NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Elkouby YM, Frank D. Wnt/β-Catenin Signaling in Vertebrate Posterior Neural Development. San Rafael (CA): Morgan & Claypool Life Sciences; 2010.
Meis3 and Cdx are genes directly regulated by Wnt/β-catenin and they each interpret its signal to activate expression of a distinct set of Hox genes, with Meis3 protein specifically inducing hindbrain fates and Cdx proteins specifically inducing spinal cord fates. One crucial question arising is: What discriminates between hindbrain and spinal cord fates downstream of the caudalizing Wnt signal? As discussed previously, Meis3 and Cdx loss-of-function phenotypes share a mirror-like image, with the Meis3 deficiency exclusively losing hindbrain fates and Cdx deficiency exclusively losing spinal cord fates (Dibner et al., 2001; Faas and Isaacs, 2009; Isaacs et al., 1998; Skromne et al., 2007). The possible mechanism that segregates these fates may be similar to the model for formation of the isthmus organizer region at the MHB (see Chapter 4, “Induction of the Midbrain–Hindbrain Border”). At the MHB, mutual repression between the midbrain Otx2 and hindbrain Gbx2 gene expression domains governs this process. The expression of Otx2 and Gbx2 is mutually exclusive, thus controlling sharp boundary formation in the MHB. While it is not clear yet what dictates the differential induction of their expression by the Wnt signal, Meis3 and Cdx genes may function similarly during the mutually exclusive determination of the hindbrain and spinal cord regions. However, factors regulating the mutual exclusive expression of these genes or the mutual repression between their products have not been determined.
Indeed, Cdx protein activity repressed hindbrain formation in zebrafish embryos. Cdx1a/Cdx4-deficient zebrafish exhibited a mirror duplication of hindbrain marker genes, which were expressed deep within the otherwise spinal cord-forming region (Shimizu et al., 2006; Skromne et al., 2007). These ectopic hindbrain-Hox-expressing regions also formed rhombomere-like structures. As the spinal cord is a nonsegmented structure, this result implies that there was a complete transformation of the spinal cord to hindbrain fates. Overexpression of HoxA7, HoxA9, and HoxB9 suppressed the hindbrain duplication in the Cdx1a/Cdx4-deficient embryos, suggesting a downstream Hox-dependent mechanism that differentiates between hindbrain and spinal cord fates. Ectopic expression of Cdx1 in the hindbrain also induced spinal cord Hox gene expression cell-autonomously, while repressing hindbrain Hox gene expression and rhombomere formation (Shimizu et al., 2006; Skromne et al., 2007). In chick embryos, it was suggested that all presumptive hindbrain and spinal cord cells are competent to respond to Cdx and FGF spinal cord-promoting activity (Bel-Vialar et al., 2002). Intriguingly, this is in agreement with a mutual exclusive mechanism regulating Meis3 and Cdx expression patterns that acts upstream of Hox activity, which somehow prevents Cdx gene expression anterior to the presumptive spinal cord region (Bel-Vialar et al., 2002).
The hindbrain duplication in Cdx1a/Cdx4-deficient zebrafish embryos also involved RA and FGF signaling (Shimizu et al., 2006; Skromne et al., 2007). Some answers have been provided from chick and zebrafish embryos, regarding differential combinatorial signaling activities that promote formation of one region over the other (Bel-Vialar et al., 2002; Nordstrom et al., 2006; Shimizu et al., 2006). In principle, Wnt/β-catenin caudalizing activity is modified by RA or FGF or both RA and FGF signals to promote and induce expression of either hindbrain Hox genes or Cdx and spinal cord Hox genes. The picture arising is of two opposing gradients of FGF and RA signaling in the hindbrain versus the spinal cord (Figure 7.1A). In zebrafish embryos, the FGF3 and FGF8 ligands are expressed in the hindbrain r4 and in the most posterior tail mesoderm, the posterior growth zone from which somites bud off. RA is synthesized by RALDH2 that is expressed in the trunk (and not tail) level paraxial mesoderm, and degraded by CyP26, expressed in the forebrain and the posterior growth zone. Hence, the presumptive hindbrain region is subjected to FGF from the anterior and RA from the posterior, while the presumptive spinal cord region is subjected, reciprocally, to RA from the anterior and FGF from the posterior (Figure 7.1A) (Nordstrom et al., 2006; Shimizu et al., 2006). As Wnt3a ligands are expressed in the paraxial mesoderm and later all along the hindbrain and spinal cord, their essential activity may, in this way, be modified by these reciprocally opposing gradients of RA and FGF signals.
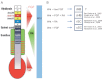
FIGURE 7.1
Downstream of Wnt: hindbrain versus spinal cord specification. (A) Scheme showing the expression patterns and secretion sources of FGF3/8, RA, and Wnt signaling molecules along the hindbrain and spinal cord. These constitute region-specific gradient–concentration (more...)
In agreement with high FGF and intermediate RA signaling in the caudal hindbrain, the ectopic expression of HoxB1, Krox20, and Val in the zebrafish hindbrain-transformed spinal cord was equally dependent on these two signals. (Shimizu et al., 2006). In addition, while Meis3 and HoxD1 are Wnt-direct targets, their expression in zebrafish embryos also requires RA signaling, and HoxD1 expression requires FGF signaling as well (Kudoh et al., 2002). This observation further supports a scenario of combined signaling activities by which Wnt, FGF, and RA drive caudal hindbrain fates. Accordingly, for the low FGF and high RA signaling scenario of the rostral spinal cord, expression of HoxB4 and HoxB5 was highly dependent on RA and much less dependent on FGF signaling (Shimizu et al., 2006). Ex vivo chick neural plate explant experiments (expressing both hindbrain and spinal cord markers in a Wnt/β-catenin-dependent manner) also support this scenario, as their treatment with both Wnt3a and RA drove these explants to express rostral spinal cord markers, while blocking the induction of caudal spinal cord markers (Nordstrom et al., 2006). Finally, corresponding to low RA and high FGF signaling in the caudal spinal cord, treatment of these explants with both Wnt3a and FGF4 biased these explants to robustly express spinal cord markers, while suppressing caudal hindbrain markers (Nordstrom et al., 2006). These different signaling scenarios and the resultant induced tissues are summarized in Figure 7.1B.
Supporting this modulation of Wnt/β-catenin by opposing gradients of RA and FGF, it was also demonstrated more generally in vivo in the chick that, while the PG1–3 Hox genes were more responsive to RA than to FGF, the posterior PG6–9 Hox genes were highly sensitive to FGF and unresponsive of RA (Bel-Vialar et al., 2002). Distinct combinations of FGF and RA signals may act on different Hox gene promoters making them more accessible and competent to respond to the Wnt signal, and thus directing proper activation of a specific Hox set in each region. Alternatively, while FGF was shown to induce various Hox genes of most PGs (Bel-Vialar et al., 2002), RA may act only on certain Hox genes and function more downstream of the Wnt and FGF signals, thus inducing or repressing a subset of the entire Wnt/FGF responsive Hox set. Indeed, RA could rescue both Wnt and FGF inhibition by inducing expression of its direct target, HoxD1, in zebrafish (Kudoh et al., 2002).
Data regarding the correct signal combination that promotes rostral hindbrain fates are missing. The FGF and RA expression patterns suggest that a combination of Wnt and FGF, in the absence of RA signaling, would account for this region. Rostral hindbrain markers were not examined in the Wnt3a/FGF4-treated chick neural plate explants, so it would be very interesting to see if their expression was induced in these conditions, in addition to the most caudal spinal cord fate. However, as both the rostral hindbrain and the caudal spinal cord seem to require a Wnt/FGF-positive, RA-negative condition, different levels of FGF may differentiate between the two. This makes sense because cells in r1–3 are exposed to an r4-secreted FGF signal, while caudal spinal cord cells are exposed to an FGF signal from the posterior growth zone, which is robust in both quantity and time exposure. Further experimentation is needed to clarify this point and to validate this model.
- Downstream of Wnt: Hindbrain or Spinal Cord? - Wnt/β-Catenin Signaling in Verteb...Downstream of Wnt: Hindbrain or Spinal Cord? - Wnt/β-Catenin Signaling in Vertebrate Posterior Neural Development
Your browsing activity is empty.
Activity recording is turned off.
See more...
