NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Shekelle P, Morton S, Rich M, et al. Pharmacologic Management of Heart Failure and Left Ventricular Systolic Dysfunction: Effect in Female, Black, and Diabetic Patients, and Cost-Effectiveness. Rockville (MD): Agency for Healthcare Research and Quality (US); 2003 Jul. (Evidence Reports/Technology Assessments, No. 82.)
This publication is provided for historical reference only and the information may be out of date.

Pharmacologic Management of Heart Failure and Left Ventricular Systolic Dysfunction: Effect in Female, Black, and Diabetic Patients, and Cost-Effectiveness.
Show detailsDescription of Evidence
Figure 3 displays the results of our literature search. As noted previously, our TEP provided us references for nine studies. Our library search identified another 315 articles. By reviewing the reference lists of those articles as we received them, we identified an additional 88 articles to assess. Thus, in total, 412 articles were selected. Of these, we were able to obtain 392 through the RAND library, the UCLA library, and a consulting firm that specializes in locating hard-to-find scientific journals. Of the 392 articles screened, 174 reported the results of randomized, controlled trials (RCTs) of beta-blockers or ACE inhibitors; these progressed to the Quality Review stage (see forms, Appendix B). Of these 174, 100 were rejected because they were not placebo controlled, did not report mortality outcomes, or did not report outcomes for a minimum of 12 weeks followup. This review process left 74 articles (see Evidence Tables 1 and 2).
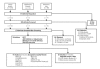
Figure
Figure 3. Flow of literature.
As mentioned in Chapter 2, many of these articles described studies that appeared to include (but did not stratify according to) our populations of interest—blacks, women, and diabetics. Thus, we attempted to correspond with the authors of all studies accepted (randomized controlled trials of beta-blockers or ACE inhibitors reporting mortality data, with a minimum of 12 week followup) in an attempt to obtain patient-level data. Of 62 authors to whom we sent letters, four agreed to send us the needed data. Ten others refused, while most others either did not reply or gave us a new contact who did not reply.
Because we were unable to obtain an acceptable response to our request for additional data, we changed our focus to trying to get the data appropriately stratified by subpopulation from the “major” RCTs, which we defined as studies with sample sizes greater than 1,000 (with one exception—we also included the CONSENSUS trial, with a sample size of 253, because it was the first ACE inhibitor study to report a mortality benefit, it was widely publicized and influential in establishing ACE inhibitor therapy for heart failure, and our TEP judged that the cardiology community would expect it to be included). By repeated efforts (including personal contacts) with original authors, examination of individual patient data for some trials obtained through the FDA (as described in the Methods section), and the serendipitous publication of subgroup results during this time period, we were able to obtain the appropriate subgroup data for all the major RCTs. These placebo-controlled RCTs are briefly described below and summarized in Evidence Tables 3 and 4.
ACE Inhibitor Studies
The Acute Infarction Ramipril Efficacy (AIRE) study assessed the effect of the ACE inhibitor ramipril on 1,986 patients with clinical evidence of heart failure after having an acute myocardial infarction. The average duration of followup was 15 months. The study reported a statistically significant reduction in all cause mortality with a relative risk of 0.73 for patients treated with ramipril. 29 Some subgroup analyses were also included.
The Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS) assessed the effect of the ACE inhibitor enalapril in 253 patients with severe heart failure (New York Heart Association class IV). The average followup period was 188 days. The study reported that at sixth months, there was a statistically significant (40%) reduction in all-cause mortality in patients treated with enalapril.30
The Survival and Ventricular Enlargement Trial (SAVE) assessed the effect of the ACE inhibitor captopril in 2,231 patients with left ventricular dysfunction (defined as an ejection fraction of 40% or less, but without overt heart failure). The average followup time was 42 months. The study reported a statistically significant 19% reduction in all-cause mortality in patients treated with captopril. 31, 32 Subgroup analyses were also presented. 32
The Survival of Myocardial Infarction Long-Term Study (SMILE) assessed the effect of the ACE inhibitor Zofenopril in 1,556 patients who had an acute anterior myocardial infarction. The duration of followup was one year. The study reported a statistically significant 22% reduction in all-cause mortality for patients treated with Zofenopril.33 The authors also reported some subgroup analyses. Although a low left ventricular ejection fraction was not a requirement for entry into this study, our TEP judged it should be included because left ventricular dysfunction is so common following anterior myocardial infarction that the enrolled population in SMILE was sufficiently similar to the other ACE inhibitor studies to justify statistical pooling. Our test of heterogeneity supported this decision.
The Studies of Left Ventricular Dysfunction (SOLVD) contained two randomized studies of the effect of the ACE inhibitor enalapril. The first study assessed the effect in 2,569 patients with New York Heart Association Class II and III heart failure and a left ventricular ejection fraction of less than or equal to 35%.24 The average period of followup was 41.4 months. The study reported a statistically significant (16%) reduction in all-cause mortality. The second SOLVD study assessed the effect of enalapril in 4,228 patients with asymptomatic left ventricular dysfunction, defined as a left ventricular ejection fraction of 35% or less. The average followup time was 37.4 months.34 The study reported a nonstatistically significant (8%) reduction in all-cause mortality in patients treated with captopril.
The Trandolapril Cardiac Evaluation (TRACE) study assessed the effect of the ACE inhibitor trandolapril in 1,749 patients with left ventricular systolic dysfunction (defined as an ejection fraction less than or equal to 35% with or without symptoms).35 The patients were followed for 24–50 months. The study reported a statistically significant reduction in mortality (22%) for patients treated with Trandolapril.
Beta-Blocker Studies
The Beta-Blocker Survival Trial (BEST) assessed the effect of the beta-blocker bucindolol in 2,708 patients with New York Heart Association functional class III or IV and a left ventricular ejection fraction of 35% or lower.36–38 The average time of followup was two years. The study reported no overall difference in mortality between treatment and placebo groups. In a subgroup analysis, nonblack patients had a statistically significant mortality benefit with a hazard ratio of 0.82.37, 38 This benefit was counterbalanced by an unexpected nonstatistically significant higher mortality rate in black patients treated with bucindolol.
The Cardiac Insufficiency Bisoprolol II Study (CIBIS-II) assessed the effect of the beta-blocker bisoprolol in 2,647 patients with New York Heart Association class III or IV heart failure and a left ventricular ejection fraction of 35% or less.39, 40 Patients were followed up for a mean of 1.3 years. The study reported a statistically significant reduction in all-cause mortality with a hazard ratio of 0.66 for patients treated with bisoprolol. Subgroup analyses were also reported.40
The Carvedilol Prospective Randomized Cumulative Survival Study Group (COPERNICUS) assessed the effect of the beta-blocker carvedilol in 2,287 patients with severe heart failure equivalent to New York Heart Association class IV and left ventricular ejection fraction of less than 25%.41 The mean period of followup was 10.4 months. The study reported a statistically significant 35% reduction in all-cause mortality for patients treated with carvedilol. Some subgroup analyses were reported.
The Metoprolol CR/XL Randomized Intervention Trial (MERIT-HF) assessed the effect of the beta-blocker metoprolol, controlled release/extended release, in 3,991 patients with New York Heart Association functional class III to IV heart failure and left ventricular ejection fraction of 40% or less.42–44 Patients were followed for a mean of one year. The study reported a statistically significant reduction in the relative risk of mortality of 0.66 for patients treated with metoprolol. Subgroup analyses were also reported.43, 44
The United States Carvedilol Heart Failure Trials were four separate studies that assessed the effect of the beta-blocker carvedilol in patients with mild, moderate, or severe heart failure and left ventricular ejection fraction of less than 35%.45, 46 A total of 1,094 patients were studied for six months or 12 months. A pooled analysis of the four studies reported a statistically significant reduction in mortality with a relative risk of 65% for patients treated with carvedilol. Results of the subgroup analysis were also reported.46
Results of Meta-Analysis
ACE Inhibitors
Gender
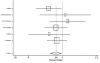
We were able to obtain gender-stratified data for all seven major studies to calculate the effect of ACE inhibitors on mortality. The seven studies were CONSENSUS, SAVE, the two SOLVD studies, SMILE, TRACE, and AIRE. Five of these studies had data sufficient to calculate both a RRR and a RHR. The data from SAVE could be used only in the RRR assessment, and the data from AIRE could be used only in the RHR assessment. In aggregate, these studies included 2,898 women and 11,674 men and lasted from six months (for CONSENSUS) to 42 months (SAVE). The pooled random-effects estimates from the six studies with relative risk data yielded values for men of 0.82 (95% CI: 0.74, 0.90) and for women of 0.92 (95% CI: 0.81, 1.04). These results are displayed in Table 6 and Figures 4 and 5. The corresponding pooled random effects estimates from the six studies with hazard ratio data yielded values for men of 0.76 (95% CI: 0.66, 0.87) and for women of 0.84 (95% CI: 0.72, 0.98) (Table 7 and Figures 6 and 7). The difference in effect between men and women approached statistical significance for the RRR (p = 0.07).
Table
Table 6. Effect of ACE inhibitors on mortality from heart failure in male and female patients (relative risk analysis).
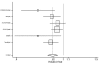
Figure
Figure 4. Effect of ACE inhibitors on mortality in male heart failure patients (relative risk analysis).
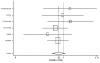
Figure
Figure 5. Effect of ACE inhibitors on mortality in female heart failure patients (relative risk analysis).
Table
Table 7. Effect of ACE inhibitors on mortality from heart failure in male and female patients (hazard ratio analysis).
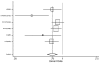
Figure
Figure 6. Effect of ACE inhibitors on mortality in male heart failure patients (hazard ratio analysis).
Figure
Figure 7. Effect of ACE inhibitors on mortality in female heart failure patients (hazard ratio analysis).
These differences between the estimates of relative risk and hazard ratios are due to the inclusion in the hazard ratio analysis of the AIRE study, which reported a slight nonsignificant mortality benefit for women compared to men treated with ramipril, as opposed to the relative risk analysis, which included the SAVE study. This study reported a distinct but nonstatistically significant increase in mortality in women relative to men treated with captopril (RRR=1.24).
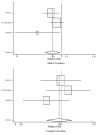
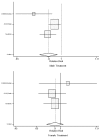
In a subgroup analysis, studies were divided into those treating symptomatic heart failure (risk ratio analysis for CONSENSUS, SOLVD-treatment, and TRACE; hazard ratio analysis AIRE, CONSENSUS, SOLVD-treatment, and TRACE) compared with those treating for asymptomatic left ventricular systolic dysfunction (risk ratio analysis for SAVE, SOLVD-prevention, and SMILE; hazard ratio analysis AIRE, SOLVD-prevention, and SMILE). The difference in efficacy between men and women is most pronounced for treatment of asymptomatic left ventricular dysfunction, where the evidence does not support or suggest a mortality benefit for women (relative risk = 0.96; 95% CI: 0.75, 1.22A, see Table 8 and Figures 8 and 9). These results are based on a pooled analysis that included 1,079 women in the symptomatic heart failure studies and 1,294 women in the asymptomatic heart failure studies. The evidence indicates that women with symptomatic heart failure benefit when treated with ACE inhibitors, although the benefit may be somewhat less that the benefit seen in men. However, the evidence calls into question whether or not women with asymptomatic left ventricular systolic dysfunction have any mortality benefit when treated with ACE inhibitors. These results are compatible with an earlier preliminary analysis of the SOLVD data.4 Additional data are needed to answer this question. In contrast, men clearly benefit when treated with ACE inhibitors for asymptomatic left ventricular systolic dysfunction.
Table
Table 8. Effect of ACE inhibitors on mortality from heart failure in male and female patients (relative risk analysis), random-effects pooled estimate (separately for prevention studies and treatment studies).
Figure
Figure 8. Effect of ACE inhibitors on mortality in male and female heart failure patients (relative risk analysis), random-effects pooled estimate, separately for prevention studies.
Figure
Figure 9. Effect of ACE inhibitors on mortality in male and female heart failure patients (relative risk analysis), random-effects pooled estimate, separately for treatment studies.
Some clinicians and patients find it easier to interpret relative risk data when they are converted to the “number needed to treat” (NNT). The NNT is the number of affected individuals who need to be given the treatment in question to achieve one successful outcome. In other words, in terms of this section, the NNT is the number of patients with heart failure or asymptomatic left ventricular systolic dysfunction who need to be treated with ACE inhibitors to prevent one death. Because the NNT depends on both the relative risk and the underlying risk, we have prepared a table that can be used to find the NNT for any common combination of these two variables (Table 9). We do not provide an NNT for each of our pooled estimates of effect. While the data presented in this report, in general, support an equal effect of ACE inhibitors regardless of underlying mortality risk, calculating an associated NNT requires a pooled absolute mortality risk. However, the mortality risk clearly varied across studies that enrolled patients with class IV heart failure (CONSENSUS) and studies that enrolled patients with asymptomatic left ventricular dysfunction, indicating that a pooled absolute mortality risk across studies would have no meaning.
Table
Table 9. Number Needed to Treat (NNT) as a function of risk ratio and population mortality risk.
Diabetes
Six studies stratified data by diagnosis of diabetes, permitting calculation of the differential effect of ACE inhibitors on mortality. These studies were CONSENSUS, SAVE, the two SOLVD studies, SMILE, and TRACE. In aggregate, these studies included 2,398 patients with diabetes and 10,188 patients without diabetes. All of these studies contributed data to our relative risk analysis; however, the SAVE study did not contain data that we could use for our hazard ratio analysis. Both analyses yielded similar results. The random-effects pooled estimate of the relative risk of mortality in patients with diabetes is 0.84 (95% CI: 0.70, 1.00) whereas the estimate of the relative risk in patients without diabetes is 0.85 (95% CI: 0.78, 0.92). The corresponding estimates for the hazard ratio are 0.73 (95% CI: 0.56, 0.95) for diabetics and 0.80 (95% CI: 0.69, 0.93) for nondiabetics. These data are presented in Tables 10 and 11 and Figures 10–13. We interpret these results as indicating that both patients with diabetes and patients without diabetes achieve reductions in mortality when treated with ACE inhibitors for heart failure.
Table
Table 10. Effect of ACE inhibitors on mortality from heart failure in diabetic and nondiabetic patients (relative risk analysis).
Table
Table 11. Effect of ACE inhibitors on mortality from heart failure in diabetic and nondiabetic patients (hazard ratio analysis).
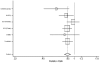
Figure
Figure 10. Effect of ACE inhibitors on mortality in nondiabetic heart failure patients (relative risk analysis).
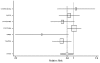
Figure
Figure 11. Effect of ACE inhibitors on mortality in diabetic heart failure patients (relative risk analysis).

Figure
Figure 12. Effect of ACE inhibitors on mortality in nondiabetic heart failure patients (hazard ratio analysis).

Figure
Figure 13. Effect of ACE inhibitors on mortality in diabetic heart failure patients (hazard ratio analysis).
Race
Three studies provided data stratified by patient race to assess the effects of ACE inhibitors on mortality. The studies with appreciable numbers of black patients were SAVE and the two SOLVD studies. The remaining ACE inhibitor studies (AIRE, CONSENSUS, SMILE, and TRACE) were conducted primarily in Scandinavian and European countries and did not include substantial numbers of black patients. SAVE did not present data that allowed us to calculate the hazard ratios, which left only two studies (the SOLVD studies), an insufficient number to pool for this analysis. Therefore, only a pooled relative risk analysis was performed, which yielded an estimate in white patients of 0.89 (95% CI: 0.82, 0.97) and an estimate in black patients of 0.89 (95% CI: 0.74, 1.06). These data are presented in Tables 12 and 13 and Figures 14 and 15. We interpret these data as indicating that there is no evidence that black patients achieve lesser or greater reductions in mortality than white patients when treated with ACE inhibitors for heart failure. Whereas the relative risk reduction in black patients did not achieve conventional levels of statistical significance, the estimate of effect is the same as the statistically significant reduction seen in white patients. Furthermore, the two estimates of effect (for black and white patients) do not differ from each other statistically. Therefore, the most likely explanation for the lack of statistical significance in the estimate for black patients is the much smaller sample size, which increases the standard error and 95% confidence intervals. These results are consistent with the analysis by the SOLVD investigators that there was not a lesser reduction in mortality among black compared to white patients in the SOLVD studies (these investigators did, however, report a difference in hospitalization rate in black patients compared to white patients).34
Table
Table 12. Effect of ACE inhibitors on mortality from heart failure in black and white patients (relative risks analysis).
Table
Table 13. Effect of ACE inhibitors on mortality from heart failure in black and white patients (hazard ratios analysis).
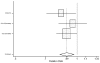
Figure
Figure 14. Effect of ACE inhibitors on mortality in white/nonblack heart failure patients (relative risk analysis).
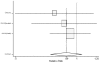
Figure
Figure 15. Effect of ACE inhibitors on mortality in black heart failure patients (relative risk analysis).
Beta-Blockers
Gender
Five studies on the effects of beta-blocker treatment on mortality stratified data by gender. The studies were CIBIS II, COPERNICUS, MERIT-HF, BEST, and US Carvedilol. The CIBIS II study contributed data only to the relative risk analysis. Bucindolol, which was the beta-blocker evaluated in BEST, was judged by our TEP to be sufficiently different in action from the other beta-blockers that the results of the BEST study should not be pooled with those of the other studies. In aggregate, the pooled studies included 2,134 women and 7,885 men. Both analyses yielded similar results. The random-effects pooled estimate for the relative risk of mortality for women was 0.63 (95% CI: 0.44, 0.91), whereas for men, the estimate was 0.66 (95% CI: 0.59, 0.75). The corresponding values for the hazard ratio analysis were 0.62 (95% CI: 0.34, 1.14) for women and 0.62 (95% CI: 0.52, 0.73) for men. Likewise, BEST reported equal effects in men and women (although in BEST, the reduction in all-cause mortality was not statistically significant). These data are presented in Tables 14 and 15 and Figures 16 – 19. Our interpretation of these data is that women and men with symptomatic heart failure have reduced mortality when treated with beta-blockers.
Table
Table 14. Effect of beta-blockers on mortality from heart failure in male and female patients (relative risk analysis).
Table
Table 15. Effect of beta-blockers on mortality from heart failure in male and female patients (hazard ratio analysis).
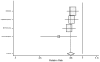
Figure
Figure 16. Effect of beta-blockers on mortality in male heart failure patients (relative risk analysis).
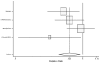
Figure
Figure 17. Effect of beta-blockers on mortality in female heart failure patients (relative risk analysis).

Figure
Figure 18. Effect of beta-blockers on mortality in male heart failure patients (hazard ratio analysis; without BEST).
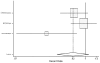
Figure
Figure 19. Effect of beta-blockers on mortality in female heart failure patients (hazard ratio analysis; without BEST).
Diabetes
Three studies stratified data by diagnosis of diabetes, permitting calculation of the differential effect of beta-blockers on mortality. In aggregate, these studies included 1,883 patients with diabetes and 7,042 patients without diabetes. The only pooled estimates that were possible were the relative risks, which yielded a value of 0.65 (95% CI: 0.57, 0.74) for nondiabetic patients and a value of 0.77 (95% CI: 0.61, 0.96) for diabetic patients. This difference in relative risk was not statistically significant; however, the 95% confidence interval was very broad. These data are presented in Tables 16 and 17 and Figures 20 and 21. Our interpretation of these data is that patients with diabetes and HF have reduced mortality when treated with beta-blockers. It is possible that the relative reduction in mortality may be less for patients with diabetes than for those without diabetes, but since the absolute risk of mortality is so much greater in diabetic patients, the absolute risk reduction is almost certainly greater for diabetic than for nondiabetic HF patients treated with beta-blockers.
Table
Table 16. Effect of beta-blockers on mortality from heart failure in diabetic and nondiabetic patients (relative risk analysis).
Table
Table 17. Effect of beta-blockers on mortality from heart failure in diabetic and nondiabetic patients (hazard ratio analysis).

Figure
Figure 20. Effect of beta-blockers on mortality in nondiabetic heart failure patients (relative risk analysis).
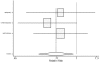
Figure
Figure 21. Effect of beta-blockers on mortality in diabetic heart failure patients (relative risk analysis).
Race
We were able to obtain race-stratified data to assess the effects of beta-blocker treatment on mortality in four studies. These studies were BEST, COPERNICUS, MERIT-HF and US Carvedilol. As mentioned above, BEST was judged to be clinically dissimilar to the other studies and was not included in the pooled analysis. The CIBIS-II study was conducted in Scandinavian and European countries and did not enroll appreciable numbers of black patients. In aggregate, the three studies included in the pooled analysis included 545 black and more than 6,000 white patients. Both the relative risk analysis and the hazard ratio analysis yielded similar results. The pooled random-effects estimate of the relative risk of the effect on mortality for black patients was 0.67 (95% CI: 0.39, 1.16), whereas for white patients, it was 0.63 (95% CI: 0.52, 0.77). The corresponding pooled estimates from the hazard ratio analysis were 0.64 (95% CI: 0.36, 1.16) for black patients and 0.59 (95% CI: 0.45, 0.76) for white patients. These data are displayed in Tables 18 and 19 and Figures 22–25.
Table
Table 18. Effect of beta-blockers on mortality from heart failure in black and white patients (relative risk analysis).
Table
Table 19. Effect of beta-blockers on mortality from heart failure in black and white patients (hazard ratio analysis).
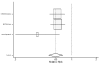
Figure
Figure 22. Effect of beta-blockers on mortality in white/nonblack heart failure patients (relative risk analysis; without BEST).
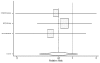
Figure
Figure 23. Effect of beta-blockers on mortality in black heart failure patients (relative risk analysis; without BEST).
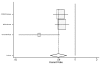
Figure
Figure 24. Effect of beta-blockers on mortality in white/nonblack heart failure patients (hazard ratio analysis without BEST).
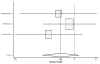
Figure
Figure 25. Effect of beta-blockers on mortality in black heart failure patients (hazard ratio analysis without BEST).
In contrast, black patients in the BEST study had a statistically significant difference in mortality compared to white patients when treated with bucindolol. In fact, the relative risk and hazard ratio for mortality exceeded 1 for black patients (although this was not statistically significant). Our interpretation of these data is that black patients are likely to have the same relative risk reduction as white patients treated with the beta-blockers bisoprolol, metoprolol, or carvedilol. Although the results for black patients were not statistically significant compared to placebo, because the point estimates of effect were similar to white patients, we judge the most likely reason for this finding to be the much smaller sample size. In contrast, bucindolol was associated with worse mortality outcomes in black patients than in white patients, and may actually increase mortality in blacks. Additional data are needed in this area.
Results of Cost-Effectiveness Analysis
Assessing Treatment of Asymptomatic Left Ventricular Dysfunction
Model Validation
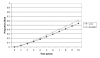
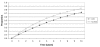
For the base-case analysis of a 55-year-old man with an ejection fraction less than 40% and no history of symptomatic heart failure, the model predicted an average life expectancy without ACE inhibitor treatment of 8.1 years (Figure 26) and a 57% five-year morbidity and mortality rate (Figure 27). These results are similar to the findings of the SOLVD prevention study.3
Base-Case Results
Treatment with ACE inhibitors improved survival and quality-adjusted survival by eight months compared to no treatment (Table 20). The lifetime cost of care was $3,718 greater for patients treated with ACE inhibitors with a cost per life year gained of $5,802 and cost per QALY gained of $5,644 compared to no treatment (Table 20).
Table
Table 20. Cost-effectiveness base-case results for assessing treatment analysis.
Sensitivity Analyses
We tested the robustness of our base-case findings by varying each of the assumptions in Table 4 over the ranges listed. Treating asymptomatic patients with ACE inhibitors provided benefit compared to waiting for symptom development and remained economically attractive (<$20,000 per QALY gained) throughout the range of every variable tested. We describe a subset of the variables tested in sensitivity analyses in the following paragraphs.
Patient Age. For the base-case analysis, we assumed an age of 55 years. For older age groups, both the cost and benefit of treatment with ACE inhibitors decreased. For an 80-year-old person, the marginal cost-effectiveness was $6,650 per QALY, which was only slightly higher than $4,666 per QALY for a 50-year old person.
Risk of Death with Heart Failure. Our base-case analysis assumed that the risk of death for patients with heart failure treated with ACE inhibitors was 6.5 times greater than the risk of death for the U.S. age-adjusted population.3 If we assumed a lower risk of death (relative risk 2.0), both costs and life expectancy increased, but the cost-effectiveness ratio remained favorable ($4,093 per QALY).
Reduction in Heart Failure Incidence. If the reduction in heart failure incidence with ACE inhibitor treatment was only half of the effect observed in the SOLVD trial, treatment cost remained less than $10,000 per QALY gained. Even when we assumed no reduction in mortality for asymptomatic patients treated with ACE inhibitors, treatment had to reduce the yearly probability of developing symptomatic heart failure from 9.8% (untreated) to only 9.5% (3% relative risk reduction) for the cost-effectiveness ratio to drop below $100,000 per QALY gained, and to 9.1% (7% relative risk reduction) for the cost-effectiveness ratio to drop below $50,000 per QALY gained.
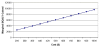
Reduction in Risk of Death for Asymptomatic Patients. In the base case, we assumed a slight improvement in survival with ACE inhibitor treatment, independent of the development of heart failure. Even when we removed this assumption, the cost-effectiveness of ACE inhibitor treatment remained only $6,474 per QALY (Figure 28).
Probability of Hospitalization if Symptomatic. We assumed that 11% of patients with heart failure would he hospitalized each year. If 15% were hospitalized each year, the cost per QALY gained dropped to $5,272. Even if hospitalizations for heart failure patients were completely eliminated by ACE inhibitor treatment, preventing heart failure ($6,539 per QALY gained) still remained cost-effective because heart failure also increases outpatient costs and worsens quality of life. Our findings were also insensitive to the probability of being hospitalized with the first episode of symptomatic heart failure.
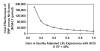
Costs. The cost-effectiveness of ACE inhibitors was insensitive to the cost of treatment. If the cost of treatment was $5 per day, the cost-effectiveness ratio remained less than $10,000 per QALY (Figure 29), and even if the cost of the ACE inhibitor were 0, treatment would not be cost saving because the improvement in survival (both before and after the development of symptoms) simply delays medical costs to older ages. If ACE inhibitors did not affect survival for asymptomatic patients with low ejection fraction, overall medical costs would be lower if the cost of ACE inhibitor treatment was less than $75 per year.
The cost of hospitalization had little effect on cost-effectiveness. Eliminating all hospitalizations did not raise the cost-effectiveness threshold above $7,400 per QALY gained. In addition, the cost of outpatient management did not affect our results. The cost per QALY gained ranged from $5,920 (if the annual outpatient cost was $200) to $5,306 (if the cost was $800). The discount rate also had little effect on the results. A discount rate of 0% resulted in $5,592 per QALY gained, compared to $5,776 per life year gained if the discount rate was 6%.
Quality of Life. We evaluated the effect of various utility values for living with heart failure on the cost-effectiveness of prevention with ACE inhibitors. In the base case, we assumed that quality-of-life utility would drop from 0.865 when asymptomatic to 0.71 when symptomatic (difference of 0.155), based on time-tradeoff utilities from the Beaver Dam Study.13
Similar results were found when we used the visual analog scale data from the SOLVD trial. In that study, the patients with asymptomatic low ejection fraction rated their quality of life at 0.68, compared with 0.60 for patients with symptoms. Using their values, we found the cost-effectiveness of ACE inhibitor treatment to be only $7,598 per QALY gained.
Assessing Screening for Reduced Left Ventricular Ejection Fraction
Base-Case Results
For a population of asymptomatic individuals, age 55 (prevalence of low ejection fraction 2.7%), we found that screening with echocardiography provided the greatest benefit but at a substantial cost. A strategy of initial screening with BNP followed by echocardiography improved outcome at a cost of only $18,300 per QALY gained compared to no screening (Table 21). If quality of life is ignored, BNP screening costs $19,000 per life-year gained compared to no screening. The number needed to screen was 77 to gain one year of life and 70 to gain one QALY.
Table
Table 21. Cost-Effectiveness base-case results for assessing screening analysis.
Because the cost-effectiveness ratio of screening with the ECG compared to no screening was greater than the ratio for BNP compared to ECG screening (extended dominance), this strategy was eliminated as a possible screening option for the base-case cohort. BNP screening demonstrated extended dominance over ECG screening, because the incremental cost-effectiveness ratio for BNP compared to ECG screening was less than the ratio for ECG screening compared to no screening. Willingness to pay for the benefits of ECG screening ensures a willingness to pay for the extra benefits of BNP screening. Similarly, strategies of relying only on the ECG or BNP to determine treatment were eliminated because they were more costly and provided fewer QALYs than the strategy using BNP followed by echocardiography. The ECG- and BNP-only strategies are not discussed further. All future references to BNP or ECG screening assumes that abnormal tests are followed by echocardiography.
Sensitivity Analyses
We tested the robustness of our base-case findings by varying each of the assumptions in Table 5 over the ranges listed. The decision to screen is primarily affected by the prevalence of low ejection fraction and the accuracy of the screening tests. The model was only mildly sensitive to the costs of screening, including echocardiography and BNP testing. The results of the sensitivity analysis are detailed here.
Prevalence of Depressed Left Ventricular Function. For the base-case analysis, we assumed an asymptomatic population of 55 and older would be screened. The prevalence of depressed ejection fraction will be higher in older populations and groups with established cardiovascular disease (Table 22). If the prevalence of low ejection fraction is at least 0.4%, the incremental cost-effectiveness of BNP screening is less than $100,000 per QALY gained (Figure 30). For the cost-effectiveness ratio with BNP screening to be less than $50,000 per QALY gained, the prevalence must be greater than 0.8%; to be under $20,000 per QALY gained, the prevalence must be 2.5%. BNP screening is never cost saving, even at 100% prevalence of disease, because treatment of asymptomatic patients with ACE inhibitors is more expensive than not treating these patients.
Table
Table 22. Cost-effectiveness literature review of prevalence of reduced ejection fration.
Test Characteristics of BNP. Past population studies of patients over 55 have indicated that the sensitivity of BNP (using a cut-off of 17.9 pg/ml) for depressed ejection fraction is 89%. If the sensitivity is actually below 65%, ECG screening is preferred (sensitivity 60%, specificity 82%, Figure 31). The specificity of BNP testing for detecting depressed left ventricular function is estimated to be 71%. Even if the specificity is 50%, the cost per QALY gained would be less than $50,000, compared to screening with the ECG (Figure 32). If the specificity is at least 70%, the ECG strategy is no longer viable (eliminated by extended dominance).
Past studies have used different cut-points for an abnormal BNP test, based on the appearance of the receiver-operator characteristics curve. However, the particular cutoff chosen may not be optimal in terms of cost-effectiveness. Using various sensitivity and specificity combinations from the MONICA patient population,20 we found that both cost of care and quality-adjusted survival improve as sensitivity increases and specificity decreases. If society is willing to pay $100,000 per QALY gained, using a low BNP threshold (24ng/ml) that produces a sensitivity near 96% with specificity near 65% is optimal. However, if society will pay only $20,000 per QALY gained, then a BNP threshold slightly above 18ng/ml (sensitivity 72%, specificity 90%) is optimal.
Cost of Testing. BNP testing remained the optimal strategy over a wide range of test costs (Figure 33). The cost per QALY gained with BNP screening (compared to ECG screening) remained less than $50,000 as long as the cost of the BNP test was less than $120.
The Medicare reimbursement for two-dimensional echocardiography has been dropping (without adjustment for inflation) in an attempt to better match actual costs of delivering treatment, as estimated by the Center for Medicare and Medicaid Services (formerly the Health Care Financing Administration). Significant disagreement exists between specialty societies and Medicare regarding the actual cost of an echocardiogram. However, even if echocardiography costs were as high as $1,000, BNP screening would still cost only $37,600 per QALY gained compared to ECG screening, and ECG screening would cost $34,200 per QALY gained compared to no screening.
ECG is similar in price to BNP testing. Therefore, the decision to use one over the other is based on the differences in test characteristics.
Impact of ACE Inhibitors for Patients with Reduced Ejection Fraction. In the base case, we estimated an increase in 0.6 QALYs for patients with low ejection fraction who take ACE inhibitors while asymptomatic compared to those who initiate treatment when they develop heart failure. If the gain in QALYs with preventive ACE inhibitor use is at least 0.3, screening with BNP costs less than $50,000 per QALY gained, compared to no screening (Figure 34).
ACE Inhibitor Use in Healthy Patients. We assumed a small decrement in quality-adjusted survival (0.001 years or 0.37 days) each year to account for potential side effects of ACE inhibitor treatment. Because no quality-of-life studies of ACE inhibitor use in healthy patients are available, the negative health impact of taking unneeded medication is unknown. However, our findings were similar over a wide range of quality-of-life decrements for ACE inhibitor treatment. The cost-effectiveness of BNP screening (compared to no screening; ECG screening was eliminated by extended dominance) ranged from $18,200 per QALY gained (for no decrease in quality adjusted survival) to $20,300 per QALY gained (for a three-day reduction per year in quality-adjusted survival) for normal patients taking ACE inhibitors.
- Results - Pharmacologic Management of Heart Failure and Left Ventricular Systoli...Results - Pharmacologic Management of Heart Failure and Left Ventricular Systolic Dysfunction
Your browsing activity is empty.
Activity recording is turned off.
See more...