NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Penson DF, Krishnaswami S, Jules A, et al. Evaluation and Treatment of Cryptorchidism [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2012 Dec. (Comparative Effectiveness Reviews, No. 88.)
This publication is provided for historical reference only and the information may be out of date.
Background
Condition
Cryptorchidism is a congenital condition in which one or both testicles are not appropriately positioned in the scrotum at birth and cannot be moved into the proper position manually. The term “cryptorchidism” literally means “hidden testicle” and is often used interchangeably with the term “undescended testicle.”1 It affects an estimated three percent of full-term male neonates and up to 30 percent of premature infants, making it the most common male genital anomaly identified at birth.2, 3 Associated conditions and consequences of cryptorchidism include hypospadias, hernia, and testicular torsion. Bilateral, nonpalpable testicles associated with hypospadias or ambiguous genitalia may represent severe developmental abnormalities (including intersexuality) that can be life threatening, warranting specific testing and treatment.1, 4 Ascertaining the correct treatment plan for cryptorchidism is therefore important and includes identifying associated conditions.
Cryptorchidism is often apparent to parents, and examination for the condition is part of general pediatric care. Therefore, boys with cryptorchidism are usually identified early in life, often within the first year. Clinical decision making about treatment is influenced by many factors, including whether or not the testicle is palpable, whether or not the condition is present unilaterally or bilaterally, the age at presentation, and coexisting medical conditions. In boys under 1 year of age whose testicle is palpable and is close to, but not quite inside, the scrotum, it may be difficult to distinguish between “true” cryptorchidism and a retractile testicle. In this case, health care providers often elect to observe the patient’s condition until he is 1 year old.
Although about 70 percent of cryptorchid testicles spontaneously descend within the first year of life (most occurring in the first three months), the number of boys whose condition persists remains constant at approximately 1 percent.5, 6 Between 1992 and 2000, there were more than 600,000 (96 per 100,000 visits in each year) physician office visits for males younger than age 18 for which cryptorchidism was the primary diagnosis.2 The annualized rate of orchiopexy was constant at 18 per 100,000 from 1994 to 1996.7 Given that the average submitted charges in 2008 for a level 3 in-office examination was $146 for new patients and $87 for established patients (according to physician payment information for value-driven health care data compiled by the Centers for Medicare & Medicaid Services) and that the charges for infant and postpubertal orchiopexy have been estimated to be $7,500 and $10,928, respectively, cryptorchidism incurs direct costs of millions of dollars annually, even by the most modest estimates.8 Cryptorchidism is, therefore, both a significant and costly health problem in the United States.
Longer-term consequences of cryptorchidism include testicular malignancy and infertility/subfertility, with stronger evidence for the etiologic role of cryptorchidism in malignancy than in disordered fertility. With regard to testicular cancer, it has been clearly established that there is a strong positive correlation between cryptorchidism and testicular cancer. An estimated ten percent of all testicular tumors develop from an undescended testicle.9 A commonly used estimate for cancer risk is that relative risk of incidence of a testicular tumor is about 40 times greater in men with cryptorchidism when compared to the general population.10 This estimate, however, was based on studies from the 1940s, when the approach to diagnosis and treatment of testicular cancer was quite different than current approaches. A more recent review of the literature estimates the relative risk of testicular cancer in men with cryptorchidism to be between two and eight times higher than men without cryptorchidism.11
Treatment Strategies
Cryptorchidism is diagnosed on physical examination and is usually fairly obvious to parents and providers when the testicle is not found in the “normal” position in the scrotum. The position of the cryptorchid testicle can vary and may be just above the scrotum, anywhere along the inguinal canal, or in the abdomen.1 Sometimes the cryptorchid testicle can be palpated and sometimes it cannot. When testicles are located just above the scrotum, it is important to distinguish between truly cryptorchid testicles and retractile testicles. The key difference is that a retractile testicle can be manually milked into a normal position while a cryptorchid testicle cannot.1 Retractile testicles are usually treated with observation, and they almost always descend into a normal position and remain there as the child grows. Some children with “low-lying” (defined as just above the scrotum) cryptorchid testes will experience spontaneous descent. In general, the further away the testicle is from the scrotum, the less likely it is to descend spontaneously into a normal position.1
Once cryptorchidism is diagnosed, treatment decisions may be guided by results of hormonal stimulation testing and/or imaging. Imaging is used to identify and locate the testicle in order to determine the optimal treatment approach. Imaging approaches include ultrasonography (US), computerized tomography (CT) scanning, routine magnetic resonance (MR) imaging and MR angiography and venography, some of which require sedation or anesthesia and are thus not without risks. The purpose of hormonal stimulation testing is to determine if viable testicular tissue is present in the setting of bilateral nonpalpable cryptorchidism. Specifically, if a boy has bilateral nonpalpable testicles, hormones such as human chorionic gonadotropin (hCG) are administered to stimulate the testicles. If increased levels of testosterone are noted after administration of human chorionic gonadotropins, it is assumed that there is at least one viable testicle somewhere in the body. If there is no testosterone response in the presence of elevated levels of follicle stimulating hormone (FSH), the boy is usually presumed to be anorchid. Imaging is often used to determine whether there is in fact a testicle and to locate it. In theory, absence of a testosterone increase in response to hormonal stimulation testing or inability to locate a testicle with imaging should preclude the need for surgery as it indicates a lack of a potentially functional testicle. However, the value and predictive power of these approaches for identifying the presence and location of a testicle is currently not well understood, and their ability to prevent unnecessary surgery is an area of clinical uncertainty.
Medical options in the treatment of cryptorchidism consist of hormones intended to increase circulating androgens. This increase in circulating androgens, in turn, is thought to potentially promote testicular descent. The two hormones that are most commonly used for the treatment of cryptorchidism are luteinizing hormone-releasing hormone (abbreviated as LHRH and also sometimes referred to as gonadotropin-releasing hormone [GnRH]) and hCG. Although used much less commonly, human menopausal gonadotropin (hMG) is also occasionally used and is thought to function in a manner similar to hCG. LHRH and its analogs and agonists can be administered intranasally, while hCG and hMG must be injected intramuscularly. This difference in mode of administration makes LHRH more acceptable in pediatric care.
The surgical options for the treatment of cryptorchidism are primarily dictated by the location and appearance of the undescended testicle. Primary orchiopexy (surgical mobilization of the testicle with placement and fixation in the scrotum) is usually performed for palpable cryptorchid testicles that are of relatively normal size and appearance that are located in the inguinal canal. In cases in which the testicle is found to be atrophic with little or no viable germ cell tissue remaining, orchiectomy is often performed. For nonpalpable testicles located just inside the internal inguinal ring or in the abdomen, surgical management is more complicated and is dependent on location in the abdomen and the length of the gonadal vessels. If the testicle is of normal size and appearance and if the vessels are of adequate length, primary orchiopexy is usually performed. If the vessels are so short as to prohibit tension-free placement of the testicle in the scrotum, a Fowler-Stephens orchiopexy is performed. This procedure entails ligating the testicular vessels. The testicular blood supply then depends on collateral circulation from the deferential artery and the cremasteric system.1
This procedure can be performed one of two ways: either as a single-stage operation, in which the vessels are ligated and the testicle is then placed into the proper position in the scrotum, or as a two-stage procedure. In a two-stage procedure the vessels are ligated in the first operation, the testicle is allowed to develop presumably better collateral circulation in its abdominal position and is then moved to the proper position in the scrotum during a second procedure, usually 3–6 months later. Both primary orchiopexy and the Fowler-Stephens procedure can be performed using laparoscopic or open surgical technique.
There remains clinical uncertainty and lack of guidance on the appropriate clinical pathway for treatment of cryptorchidism. This uncertainty includes selecting the optimal approach to treatment planning (imaging vs. no imaging; hormonal stimulation testing or not) and intervention (surgical vs. hormonal, one-stage vs. two-stage Fowler Stephens, various modifications of each of the surgical techniques, and open vs. laparoscopic approach). The immediate goal of most interventions for cryptorchidism is to reposition the undescended gonad in a “normal” position in the scrotum. Intermediate outcomes include psychological benefits in terms of body image, and long term goals include preservation of fertility and prevention of testicular malignancy. All of these outcomes are important to patients. While there is some preliminary evidence that medical treatment with hormones, such as LHRH or hCG, may result in descent of the cryptorchid testicle into the scrotum, most children with cryptorchidism ultimately undergo surgical treatment for the condition. The standard urology textbook, Campbell-Walsh Urology, considers that “early surgical repositioning of the testicle into the scrotum before the onset of histopathological changes can reduce the risk of subfertility,” but this statement has not been systematically considered.1 Although many clinicians advocate early orchiopexy to reduce the risk of testicular cancer associated with cryptorchidism, the published literature offers conflicting results.10, 11 This topic was, therefore, nominated by a professional organization interested in developing guidelines for their clinical members on how to treat this condition.
Scope of the Review
This review focuses on the effectiveness of imaging for identifying and correctly locating testicles, on the use of hormonal stimulation for treatment planning and hormones for achieving testicular descent, and on choices among surgical treatments, including surgical approach (open vs. laparoscopic). We focus on treatment of pre-pubescent boys who have been diagnosed with cryptorchidism, and sought information on both short term outcomes (testicular descent) and longer-term outcomes (body image and quality of life, fertility and cancer). Orchiectomy is not reviewed in this report because the report focuses only on procedures to maintain testicular tissue and viability.
Key Questions
We have synthesized evidence in the published literature to address the following Key Questions (KQs):
- KQ1a.
For determining a course of treatment, is imaging equivalent to laparoscopy in determining the presence and location of a nonpalpable testicle?
- KQ1b.
In male children with bilateral, nonpalpable testicles, does the use of hormonal stimulation testing reduce the need for surgery as part of a treatment plan?
- KQ2.
What is the effectiveness of initial hormonal therapy (human chorionic gonadotropin or LHRH) for the treatment of cryptorchidism for outcomes, including but not limited to:
- Further surgical intervention
- The effect on infertility/subfertility
- The development of testicular malignancy
- The size, location, and function of the testicles
- KQ3.
What is the effectiveness of surgical therapies (one-stage vs. two-stage, laparoscopic vs. open approach) for the treatment of cryptorchidism for outcomes including but not limited to:
- Further surgical intervention
- The effect on infertility/subfertility
- The development of testicular malignancy
- The size, location and function of the testicles
- KQ4.
How does the age at presentation, physical presentation of cryptorchidism (unilateral vs. bilateral, palpable vs. nonpalpable, anatomic location) and occurrence of associated abnormalities (e.g., hernia) modify diagnosis, treatment, and outcomes?
- KQ5.
What is the nature and frequency of harms associated with workup or treatment for cryptorchidism?
The relevant population, interventions, comparators, outcomes, timing, and settings (PICOTS) are shown in Tables 1 and 2.
Table 1
PICOTS for KQs 1a and 1b.
Table 2
PICOTS for KQs 2–5.
Analytic Framework
We developed the analytic framework (Figure 1) based on clinical expertise and refined it with input from our key informants and technical expert panel members. It outlines potential areas in which to target a review of the effectiveness of treatments for cryptorchidism in children with cryptorchidism. The framework depicts how workup evaluations can affect the route of treatment in prepubescent males presenting with cryptorchidism. It also summarizes how treatments for cryptorchidism may result in intermediate outcomes such as change in testicular position, size, or appearance; pain; and need for further intervention, as well as long-term outcomes such as effects on fertility and endocrine function and the development of cancer. Also, adverse events may occur at any point after the workup of a patient.
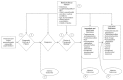
Figure 1
Analytic framework of treatments for cryptorchidism.
Organization of This Evidence Report
The Methods chapter describes our processes including our search strategy, inclusion and exclusion criteria, approach to review of abstracts and full publications, and methods for extraction of data into evidence tables, and compiling evidence. We also describe our approach to grading the quality of the literature and to describing the strength of the body of evidence.
The Results chapter presents the findings of the literature search and the review of the evidence by KQ, synthesizing the findings across strategies.
The final section of the report discusses the results and enlarges on the methodologic considerations relevant to each KQ. We also outline the current state of the literature and challenges for future research in the field.
The report includes a number of appendices to provide further detail on our methods and the studies assessed. The appendixes are as follows:
- Appendix A: Exact Search Strings and Results
- Appendix B: Sample Abstract and Full-Text Review Forms
- Appendix C: Excluded Studies
- Appendix D: Evidence Tables
- Appendix E: Quality of the Literature
- Appendix F: Applicability
We also include a list of abbreviations and acronyms at the end of the report.
Uses of This Evidence Report
We anticipate this report will be of primary value to organizations that develop guidelines for clinical practitioners and to health care providers who take care of male infants. Interested organizations would include the American Academy of Pediatrics, the American Academy of Family Physicians; and the partner in this report, the American Urological Association. Treatment of cryptorchidism is generally provided by pediatric surgeons and urologists, but diagnosis and decisions about care trajectories may take place in general pediatrics and family practice. This report can bring providers up to date about the current state of evidence, and it provides an assessment of the quality of studies that aim to determine the outcomes of treatments for cryptorchidism. It will be of interest to parents concerned about the health of their infants and facing treatment choices around care for their children with cryptorchidism.
Researchers can obtain a concise analysis of the current state of knowledge in this field. They will be poised to pursue further investigations that are needed to advance research methods, understand risk factors, develop new treatment strategies, and optimize the effectiveness and safety of clinical care for boys with cryptorchidism.
- Introduction - Evaluation and Treatment of CryptorchidismIntroduction - Evaluation and Treatment of Cryptorchidism
Your browsing activity is empty.
Activity recording is turned off.
See more...
