NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Nelson HD, Tyne K, Naik A, et al. Screening for Breast Cancer: Systematic Evidence Review Update for the US Preventive Services Task Force [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2009 Nov. (Evidence Syntheses, No. 74.)
This publication is provided for historical reference only and the information may be out of date.

Screening for Breast Cancer: Systematic Evidence Review Update for the US Preventive Services Task Force [Internet].
Show detailsKey Questions and Analytic Framework
The USPSTF and Agency for Healthcare Research and Quality (AHRQ) developed the key questions that guided the update. Investigators created an analytic framework incorporating the key questions and outlining the patient population, interventions, outcomes, and harms of the screening process (Figure 1). The target population includes women without preexisting breast cancer and not considered at high risk for breast cancer based on extensive family history of breast or ovarian cancer or other personal risk factors, such as abnormal breast pathology or deleterious genetic mutations. Key questions include:
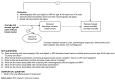
Figure 1
Analytic Framework and Key Questions.
- 1a. Does screening with mammography (film and digital) or MRI decrease breast cancer mortality among women age 40–49 years and ≥70 years?
- 1b. Does CBE screening decrease breast cancer mortality? Alone or with mammography?
- 1c. Does BSE practice decrease breast cancer mortality?
- 2a. What are the harms associated with screening with mammography (film and digital) and MRI?
- 2b. What are the harms associated with CBE?
- 2c. What are the harms associated with BSE?
Harms include radiation exposure, pain during procedures, patient anxiety and other psychological responses, consequences of false-positive and false-negative tests, and overdiagnosis. Overdiagnosis refers to women receiving a diagnosis of invasive or noninvasive breast cancer who had abnormal lesions that were unlikely to become clinically evident during their lifetimes in the absence of screening.55 Overdiagnosis may have more effect on women with shorter life expectancies because of age or comorbid conditions.
An additional contextual question on the cost effectiveness of screening is also included. Contextual questions are addressed as a narrative, not systematic, review of relevant studies. The purpose of the cost effectiveness question is to provide background information.
Search Strategies
We searched the Cochrane Central Register of Controlled Trials and Cochrane Database of Systematic Reviews (through the 4th Quarter 2008) and the MEDLINE database (January 1, 2001 to December 1, 2008) for relevant studies and meta-analyses (Appendix B1). We also conducted secondary referencing by manually reviewing reference lists of key articles and searching citations by using Web of Science,56 particularly searching for follow-up data from screening trials cited in the previous evidence review.2, 3 Appendix B2 shows our search results.
Study Selection
We selected studies on the basis of inclusion and exclusion criteria developed for each key question. Studies identified from our searches that did not meet inclusion criteria are listed in Appendix B3. To determine the effectiveness of screening, we included randomized controlled trials and updates to previously published trials of screening with mammography (film and digital), MRI, CBE, or BSE with breast cancer mortality outcomes published since 2001. One trial was translated into English from Russian for this update.57 We also reviewed meta-analyses that included studies with mortality data. We excluded studies other than controlled trials and systematic reviews or those without breast cancer mortality as an outcome.
We determined harms of screening by using evidence from several study designs and data sources. For mammography, we focused our searches on recently published systematic reviews and meta-analyses of radiation exposure, pain during procedures, patient anxiety and other psychological responses, consequences of false-positive and false-negative tests, and overdiagnosis. We also conducted specific searches for primary studies published more recently than the included systematic reviews and meta-analyses. In addition, we evaluated data from the BCSC, which is a collaborative network of 5 mammography registries and 2 affiliated sites with linkages to pathology and/or tumor registries across the United States, that is sponsored by the National Cancer Institute.58, 59 These data draw from community samples that are representative of the larger, national population and may be more applicable to current practice in the United States than other published sources. Data include a mix of film and digital mammography. For harms of CBE and BSE, we reviewed screening trials of these procedures that reported potential adverse effects, utilized recently published systematic reviews, and conducted focused searches.
We included studies of the cost effectiveness of screening that were relevant to the key questions and target population (Appendix C1). We excluded studies evaluating the cost of improving screening rates (e.g., post-card reminder versus telephone reminder), dual review of screening mammography, screening education programs, or studies of patients with a history of breast cancer or who were at high risk for developing breast cancer. We highlighted studies that expressed outcomes in quality-adjusted life-years (QALY). The QALY incorporates changes in length and quality of life, expressed as the extra dollars (cost per QALY ratio) required to achieve 1 extra QALY.60 A year in perfect health is considered equal to 1.0 QALY.
Data Abstraction and Quality Rating
We abstracted details about the patient population, study design, analysis, follow-up, and results. By using predefined criteria developed by the USPSTF,61 two investigators rated the quality of each study as good, fair, or poor (described in Appendix B4 and B5) and resolved discrepancies by consensus. We included only systematic reviews rated good quality in the report and randomized controlled trials rated fair or good quality in the meta-analysis.
Meta-analysis of Mammography Trials
We updated the 2002 meta-analysis to include new findings from published trials of mammography screening compared with control participants for women age 40–49 years that reported relative risk (RR) reduction in breast cancer mortality. We conducted similar updates for other age groups for context. We used breast cancer mortality results from trials to estimate the pooled RR. We calculated estimates from a random-effects model under the Bayesian data analytic framework by using the RBugs package in R,62, 63 the same model as that used in the previous report.2 Appendix B6 provides additional details. We used funnel plots to assess publication bias and L’Abbé plots to assess heterogeneity.
Analysis of Breast Cancer Surveillance Consortium Data
Background information and additional details about methods of the BCSC are described in Appendix B7. We obtained data from 600,830 women age 40 years or older undergoing routine mammography screening from 2000–2005 at the BCSC sites from the BCSC Statistical Coordinating Center and stratified it by age in decades. Routine screening was having at least one mammography examination within the previous 2 years, which is consistent with current USPSTF recommendations. For women with several mammography examinations during the study, one result was randomly selected to be included in the calculations. These data constitute selected BCSC data intended to represent the experience of a cohort of regularly screened women without preexisting breast cancer or abnormal physical findings.
Variables include the numbers of positive and negative mammography results and, of these, the numbers of true-negative and false-negative results based on follow-up data within 1 year of mammography screening. A positive mammography result was defined according to standardized terminology and assessments of the American College of Radiology Breast Imaging Reporting and Data System (BI-RADS) manual used by the BCSC.64 These include four categories: needs additional evaluation (category 0), probably benign with a recommendation for immediate follow-up (category 3), suspicious (category 4), or highly suggestive of malignancy (category 5).65 For women who had a positive screening mammography result, we evaluated data on the number of women undergoing additional imaging and biopsy, and diagnoses including invasive cancer, DCIS, and negative results. We considered additional imaging procedures and biopsies done within 60 days of the screening mammography to be related to screening. From these data, we calculated age-specific rates (numbers per 1000 women per round) of invasive breast cancer, DCIS, false-positive and false-negative mammography results, additional imaging, and biopsies. We based true-positive and true-negative mammography results on invasive and noninvasive cancer diagnosis. Rates of additional imaging and rates of biopsies may be underestimated because of incomplete capture of these examinations by the BCSC. We conducted a sensitivity analysis of missing values, although this does not include records that were unavailable to the BCSC.
External review
We distributed a draft of the systematic review for review by external experts not affiliated with the USPSTF (listed in Appendix B8).
- Methods - Screening for Breast CancerMethods - Screening for Breast Cancer
Your browsing activity is empty.
Activity recording is turned off.
See more...
