The sympathetic nervous system plays an important role in the control of visceral functions critical for homeostasis. The sympathetic nervous system is tonically active. One of the critical components in its control of the homeostatic regulation of arterial pressure and sodium balance is related to the fact that the sympathetic nervous system can rapidly change its activity in response to information from mechanosensitive (pressure, volume) and chemosensitive receptors to coordinate the functions of the various components of the cardiovascular system for the maintenance of homeostasis. This concept has been readily accepted with respect to the control of the general circulation [181]. However, the critical role of the sympathetic nervous system in the control of renal function was long questioned. Not until the early 1970s was it generally accepted that changes in sympathetic activity within the physiological range could alter not only renal blood flow but also sodium/water excretion and renin release, all important components in the maintenance of homeostasis [58]. Studies in anesthetized dogs, summarized in Figure 1.1, showed that graded increases in renal sympathetic nerve activity result in graded increases in renin secretion rate and graded decreases in urinary sodium excretion and renal blood flow. Importantly, renin secretion rate is increased at very low intensities of renal nerve stimulation that do not alter tubular reabsorption of sodium or renal blood flow. Slightly higher intensities of renal nerve stimulation result in further increases in renin secretion in rats and decreases in urinary sodium excretion in the absence of any changes in renal blood flow. At intensities of renal nerve stimulation when renin secretion rate is near maximum, tubular sodium excretion increases further, and renal blood is starting to decrease. Taken together, these studies suggest that renal blood flow is not modulated by changes in renal sympathetic nerve activity within the physiological range, i.e., at rest. However, most likely, renin secretion rate and, to some extent, urinary sodium excretion are modulated by small changes in the renal sympathetic nerve activity occurring during the course of the day and night. Also, there is now considerable evidence that renal sympathetic nervous activity is increased in pathological conditions and contributes importantly to a variety of cardiovascular diseases, as will be discussed later in the book.
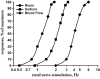
FIGURE 1.1
Graded increases in renal nerve stimulation result in graded increases in renin secretion rate (circles), graded decreases in urinary sodium excretion (squares), and graded decreases in renal blood flow. Data are depicted as percent of maximal response (more...)
Although the understanding of the importance of the renal sympathetic nervous control of renal function was slow in coming, there were studies in the anesthetized dog suggesting a role for the renal nerves in the control of renal function as early as in the 1850s [58]. These studies by Claude Bernard [21] showed that removal of the sympathetic nervous control of one kidney resulted in increased urine outflow from that same kidney. These findings were subsequently followed by studies in the anesthetized dog in the late 1880s that showed that acute renal denervation increased renal volume. Conversely, increases in renal sympathetic nervous activity decreased renal volume [28]. Thus, at the end of the 19th century, the renal nerves were known to contain fibers which upon activation decreased renal blood flow and urinary flow rate. In addition, in the setting of anesthesia and surgery, transection of the renal nerves increased renal blood flow and urinary flow rate.
Over the subsequent years, several studies provided compelling evidence for a clear effect of renal denervation on renal excretory function in anesthetized animals but less evidence for a persistent effect following recovery from anesthesia [58]. Hence, it was concluded that the effects of renal denervation were only apparent during stressful conditions, such as anesthesia and surgery, when there is heightened activity of the sympathetic nervous system [225].
Early human studies in identical twins following kidney transplantation appeared to support the notion that the renal nerves played little, if any, role in the control of renal function in nonstressed conditions, i.e., in the absence of acute anesthesia and/or surgery. Studies performed 56–922 days posttransplantation showed that the ability of renal transplant donors and recipients to achieve external sodium balance and modulate plasma renin activity and aldosterone secretion on high and low dietary sodium intake was similar in the donors and recipients [23]. However, the possibility that there was reinnervation of the transplanted kidney could not be ruled out. Subsequent studies in human renal transplants showed that, indeed, beginning regeneration of renal nerves may occur as early as 28 days after transplantation. The number of regenerating renal nerves increases as the survival of the graft increases.
Since the demonstration of direct adrenergic innervation of the juxtaglomerular cells, renal tubular cells, and renal vasculature [4–12, 171], there is now firm anatomical support for the renal sympathetic nerves exerting control on all aspects of kidney function (Figure 1.1). Furthermore, numerous studies in hypertensive animals [58] and, recently, also in hypertensive humans [73, 164, 217] have shown that removing the renal sympathetic nerves decreases arterial blood pressure suggesting their importance in cardiovascular function. The readers are referred to a number of comprehensive reviews that provide further details on the renal neural control of renal function beyond what is discussed in this book [48–53, 58, 59, 121, 181, 232].
Publication Details
Copyright
Publisher
Morgan & Claypool Life Sciences, San Rafael (CA)
NLM Citation
Kopp UC. Neural Control of Renal Function. San Rafael (CA): Morgan & Claypool Life Sciences; 2011. Chapter 1, Introduction.
