- Yap KL, Kim J, Truong K, Sherman M, Yuan T, Ikura M. Calmodulin target database. J Struct Funct Genomics. 2000;1:8. [PubMed: 12836676]
- 99.
- Yuen EY, Jiang Q, Chen P, Gu Z, Feng J, Yan Z. Serotonin 5-HT1A receptors regulate NMDA receptor channels through a microtubule-dependent mechanism. J Neurosci. 2005;25:5488. [PMC free article: PMC6724987] [PubMed: 15944377]
- Constitutive activity of serotonin 2C receptors at G protein-independent signaling: modulation by RNA editing and antidepressants.[Mol Pharmacol. 2010]Constitutive activity of serotonin 2C receptors at G protein-independent signaling: modulation by RNA editing and antidepressants.Labasque M, Meffre J, Carrat G, Becamel C, Bockaert J, Marin P. Mol Pharmacol. 2010 Nov; 78(5):818-26. Epub 2010 Aug 10.
- Serotonin 5-HT receptor blockade enhances Ca(2+)/calmodulin-dependent protein kinase II function and membrane expression of AMPA receptor subunits in the rat hippocampus: implications for memory formation.[J Neurochem. 2005]Serotonin 5-HT receptor blockade enhances Ca(2+)/calmodulin-dependent protein kinase II function and membrane expression of AMPA receptor subunits in the rat hippocampus: implications for memory formation.Schiapparelli L, Del Río J, Frechilla D. J Neurochem. 2005 Aug; 94(4):884-95.
- NMR structural study of the intracellular loop 3 of the serotonin 5-HT(1A) receptor and its interaction with calmodulin.[Biochim Biophys Acta. 2011]NMR structural study of the intracellular loop 3 of the serotonin 5-HT(1A) receptor and its interaction with calmodulin.Chen AS, Kim YM, Gayen S, Huang Q, Raida M, Kang C. Biochim Biophys Acta. 2011 Sep; 1808(9):2224-32. Epub 2011 May 24.
- Review Molecular and functional characterization of proteins interacting with the C-terminal domains of 5-HT2 receptors: emergence of 5-HT2 "receptosomes".[Biol Cell. 2004]Review Molecular and functional characterization of proteins interacting with the C-terminal domains of 5-HT2 receptors: emergence of 5-HT2 "receptosomes".Gavarini S, Bécamel C, Chanrion B, Bockaert J, Marin P. Biol Cell. 2004 Jun; 96(5):373-81.
- Review Calcium-Sensing Receptor: Trafficking, Endocytosis, Recycling, and Importance of Interacting Proteins.[Prog Mol Biol Transl Sci. 2015]Review Calcium-Sensing Receptor: Trafficking, Endocytosis, Recycling, and Importance of Interacting Proteins.Ray K. Prog Mol Biol Transl Sci. 2015; 132:127-50. Epub 2015 Apr 25.
- Calmodulin Is a 5-HT Receptor-Interacting and Regulatory Protein - Serotonin Rec...Calmodulin Is a 5-HT Receptor-Interacting and Regulatory Protein - Serotonin Receptors in Neurobiology
Your browsing activity is empty.
Activity recording is turned off.
See more...
