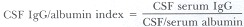Definition
Cerebrospinal fluid (CSF) is a biologic fluid, formed mainly in the ventricular choroid plexus, distributed within the ventricular system, basal cisterns, and subarachnoid space. Analysis of the CSF provides invaluable diagnostic information because diseases take place either within its bounding membranes (e.g., meningitis) or in the adjoining parameningeal structures of the brain (e.g., brain abscess).
Table 74.1 lists normal lumbar CSF values for adults. A detailed discussion of normal values and the factors affecting them can be found in Fishman (1980).
Table 74.1
Normal Values for Adults (Lumbar CSF).
Technique
There are multiple indications for performing a lumbar puncture. Examination of the CSF is useful in patients suspected of having meningitis, encephalitis, neurosyphilis, subarachnoid hemorrhage, multiple sclerosis, Guillain-Barré syndrome, meningeal carcinomatosis, and many other processes that may involve the CNS. Lumbar punctures are often performed on patients with unexplained seizures, fever of unknown origin, dementia, and acute confusional states. CSF examination is performed as follow-up in various meningitides and as a preface to instituting anticoagulant therapy. Therapeutically the technique is used in the treatment of certain diseases, and diagnostically for the introduction of contrast material. This list is incomplete and does not address complex issues related to if and when to perform the test in the various conditions listed above.
Contraindications must be considered with care before performing the lumbar puncture. The procedure is absolutely contraindicated when there is infection in the skin overlying the access site because of the likelihood of producing purulent meningitis.
Papilledema is a relative contraindication. When the papilledema is caused by pseudotumor cerebri, lumbar puncture is not contraindicated and may be used as part of the therapy. Papilledema caused by an intracranial mass lesion is another matter. Mass lesions cause distortion and obstruction of the ventricular system. Interference with intracranial pressure dynamics, in the face of such displacement, can result in the production of or exacerbation of tentorial or cerebellar pressure cones, leading to death due to brainstem compression. Deterioration can be immediate, or occur within 12 hours (Duffy, 1969). The availability of computed tomography (CT) has solved to a large degree the dilemma previously faced by clinicians seeing a patient with papilledema in whom the diagnosis was obscure. A lumbar puncture can be performed with some degree of safety in the presence of papilledema when a CT scan demonstrates there is no obstruction or displacement of the ventricular system and no evidence of a mass lesion.
A bleeding diathesis is a relative contraindication to lumbar puncture. A platelet count of 50,000 or lower greatly increases the possibility of a spinal epidural hematoma occurring after the procedure. The platelet count, prothrombin, and partial thromboplastin times should be evaluated in susceptible patients. Appropriate replacement therapy should be instituted and maintained a suitable time in the event of abnormalities. These statements also apply to patients who are anticoagulated for therapeutic reasons.
Another relative contraindication is severe pulmonary disease or respiratory difficulty in the patient. The optimum position of the patient significantly decreases pulmonary function. Lumbar puncture should be approached with great caution in the hypoxic patient. Appropriate respiratory support measures should be undertaken before the procedure. An assistant should monitor respiratory function at every moment while the spinal tap is being performed.
Performance of the lumbar puncture begins with carefully explaining to the patient and/or the family the indications, complications, and technique of the procedure. Many patients have an ingrained fear of lumbar puncture, based on anecdotal accounts of various mishaps. Fear of paralysis ranks high on the list of patient concerns. A painstaking explanation of the procedure couched in language understandable to the particular patient will usually alleviate this and other anxieties.
The technique is simple, but success often eludes the operator who is not meticulous about the position of the patient and the needle. Three essential features must be observed:
- The patient must be precisely horizontal.
- The back of the patient must be exactly perpendicular to the bed or table.
- The needle must be inserted in the exact midline parallel to the horizontal plane.
Conscientious adherence to these points will usually mean an effortless and virtually painless procedure.
The patient is positioned on a hard surface (bed or table) on his or her side, with the craniospinal axis parallel to the floor. Any elevation of the head above the level of the spinal needle may falsely elevate the CSF pressure. An assistant is helpful in maintaining the neck and thighs acutely flexed and at the same time keeping the back in the vertical plane. The greater the degree of flexion, the greater the likelihood of success—but bear in mind the cautions above with respect to hypoxic patients! The operator should check the position of the patient with respect to the horizontal, and the perpendicularity of the back to the horizontal plane after positioning. The importance of this cannot be too strongly emphasized.
Sterile technique is mandatory. After the area has been prepared, local anesthesia should be used. A small bleb should be made in the exact midline, precisely where the spinal needle will be inserted. It is not necessary to infiltrate deeper than the subcutaneous tissues. If the spinal needle is in the exact midline plane, there will be no pain. The occurrence of pain after the skin has been penetrated immediately alerts the clinician to the fact that the spinal needle is not properly positioned.
The optimal entry site is the midline of the L3–4 interspace (crossed by a line connecting the tops of both iliac crests). The two lower spaces are also usable.
A 20 or 22 gauge needle is recommended. A stylet is necessary. Epidermoid tumors are occasionally present in the midline. The stylet prevents the implantation of these tumor cells into the subarachnoid space. The bevel of the needle is pointed upward (toward the ceiling in the horizontal patient) so that the point slips in between the fibers of the dura, leaving the smallest possible hole. The midline is the necessary entry site. This site avoids the nerve roots, which are fixed laterally as they exit the spinal canal. The importance of lining the needle up at an exact right angle to the patient's body has been emphasized above. As soon as the needle penetrates the skin, the hub is aimed at the umbilicus. Any pain on the part of the patient is an indication that the needle is not in the exact midline, which is where it should be for a successful procedure. If there is pain, reassess the position of the needle, withdrawing into the subcutaneous tissue in order to redirect it, if necessary.
Slowly advance the needle till a "snap" or "give" is felt at approximately 3 to 5 cm depth. This sensation is created by penetration of the ligamentum flavum. The stylus is withdrawn. If no CSF is obtained, rotate the needle 90 degrees. If this is unsuccessful, withdraw the needle to the subcutaneous plane and realign it.
As soon as CSF appears in the hub, the three-way stopcock is inserted and the CSF manometer is attached rapidly, in order to avoid significant fluid loss that can falsely lower the CSF pressure.
The CSF should rise and fall in a gentle rhythm if the needle is correctly placed. There are two rhythms to the oscillations: a 2 to 5 mm variation with heartbeat, and a 4 to 10 mm variation with respiration. If there is any question about the free flow of the fluid, have the assistant gently but firmly compress the patient's abdomen. The pressure should immediately rise 5 to 15 mm (due to increased pressure in the venous system) and then rapidly fall as the abdominal pressure is released. If it appears there is partial obstruction to fluid flow, gingerly rotate the needle and see if there is free flow (a nerve fiber may be blocking the aperture). If this maneuver is unsuccessful, then tinker gently with the position of the needle.
Note the opening pressure. If it is above normal, several maneuvers should be performed to make sure the pressure is not falsely elevated. The usual cause of a falsely elevated pressure is abdominal compression, with a resulting elevation of venous pressure, due either to position or anxiety with a consequent tensing of the abdominal musculature. Have the assistant gently straighten the legs. In most cases the pressure will decrease to normal. If this does not work, give the patient a distracting task—enumerate the months of the year, names of children, etc. Most false elevations of pressure will be taken care of with these stratagems. The record of every lumbar puncture with an elevated pressure should contain the notation of the procedures performed to ascertain the correctness of the pressure.
If the opening pressure is felt to be truly greater than 300 mm, then only 2 ml of CSF should be removed, and the procedure terminated. This is a prudent measure, even though the hydrodynamic changes that cause herniation in the face of elevated pressure are due to CSF leakage through the dural rent created by the needle and not to the amount removed for laboratory examination. The leakage can continue for many hours. The patient should be placed in an intensive care unit and observed closely for signs of herniation for the next 24 hours. A CT scan of the head should be done immediately if one was not done prior to the lumbar puncture. Hyperventilation and mannitol therapy should be instituted if appropriate.
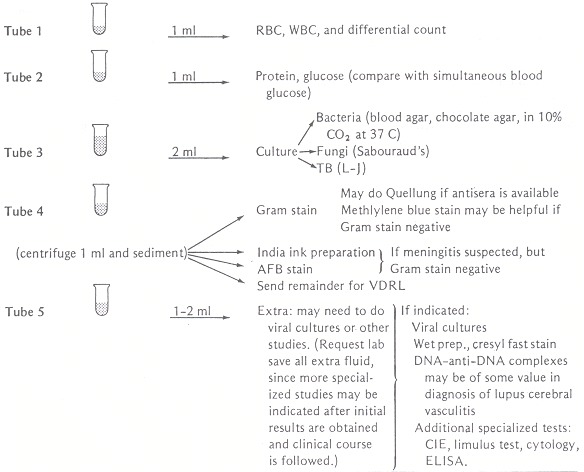
Ten to 15 ml of fluid may be removed from the adult patient and apportioned into tubes as outlined in Figure 74.1.

Figure 74.1
Studies indicated for evaluation of CSF. From Shulman JA, Schlossberg D. Handbook of differential diagnosis in infectious diseases. New York: Appleton-Century-Crofts, 1980;30. By permission.
The closing pressure reading should be recorded, as the drop from the opening pressure can give a rough estimate of pressure–volume relationships.
There are several other techniques for obtaining CSF that can be done in special situations. These include a lateral cervical puncture, cisternal puncture, and lateral lumbar puncture (Fishman, 1980).
The complications of lumbar puncture are relatively few in number, albeit either unpleasant and/or dangerous.
Brain herniation is the most serious complication. Both uncal and cerebellar herniation may occur. The incidence in the literature is variously reported at 1 to 15% (Fishman, 1980). The mechanism appears to be a rostrocaudal displacement of the brain in the face of a partial obstruction to CSF flow that existed prior to the lumbar puncture. Therapeutic maneuvers have been discussed above.
Headache after the tap is the most common complication. Tourtellotte and associates (1964) reported an extensive review of the literature. The incidence after spinal anesthesia is around 10%. The onset occurs in 15 minutes to 4 days after the procedure, usually lasting 4 to 8 days. They are usually frontal (often retro-orbital) and are described as pounding. The headache occurs whenever the patient sits or stands, and disappears with recumbency. The use of small-gauge needles in performing the procedure has greatly lessened the occurrence of headaches. In severe cases a spinal epidural patch with the patient's own blood is highly effective in relieving the headache. Studies relating immobility in bed after the procedure to occurrence of postspinal headache have been contradictory.
Diplopia due to unilateral or bilateral sixth nerve palsy occurs in a small percentage of patients. The lowered CSF pressure causes the sixth nerve to stretch as it courses over the petrous bone. The diplopia is usually transient, although on rare occasions it may be permanent. A patch is useful for a few days. If the injury is permanent, then consultation with an ophthalmologist is necessary, as certain measures (such as application of botulinum toxin) can be quite helpful.
Subarachnoid hemorrhage, or a "traumatic" tap, is common. The needle penetrates some of the small equina vessels, causing the hemorrhage. Xanthochromic spinal fluid, a fever, and stiff neck may ensue later, mimicking a spontaneous subarachnoid bleed.
A spinal epidural hematoma or spinal subdural hematoma may occur due to nicking of the spinal radicular arteries.
Other and more rare complications are discussed in Fishman (1980).
Examination of the CSF begins immediately after collection of the specimen. The fluid is observed for turbidity; normal fluid is clear. The color of the fluid is noted next. This must be done within 30 minutes of collection of the fluid, since red cell lysis after this time can affect the results. This is a vital piece of information that often provides the sole objective clue to the diagnosis of subarachnoid hemorrhage. Take a glass tube of the CSF and prepare an identical test tube filled with the same amount of water. Look down the long axes of the tubes against a white background. If any difference is perceived between the two tubes, then there is an abnormal pigment in the CSF specimen. Xanthochromia is the usual abnormal color. Diagnostic considerations that apply to xanthochromia are given below.
A gram stain, AFB (acid-fast bacillus) stain for tuberculosis, and India ink examination should be done promptly on the CSF. The gram stain has the virtue of providing an instant, almost exact etiologic diagnosis in bacterial meningitis, thus permitting much needed specific therapy, in 60 to 80% of cases of bacterial meningitis. For undetermined reasons, the yield in Listeria monocytogenes meningitis is much lower (30%). False positive results have been reported on rare occasions (Musher and Schell, 1973). The yield of the gram stain may be improved by use of the cytospin slide centrifuge (Peterson et al., 1980).
The India ink examination has the charm of an immediate, specific diagnosis of cryptococcal meningitis within minutes of obtaining CSF. Unfortunately it is positive in only 50% of cases of cryptococcal meningitis (up to 80% in AIDS patients). Be sure to use well-filtered India ink in order to avoid false positive results. Another source of false positive results is misinterpretation of small mononuclear cells as yeasts. This can be avoided by the experienced observer by noting the highly refractile cell wall and internal structure of the cryptococci (not seen in lymphocytes) and the fact that the interface between the ink and the cells is blurred when they are leukocytes. To be more specific, only budding yeast cells seen on India ink examination shall be considered cryptococci.
Sensitivity of the AFB stain has varied widely in different reported series, ranging from 9% to as high as 91%. The latter yield was reported by Stewart (1953) with the "stacking" method: After centrifuging 10 to 20 ml of CSF (at 250 rpm/30 min) all but a few drops are removed. Then a thick smear containing the whole of the pellet is stained and the slide examined for 30 to 90 minutes. Newer approaches such as adenosine deaminase and tuberculostearic acid are being developed, since the diagnosis of tuberculosis meningitis is difficult. Only time will tell.
The tubes are sent for laboratory examination according to the schema outlined in Figure 74.1. Examination of the fluid for white cells and red cells must be done within 1 hour of collection, before the cells have been lysed.
A procedure note should be written in the chart after the lumbar puncture is completed. This note should contain the following information:
- Indications for procedure
- Complications (if any)
- Technique
- Condition of patient after procedure
- Opening pressure with statement about dynamics
- Closing pressure
- Turbidity
- Color: state "colorless" if this is the case
- Laboratory values available when note written as well as a list of tests ordered
- Analysis of information obtained
Further laboratory information and analyses should be entered as they are obtained. It is of some benefit to save in the refrigerator at 18°C an extra tube of CSF to be analyzed if further tests are indicated after the initial evaluation.
Basic Science
There are a number of excellent reviews of the basic science of the CSF (Fishman, 1980; Cutler and Spertell, 1982; McComb, 1983; Davson, 1967). CSF is secreted by the choroid plexus at the rate of about 500 ml per day, 0.35 ml per minute, and a turnover of 14% per hour. The usual volume is 150 ml. Formation occurs as a result of a two-step process. Fluid is first filtered through the core capillaries of the choroid plexus into the extracellular space surrounding the choroidal cells. This fluid is a plasma ultrafiltrate. Then sodium is actively transported by sodium–potassium activated adenosine triphosphatase (ATPase) across the choroidal cells into the CSF; water follows down an osmotic: gradient. Cholinergic stimulation increases production and adrenergic stimulation decreases production. Drugs that inhibit sodium–potassium ATPase or carbonic anhydrase decrease production. Furosemide also slows CSF production due to its effect on chloride flux. Some CSF is apparently produced in the ependyma of the brain, in addition to the choroid plexus.
The arachnoid granulations, which penetrate the major dural venous sinuses, constitute the major resorptive sites. They are also present in the epidural veins around the spinal nerve roots; whether or not CSF is absorbed at these sites is unknown. These structures are herniations of the arachnoid membrane. CSF is absorbed in bulk through the arachnoid villi by mechanisms that are incompletely understood. It has recently been proposed that multiple vacuoles in I he arachnoid villous membrane allow the vesicular transport of CSF in bulk. In addition to the bulk resorption, many solutes are actively transported by the choroid plexus and other cells, such as capillary endothelial cells. Finally, solutes can disappear by diffusion into the brain and brain capillaries.
The brain is protected from macrormolecules by the blood–brain barrier. The morphologic barrier is provided by endothelial tight junctions. Several areas of the brain, however, have "windows" or sections that do not have tight junctions: portions of the hypothalamus; the area postrema; the subfornical and subcommissural organs (Fishman, 1980). In these areas the plasma has closer proximity to osmoreceptors and chemoreceptors in the brain. The molecular weight of solutes greatly influences their admission into the CSF. Other solute properties that are important include lipid solubility, ionization, and protein binding. Certain solutes are actively transported by specific systems in brain endothelial cells.
Clinical Significance
Analysis of the CSF provides invaluable insight into pathologic derangements of the nervous system. The sequence of the discussion follows the usual order of examination of the CSF.
Pressure
The usual range of opening pressure in the literature is 50 to 200 mm CSF. Generally pressures over 180 are considered to be abnormal. The causes of intracranial hypertension are manifold, ranging from pseudotumor cerebri to intracranial neoplasms. Meningitis, subarachnoid hemorrhage, elevated central venous pressure and a host of other conditions elevate CSF pressure. A reason must be found in every case. Table 74.2 presents a highly useful classification of various causes of brain edema.
Table 74.2
Causes of Brain Edema.
Comparison of the opening and closing pressures provides a crude estimation of the volume of the CSF reservoir. A large fall in pressure indicates a very small reservoir (e.g., opening pressure 80 mm, closing pressure after removal of 1 ml, zero), while very little change after removal of a large amount of fluid indicates a large reservoir. This observation was of greater value when techniques such as CT scanning and nuclear magnetic resonance imaging were not available.
Clarity
The normal CSF is crystal clear. The occurrence of pleocytosis is the usual reason for cloudy fluid. At least 200 white cells per cubic millimeter can be present without altering the clarity. Over 500 white cells per cubic millimeter usually produces cloudiness. Red cell concentrations between 500 and 6000 per cubic millimeter can cause the fluid to appear cloudy, while concentrations of over 6000 give a grossly bloody appearance. A markedly elevated protein can also alter the clarity of the CSF.
The clarity of the fluid is of little clinical use, except to provide an immediate indication of abnormality of the CSF. A very useful point to remember is that a large number of cells can be present without affecting the clarity.
Color
The presence or absence of color, usually xanthochromia, in the CSF is a crucial observation. Xanthochromia commonly indicates spontaneous subarachnoid hemorrhage. A variety of conditions, however, can also produce xanthochromia: a traumatic tap; bilirubin due to jaundice; protein; etc. Table 74.3 summarizes factors which need to be considered in the analysis of xanthochromia.
Table 74.3
Analysis of Xanthochromic CSF.
Cells
Analysis of the cellular components of the CSF is the single most valuable indicator of infectious diseases of the nervous system. Infections of the nervous system produce what Shulman and Schlossberg (1980) have described as three basic CSF types. Using these types as a guide, the clinician can come up with a list of the most likely diagnoses. Clinical information and other tests can help to identify the specific diagnosis. Tables 74.4, 74.5, and 74.6 summarize this information. The discussion that follows is anchored to these tables.
Table 74.4
Description of CSF Types.
Table 74.5
Differential Diagnosis of Infectious Causes of CSF Pleocytosis.
Table 74.6
Differential Diagnosis of Noninfectious Causes of CSF Pleocytosis.
Type A fluid is characterized by 500 to 20,000 WBC, 90% of which are polymorphonuclear leukocytes, low CSF sugar, and protein elevated to 100 to 500 mg/dl. Bacterial meningitis is the most common cause of this CSF formula. A gram stain of the CSF sediment will reveal the causative organism in up to 80% of cases. An exception is Listeria monocytogenes, which will have a positive gram stain in only 30% of cases. In those cases where the gram stain is non-revealing, the patient's age and the clinical and epidemiologic setting may offer clues to the etiologic agent.
Partially treated bacterial meningitis (Dalton and Allison, 1968) on rare occasions (5%) will have type C fluid characteristics due to incomplete treatment with empirical antibiotics. Most such CSF specimens will retain type A characteristics, with some decrease in sensitivity of gram stain (by 25%) and culture (by 30%) as compared to untreated patients. Also noteworthy is the fact that gram-positive organisms tend to appear as gram negative after exposure to antibiotics. The mean CSF" pleocytosis, protein, and glucose level are not significantly affected by prior empirical therapy. Multiple factors play a role in the effect of prior antibiotic: therapy on the identification of the etiologic agent, such as sensitivity of the organism to the empirically administered antibiotic and CSF penetration of the antimicrobial that has been used.
Acute bacterial meningitis will on rare occasions produce minimal or even absent CSF abnormalities. This so-called normocellular or developing bacterial meningitis occurs most commonly in immunosuppressed patients. It is fortunately quite uncommon.
There are a number of tests that may be valuable aids in the diagnosis of bacterial meningitis. An excellent review on the clinical relevance of rapid diagnostic techniques for detection of bacterial polysaccharide antigens has been published (Kejlan, 1983). A recent paper reviews rapid but nonspecific techniques in the evaluation of bacterial meningitis, such as C-reactive protein, LDH isoenzymes, gas-liquid chromatography (for "chemotyping" etiologic agents by means of analysis of lipid, carbohydrate, and lipopolysaccharide components of bacterial cells) and Limulus assay (Martin, 1983).
Primary amebic meningoencephalitis (Duma et al., 1971) is a rare but devastating condition, caused by free-living amebas. The disease is classically associated with fresh water exposure (swimming in ponds and lakes in the summer). The organisms are thought to penetrate through the nasal mucosa; hypo-osmia is frequently reported. After a brief incubation an acute, rapidly progressive meningoencephalitis ensues. The classic epidemiology, absence of organisms on gram stain and eventually on culture, and a hemorrhagic component to the fluid should promptly suggest the diagnosis, which can be confirmed by wet mounts of the CSF revealing motile trophozoites. Amphotericin B therapy, both systemically and intrathecally, has occasionally met with some success.
Ruptured brain abscess is a medical catastrophe with an extremely high mortality. The diagnosis is suggested by a gram stain showing multiple types of organisms, with tinctorial characteristics suggestive of anaerobes, an extremely high protein level, and the eventual isolation of multiple organisms, including anaerobes.
Nonsteroidal anti-inflammatory agent (NSAIA) meningitis is a peculiar and rare entity that has been described recently. NSAIA is seen predominantly but not exclusively in patients with collagen vascular diseases (Ballas and Donta, 1983). At variable intervals after initial exposure to a different NSAIA, patients develop a clinical picture compatible with acute meningitis. CSF examination shows pleocytosis (as high as 2000 WBC/mm3 [white blood cells per cubic millimeter]) with marked predominance of polymorphonuclear leukocytes (PMLs) (as high as 100%), variable protein elevation (as high as 50 mg/dl), and minimal or no drop in glucose. This is obviously a diagnosis of exclusion. Most patients need empiric antibiotic coverage for acute bacterial meningitis until the diagnosis is established in retrospect.
Other drugs that have been associated with neutrophilic pleocytosis include sulfonamides and isoniazid. Chronic neutrophilic meningitis (Peacock et al., 1984), albeit rare, may become more common as the number of chronically immunosuppressed hosts increases.
Type B fluid is characterized by 25 to 500 WBC that are mononuclear cells (but may be PMLs early in the course of the disease), a low or occasionally normal CSF sugar, and a protein of 50 to 500 mg/dl. This fluid is characteristic of tuberculosis and other granulomatous meningitides.
Tuberculous meningitis (Molavi and LeFrock, 1985) usually has an insidious presentation; it can present more acutely, usually in children. Although the hallmark is a mononuclear pleocytosis, the CSF on occasion will have an early PMN predominance. If this is the case, there will be a gradual shift to mononuclear cells over 7 to 10 days.
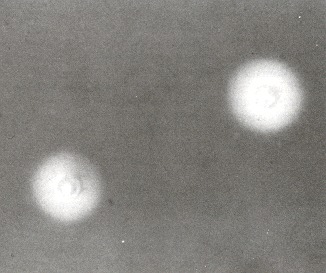
Fungal meningitis can be produced by a variety of fungal agents, the most frequent of which are cryptococci, histoplasma, coccidioides, and Candida. Usually seen in immunosuppressed individuals and/or in highly endemic areas (in the Ohio valley for histoplasma, southwestern U.S. for coccidioides), they present acutely or in an indolent fashion. In the case of cryptococcal meningitis, an India ink preparation will show the characteristically encapsulated yeasts in 50% of patients (Figure 74.2).
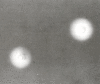
Figure 74.2
India ink preparation of cerebrospinal fluid showing two Cryptococcus neoformans cells. Note the consistent central position of the yeast cell, surrounded by the capsule. Most white blood cells, which may resemble cryptococcal cells superficially, will (more...)
A number of serological tests can aid in the diagnosis of certain of these causes of meningitis. The serological test for cryptococcal antigen detection is remarkably accurate. It has a sensitivity of 95% when compared to the gold standard, isolation of the fungi, and high specificity when the possible interference by rheumatoid factor is addressed by appropriate absorption steps. The cryptococcal antigen titer is reported to be of prognostic value and sequential antigen levels are frequently used in evaluation of therapeutic response (Diamond and Bennett, 1974). The antigen test has the advantage of speed (as opposed to a culture that may take weeks to become positive). Histoplasma and coccidioides CSF serology is also quite useful (Plouffe and Fass, 1980).
Sarcoidosis (Delaney, 1977) often has meningeal involvement. The characteristic picture is a mild to moderate pleocytosis that is almost mononuclear, in the range of 10 to 300 WBC/mm3, mild to moderate protein elevation (between 50 and 200 mg/dl), and hypoglycorrhachia (seen in 18% of patients). This latter abnormality is seen more commonly with diffuse involvement of the meninges, hence patients with sarcoid involvement restricted to the hypothalamus are more likely to have a normal CSF.
Meningeal carcinomatosis (Dyken, 1975) may be an elusive diagnosis, even in the setting of a patient with a known primary malignancy. The crux of the diagnosis is the demonstration of neoplastic cells in the cerebrospinal fluid. The development of improved cytologic techniques has facilitated antemortem diagnosis. The most common primary tumors reported to cause meningeal carcinomatosis are breast carcinoma, lymphomas, lung carcinoma, and pancreatic carcinoma (Olson et al., 1974). The CSF opening pressure is usually elevated, although it can be normal early in the course of meningeal involvement. An abnormally low opening pressure should bring to mind the possibility of a complete spinal block, which can occur late in meningeal carcinomatosis. The CSF protein is variably elevated. Hypoglycorrhachia, the mechanism of which is not clear, is observed in the majority of cases.
Type C fluid is characterized by 5 to 1000 WBC/mm3, a mononuclear pleocytosis (may be PMLs early), normal glucose (rarely quite low), and a protein less than 100 mg/dl. A heterogeneous group of disorders can produce this CSF formula.
Parameningeal infections comprise an important group of diseases that need to be kept high in the differential diagnosis; otherwise they may go undetected. The CSF is but an initial stepping stone to their detection. Analysis of the fluid does not make the diagnosis, but begets the specific diagnostic procedure (i.e., sinus/spine/skull films, CAT of the head, etc.). The differential diagnosis of parameningeal infection includes brain abscess, subdural abscess, cerebral epidural abscess, cerebral thrombophlebitis, spinal epidural abscess, otitis, sinusitis, retropharyngeal abscess, and others. A paramount point to remember in parameningeal infections is that the lumbar puncture is not only nondiagnostic but fraught with complications in patients with the classic parameningeal infection, a brain abscess.
Listeria monocytogenes meningitis (Niedman, 1980) in a significant minority of cases (about 20%) will present with a type C CSF. This peculiar gram-positive rod with characteristic "tumbling motility" exhibits a marked neurotropism. Up to 75% of cases of listeriosis in adults reported to the Centers for Disease Control in 1971 had meningitis. The organism has a propensity to afflict those at the extremes of life and immunosuppressed hosts, particularly renal transplant recipients. However, in the review cited, consisting of 94 cases, 30% occurred in apparently healthy individuals.
Syphilis remains a major health problem. The lumbar puncture is often necessary in order to make the diagnosis and guide therapy.
Secondary syphilis that is clinically apparent has CNS involvement in 1% of cases, usually mimicking the syndrome of aseptic meningitis. Other clinical presentations include transverse myelitis, cranial nerve palsies, papilledema, thrombosis of cerebral arteries, perceptive deafness, and iritis. However, between 5 and 15% of patients with secondary syphilis and no CNS symptomatology will have an abnormal CSF as manifested by pleocytosis or elevated protein levels. Five percent will have positive CSF serology ("asymptomatic secondary neurosyphilis"). In spite of these figures, some experts believe CSF examination in individuals with secondary syphilis and no CNS symptomatology is not necessary, as adequate treatment of secondary lues (benzathine penicillin G, 2.4 million units IM × 1) prevents progression to classic (tertiary stage) neurosyphilis. In this context, the main value of doing a diagnostic LP in the rare patient with symptomatic neurologic disease is to exclude other causes of the picture of "aseptic meningitis." There may also be real value in doing follow-up examination of the CSF.
Tertiary (classic) neurosyphilis spans a wide spectrum of clinical presentations, artificially divided into four syndromes: asymptomatic; meningovascular; parenchymatous (dementia paralytica or general paresis); and tabes dorsalis.
Asymptomatic neurosyphilis by definition consists of an abnormal CSF in the absence of clinical CNS involvement. Abnormalities of the CSF consist of lymphocytic pleocytosis (<100 cells/mm3), normal or slightly elevated protein (<100 mg/dl), and positive nontreponemal test in over 90% of cases. In the pre-penicillin era, asymptomatic neurosyphilis was reported to progress to clinical neurosyphilis in 23 to 87% of cases.
Meningovascular neurosyphilis is defined as ischemic injury to any part of the CNS due to syphilitic endarteritis. It constitutes approximately 10% of cases of neurosyphilis, occurring 5 to 12 years after acquisition of the infection. Untreated, it may progress to parenchymatous disease. The CSF reveals mild (10 to 100 cells/mm3) lymphocytic pleocytosis, protein in the range of 40 to 250 mg/dl, and a positive VDRL.
Parenchymatous neurosyphilis, or general paresis, is syphilitic meningoencephalitis. It has an incubation period of 15 to 20 years and is a composite of neurologic and psychiatric manifestations that span the gamut. Of note, seizures (seen in up to 20% of cases) may be the presenting manifestation. The CSF shows essentially the same characteristics as described for the meningovascular type.
Tabes dorsalis is currently the most uncommon form of neurosyphilis. In the pre-penicillin era it accounted for one-third of cases. Its incubation period is in the range of 20 to 25 years. Lightning pains, visceral crises, broad-based stamping gait, optic atrophy, Charcot joints, and pupillary abnormalities are the salient features of this syndrome, reflecting syphilitic damage to posterior roots and posterior column dysfunction. The cell count may be normal in up to 50% of cases and the CSF VDRL nonreactive in a third of patients.
In the evaluation of patients suspected of having neurosyphilis it is well to remember the insensitivity (nonreactive in 30%) and nonspecificity of the blood nontreponemal tests. Ten percent of individuals over 70 years old have a biologic false positive nontreponemal test. These patients are the ones usually being worked up for dementia. Such patients should have a treponemal test done (with or without a nontreponemal test).
The FTA-Abs test is not recommended in the CSF as it is extremely sensitive. Consequently, minimal contamination of the CSF with FTA-Abs positive blood, at concentrations not detectable by the naked eye, will give false positive results. Unfortunately, since the CSF nontreponemal test may be falsely negative, there is no study in the literature that investigates the specificity of a CSF FTA test when there is absolutely no contamination as ascertained by no RBC in the CSF fluid.
Rocky Mountain spotted fever (RMSF) may cause type C CSF. A stiff neck in patients with RMSF may be due to meningitis or to myalgias of the cervical musculature. In a series from North Carolina (Kaplowitz et al., 1981), CSF pleocytosis was present in 38%, with WBC ⩾ 100/mm3 in 7 out of 63 patients. A PMN predominance was seen in 50% of cases (including all those CSF with WBC > 100/mm3). Modest depression of CSF glucose was present in 5 out of 62; 4 of these 5 patients died. Protein ranging from 100 to 300 mg/dl was present in 6 out of 62. When severe, RMSF may resemble meningococcemia.
Toxoplasmosis (Luft and Remington, 1985) of the CNS can produce different syndromes, depending on the host. In immunocompetent individuals CNS involvement is rare, presenting as part of a syndrome of diffuse and uncontrolled dissemination or as active infection limited to the CNS. In immunosuppressed hosts (lymphoproliferative neoplasms, organ transplant recipients, AIDS and miscellaneous immunosuppressive states such as collagen vascular diseases and hemochromatosis), toxoplasma can produce a severe necrotizing encephalitis. The third clinical setting is that of congenital toxoplasmosis. The CSF routine examination (cells, protein, and glucose) is that of type C fluid without specific diagnostic features. Approaches to a specific diagnosis (isolation of the organism by mouse inoculation, immunofluorescent staining of spun CSF, toxoplasma antibody titers in CSF) have met with insensitivity and nonspecificity problems. The current approach is to evaluate the local production of antibodies within the CNS.
Herpes simplex I (HSV-I) meningoencephalitis, while a rare complication of HSV-I infection, is probably the most common acute nonepidemic viral encephalitis in the United States (Whitley, 1981). This observation, coupled with the availability of specific therapy, has made HSV-I meningoencephalitis a very important diagnosis to confirm or exclude. Unfortunately, the CSF offers little help in this regard. Abnormalities are variable; a moderate pleocytosis with PMN and lymphocytes is seen. Some authors report the presence of RBCs in most cases, as a manifestation of the necrotizing nature of the disease. Mild elevation of protein and normal glucose levels complete the nonspecific findings. The final diagnosis currently rests on the brain biopsy with histologic and cultural techniques. A high index of suspicion and a vigorous and prompt diagnostic approach are required, as the earlier the diagnosis is made and therapy instituted, the better the outcome.
Viral meningitides also produce type C CSF. The term "aseptic" is a misnomer, as the etiologic agents usually implicated in this syndrome are infectious agents, albeit non-treatable. Enteroviruses account for over 50% of cases. Other agents that can produce the same clinical and CSF picture include flaviviruses (St. Louis encephalitis agent), mumps (late winter–spring), herpes simplex, lymphocytic choriomeningitis (LCM), and the human immunodeficiency virus (HIV). Helpful epidemiologic clues as to the viral nature of the etiology are occurrence in the summer months and age of the patients (usually <40 years old), but the diagnosis most of the time is one of exclusion. Not infrequently, the initial LP shows a PMN predominance that on repeat LP within 12 to 48 hours will demonstrate a shift toward mononuclear predominance. Hypoglycorrhachia may occur in meningitis due to mumps, herpes simplex, and herpes zoster infections. A rash (maculopapular or petechial), creating confusion and concern about meningococcemia, may be seen with Echo and Coxsackie viruses. An etiologic diagnosis may be attempted by cultural (CSF, throat, and stool swabs) and serologic (acute and convalescent) tests.
Human immunodeficiency virus (HIV), the causative agent of AIDS, can affect the CNS (and thus the CSF) in several ways.
- HIV may invade the CNS very early (during the acute infection). This process can be asymptomatic, can cause an "aseptic" meningitis or, uncommonly, an actue encephalopathy. A mild lymphocytic pleocytosis and a normal or mildly elevated protein are seen. HIV cultures are positive in the majority of such patients.
- After seroconversion and before overt immunosuppression, asymptomatic CSF abnormalities are not uncommon (~40%). These consist of low grade lymphocytic pleocytosis (<30/mm3) and mild protein elevation. HIV can be isolated from CSF in 0–50% of such individuals.
- Patients with ARC (AIDS-related-complex) may have bouts of acute or chronic meningitis with or without cranial neuropathies secondary to HIV (which can be isolated from the CSF in the majority). Normal to marked elevation of cells and mild elevation of proteins characterize this subset.
- Patients with AIDS (overt immunosuppression) can have two types of CNS diseases.
- CNS diseases directly caused by HIV (AIDS dementia or a symmetric distal sensory neuropathy). A minority of demented patients will have pleocytosis (<15/mm3). Protein elevation can be seen in up to 80% of cases (even in the absence of dementia or neuropathy). CSF isolation of HIV is possible in up to two-thirds of patients with AIDS, regardless of presence of dementia.
- The immunosuppression induced by HIV opens the AIDS patient to many CNS infections, mainly cryptococcal meningitis and toxoplasmosis. Also, neurosyphilis, atypical in its course and response to therapy, seems to be associated with HIV infection.
In HIV-infected individuals, the likelihood of CSF pleocytosis being related directly to HIV depends on the degree of pleocytosis (cell counts >100/mm3 should bring to mind a cause other than HIV) and the degree of immunosuppression, as assessed by T4 cell counts (e.g., in patients with depressed T4 cells, an opportunistic infection has to be strongly considered).
A problem that arises often is distinguishing between a traumatic tap superimposed on a normal CSF and a traumatic tap superimposed on a CSF that was abnormal before the tap was performed. The question comes down to whether the WBC in the CSF are there because they are found in the blood brought into the CSF by the traumatic tap, or whether there are WBC both due to the trauma and due to an abnormal process preexisting in the CSF. This question can be answered crudely by comparing the ratio of WBC to RBC in the peripheral blood to the ratio of WBC to RBC in the CSF.
Suppose the peripheral WBC is 10,000 with a RBC of 4,000,000. The ratio is derived by dividing 10,000 by 4,000,000 = 1 WBC for every 400 RBC. If the CSF has 20,000 RBC, then 50 WBC are "allowed" if the tap is traumatic. If more than 50 WBC are present in the CSF, then there were preexisting WBC in the CSF.
The difficulty of sorting out a traumatic tap from a spontaneous subarachnoid hemorrhage has been discussed above.
Proteins
CSF proteins are derived from serum proteins with the exception of the trace proteins and some beta globulins. Serum proteins enter the CSF by means of pinocytosis across the capillary endothelial cells of the brain and spinal cord. Clinical usefulness of CSF proteins is presently limited to the measurement and characterization of total protein and IgG. Three pathological conditions can cause abnormalities of the CSF proteins:
- Increased entry of plasma proteins due to increased permeability of the blood–brain barrier. The composition and pattern of CSF proteins will in this case reflect that of the plasma proteins, whether normal or abnormal.
- Local synthesis of proteins within the central nervous system. Clinical interest is limited to IgG currently.
- Impaired resorption of CSF proteins by the arachnoid villi.
Elevated CSF total protein is highly suggestive of neurologic disease. Elevation indicates increased endothelial cell permeability. Many diseases elevate the CSF protein to some degree, as seen in Table 74.7. Total protein over 500 mg/dl is seen in meningitis, cord tumor with spinal block, and bloody CSF (Fishman, 1980). Each 1000 RBC/mm3 raises the CSF protein 1.5 mg/dl (see Table 74.3). Peripheral neuropathy due to various causes (e.g., diabetes) is a frequent diagnosis in patients with modestly elevated total protein. The usefulness of protein in the diagnosis of meningitis has been discussed above.
Table 74.7
The Total Protein Content of the Lumbar Cerebrospinal Fluid from 4157 Patients.
CSF that clots due to protein over 1000 mg/dl is caused by complete spinal block, usually caused by a tumor. Froin's syndrome is the eponym. The fluid is usually intensely xanthochromic.
IgG concentration in the CSF is normally 4.6 ± 1.9 mg/dl (Fishman, 1980). It is the principal immunoglobulin in the CSF. Under normal conditions the CSF IgG is derived from the plasma. Local synthesis within the central nervous system occurs in a variety of disorders: multiple sclerosis, neurosyphilis, subacute sclerosing panencephalitis, progressive rubella encephalitis, viral meningoencephalitides, sarcoidosis, etc. The majority of these conditions are inflammatory disorders. In addition to quantification, IgG can be characterized by agar gel electrophoresis and isoelectric focusing for the identification of oligoclonal banding. Up to 90% of cases of confirmed multiple sclerosis have elevated gamma globulin and/or oligoclonal bands. Oligoclonal bands represent a qualitative change in IgG. Each band is presumed to be protein derived from the response of immunocompetent cells to a single antigen. These bands occur in the inflammatory conditions enumerated previously as causing elevated IgG. The appearance of oligoclonal bands in the CSF in the absence of similar bands in the serum is an indication of gamma globulin production in the central nervous system even when quantified levels of gamma globulin are normal (Fishman, 1980).
The difficulty in assessing IgG levels in the CSF lies in distinguishing whether elevated levels are due to increased permeability of the blood–brain barrier, or whether there is local synthesis in the central nervous system. Table 74.8 outlines the most useful method of making this determination.
Table 74.8
Fractionation of CSF Protein.
Excellent recent reviews of CSF proteins can be found in Fishman, 1980; Cutler and Spertell, 1982; Hershey and Trotter, 1980.
Glucose
The usual CSF glucose is 60 to 80% of the plasma glucose. Values under 45 mg/dl can usually be considered abnormal; values under 40 mg/dl are almost without exception abnormal. Glucose enters the CSF largely through active transport. Glucose molecules are shuttled across capillary endothelium, choroid plexus cells, neurons, and supporting cells by this mechanism (Fishman, 1980). Some glucose crosses by way of simple diffusion. It takes about 4 hours for CSF glucose levels to reach equilibrium with plasma levels. Glucose is utilized for energy by cellular elements close to the CSF; this is the principal means of glucose removal. It also enters the venous system by way of the bulk flow of CSF.
The most common cause of lowered CSF glucose (hypoglycorrhachia) is meningitis: bacterial, tuberculous, fungal, amebic, acute syphilitic, chemical, and certain of the viral meningitides (mumps, herpes simplex, and herpes zoster). In acute bacterial meningitis the CSF glucose level may remain depressed for approximately 1 to 2 weeks following appropriate therapy, at a time when cells and protein levels are essentially back to normal.
Lowered CSF glucose occurs in about 15% of cases of subarachnoid hemorrhage, reaching a nadir 4 to 8 days after the bleed.
Meningeal carcinomatosis also produces hypoglycorrhachia. A large variety of tumors have been implicated. Cytologic examination of the fluid is often the key to diagnosis.
Other causes of lowered CSF glucose include sarcoidosis, cysticercosis, trichinosis, and rheumatoid meningitis (Fishman, 1980).
References
- Ahlskog JE, O"Neill BP. Pseudotumor cerebri. Ann Intern Med. 1981;97:249. [PubMed: 7049034]
- Ballas ZK, Donta ST. Sulindac-induced aseptic meningitis. Arch Intern Med. 1983;142:165. [PubMed: 7053720]
- Cutler RWP, Spertell RB. Cerebrospinal fluid: a selective review. Ann Neurol. 1982;11:1–10. [PubMed: 6120677]
- Dalton HP, Allison MJ. Modification of laboratory results by partial treatment of bacterial meningitis. Am J Clin Pathol. 1968;49:410–13. [PubMed: 4384575]
- Daniel TM. New approaches to the rapid diagnosis of tuberculous meningitis. J Infect Dis. 1987;155:599. [PubMed: 3102626]
- Davson H. Physiology of the cerebrospinal fluid. London: Churchill, 1967.
- Delaney P. Neurologic manifestations in sarcoidosis. Ann Intern Med. 1977;87:336. [PubMed: 197863]
- Diamond RD, Bennett JE. Prognostic factors in cryptococcal meningitis. A study of 111 cases. Ann Intern Med. 1974;80:176–81. [PubMed: 4811791]
- Duffy GP. Lumbar puncture in the presence of raised intracranial pressure. Br Med J. 1969;1:407–90. [PMC free article: PMC1981862] [PubMed: 5763958]
- Duma RG, Rosenblum WI, McGehee RF. et al. Primary amoebic meningoencephalitis caused by naegelia. Ann Intern Med. 1971;74:923–31. [PubMed: 5580639]
- Dyken PR. Cerebrospinal fluid cytology: practical clinical usefulness. Neurology. 1975;25:210. [PubMed: 1089912]
- Eng RHK, Seligman SJ. Lumbar puncture-induced meningitis. JAMA. 1981;245:1456. [PubMed: 7206149]
- Fishman RA. Cerebrospinal fluid in diseases of the nervous system. Philadelphia: W.B. Saunders, 1980.
- Hershey LA, Trotter JL. The use and abuse of the cerebrospinal fluid IgG profile in the adult: a practical evaluation. Ann Neurol. 1980;8:426–34. [PubMed: 6776879]
- Hollander H. Cerebrospinal fluid normalties and abnormalties in individuals infected with human immunodeficiency virus. J Infect Dis 1988;158(4) [PubMed: 3049840]
- Hyslop NE, Montgomery WW. Diagnosis and management of meningitis associated with cerebrospinal fluid leaks. In: Remington JS, Swartz MN, eds. Current clinical topics in infectious diseases. New York: McGraw-Hill, 1982;3:254.
- Johnson KP. Cerebrospinal fluid and blood assays of diagnostic usefulness in multiple sclerosis. Neurology. 1980;30:106. [PubMed: 6156430]
- Kaplowitz LG, et al. Rocky Mountain spotted fever: a clinical dilemma. In: Remington JS, Swartz MN, eds.Current clinical topics in infectious diseases. McGraw-Hill,1981;2:89.
- Kejlan SL. Antigen detection in cerebrospinal fluid—pros and cons. AmJ Med 1983; July 28 (Suppl):109. [PubMed: 6410914]
- Kjeldsberg CR, Krieg AF. Cerebrospinal fluid and other body fluids. In: Henry JB, ed. Clinical diagnosis and management by laboratory methods. 17th ed. Philadelphia: W.B. Saunders, 1984.
- Kolmel HW. Atlas of cerebrospinal fluid cells. New York: Springer-Verlag, 1976.
- Kuberski T. Eosinophils in the cerebrospinal fluid. Ann Intern Med. 1979;91:70. [PubMed: 380429]
- Laurent JP. Subarachnoid hemorrhage. In: Woud J, ed. Neurobiology of the cerebrospinal fluid. New York: Plenum, 19XX; chap. 20.
- Luft BJ, Remington JS. Toxoplasmosis of the central nervous system. In: Remington JS, Swartz MN, eds. Current clinical topics in infectious diseases. New York: McGraw-Hill,1985;6:315.
- Martin WJ. Rapid and reliable techniques for the laboratory detection of bacterial meningitis. Am J Med 1983; July 28 (Suppl): 119–23. [PubMed: 6349338]
- McArthur JC. Neurologic manifestations of AIDS. Medicine. 1987;66:407. [PubMed: 3316921]
- McComb JG. Recent research into the nature of cerebrospinal fluid formation and absorption. J Neurosurg. 1983;59:369–83. [PubMed: 6886750]
- Molavi A, LeFrock JL. Tuberculosis meningitis. Med Clin North Am. 1985;69:315–31. [PubMed: 3990437]
- Morley JB, Reynolds EH. Papilledema and the Landry-Guillain-Barré syndrome. Case reports and a review. Brain. 1966;89:205. [PubMed: 5939039]
- Musher DM, Schell RF. False positive gram stains of cerebrospinal fluid. Ann Intern Med. 1973;79:603–4. [PubMed: 4127061]
- Niedman R. Listeriosis in adults. A changing pattern. Rev Infect Dis. 1980;2:207. [PubMed: 6771866]
- Olson ME. et al. Infiltration of the leptomeninges by systemic cancer. A clinical and pathological study. Arch Neurol. 1974;30:122. [PubMed: 4405841]
- Peacock JE, McGinnis MR, Cohen MS. Persistent neutrophilic meningitis. Report of four cases and review of the literature. Medicine. 1984;63:379–95. [PubMed: 6390082]
- Peterson LR. et al. Comparison of gram stains from infected body fluids prepared by cytospin centrifugation, conventional centrifugation and direct application. Clin Res. 1980;30:778.
- Plouffe JF, Fass RJ. Histoplasma meningitis: diagnostic value of cerebrospinal fluid serology. Ann Intern Med. 1980;92:189–91. [PubMed: 7352724]
- Powers WJ. Cerebrospirial fluid to serum glucose ratios in diabetes mellitus and bacterial meningitis. Am J Med. 1981;71:217–20. [PubMed: 7258216]
- Prokesch RC. et al. Cerebrospinal fluid pleocytosis after seizures. South Med J. 1983;76:322. [PubMed: 6828899]
- Shulman JA, Schlossberg D. Handbook of differential diagnosis in infectious diseases. New York: Appleton-Century-Crofts, 1980;25–42.
- Stewart SM. The bacteriologic diagnosis of tuberculosis meningitis. J Clin Pathol. 1953;6:241–242. [PMC free article: PMC1023629] [PubMed: 13084774]
- Swartz MN. Neurosyphilis. In: Holmes KK, Mandl P, Sjarling PF, Wiesner PJ. eds. Sexually transmitted diseases. New York: McGraw-Hill, 1984.
- Tourtellotte WW, Haerer AF, Heller GL, et al. Post-lumbar puncture headaches. Springfield: Charles C Thomas, 1964.
- Whitley R. Diagnosis and treatment of herpes simplex encephalitis. Annu Rev Med. 1981;32:335. [PubMed: 7013672]
- Wilson CB. Rapid tests for the diagnosis of bacterial meningitis. In: Remington JS, Swartz MN, eds. Current clinical topics in infectious diseases. New York: McGraw-Hill,1986;7:134.
Publication Details
Author Information and Affiliations
Authors
Rafael Jurado and H. Kenneth Walker.Copyright
Publisher
Butterworths, Boston
NLM Citation
Jurado R, Walker HK. Cerebrospinal Fluid. In: Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd edition. Boston: Butterworths; 1990. Chapter 74.