NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Baron S, editor. Medical Microbiology. 4th edition. Galveston (TX): University of Texas Medical Branch at Galveston; 1996.
General Concepts
Mycobacterium tuberculosis Complex: Mycobacterium tuberculosis, M bovis
Clinical Manifestations
Tuberculosis primarily affects the lower respiratory system and is characterized by a chronic productive cough, low-grade fever, night sweats, and weight loss.
Structure
Mycobacteria are slender, curved rods that are acid fast and resistant to acids, alkalis, and dehydration. The cell wall contains complex waxes and glycolipids. Multiplication on enriched media is very slow, with doubling times of 18 to 24 hours; clinical isolates may require 4 to 6 weeks to grow.
Classification and Antigenic Types
On the basis of growth rate, catalase and niacin production, and pigmentation in light or dark, mycobacteria are classified into members of the Mycobacterium tuberculosis complex (M tuberculosis, M bovis, M africanum, M microtii) and nontuberculous species. Gene probe technology now facilitates this distinction.
Pathogenesis
Tuberculous mycobacteria enter the alveoli by airborne transmission. They resist destruction by alveolar macrophages and multiply, forming the primary lesion or tubercle; they then spread to regional lymph nodes, enter the circulation, and reseed the lungs. Tissue destruction results from cell-mediated hypersensitivity.
Host Defenses
Susceptibility is influenced by genetic and ethnic factors. Acquired resistance is mediated by T lymphocytes, which lyse infected macrophages directly or activate them via soluble mediators (e.g., gamma interferon) to destroy intracellular bacilli; antibodies play no protective role.
Epidemiology
M tuberculosis is contagious, but only 5–10 percent of infected normal individuals develop active disease. Tuberculosis is most common among the elderly, poor, malnourished, or immunocompromised, especially persons infected with human immunodeficiency virus (HIV). Persistent infection may reactivate after decades owing to deterioration of immune status; exogenous reinfection also occurs.
Diagnosis
Recent infection with M tuberculosis results in conversion to a positive Mantoux skin test with purified protein derivative (PPD). A diagnosis of active disease is based on clinical manifestations, an abnormal chest radiograph, acid-fast bacilli in sputum or bronchoscopic specimens and recovery of the organism. Assays based upon amplification of mycobacterial genes in clinical specimens are currently being tested.
Treatment and Control
Therapy consists of a 6 to 9 month course of isoniazid, rifampin, pyrazinamide and ethambutol. Additional drugs may be used if drug resistance is suspected (e.g., infection in Southeast Asian immigrants or in areas where outbreaks of drug-resistant tuberculosis have been documented). If the patient is HIV-positive, treatment for longer periods (9–12 months) is recommended. PPD conversion without other signs or symptoms may warrant prophylactic isoniazid therapy for 6 months. M bovis BCG vaccine is used in more than 120 countries, but its efficacy is controversial. Although BCG has not been used routinely in the U.S., the current epidemic has prompted a reevaluation of its use, especially in high-risk populations.
Nontuberculous Mycobacteria
Clinical Manifestations
Patients exhibit lower respiratory disease similar to tuberculosis ( M kansasii, M avium-intracellulare), cervical lymphadenitis ( M scrofulaceum), skin and soft tissue infections ( M ulcerans, M marinum), or disseminated disease in persons infected with HIV.
Structure
Nontuberculous mycobacteria resemble other mycobacteria. Multiplication is similar to that of other mycobacteria, except for rapidly growing strains (e.g. M. fortuitum).
Classification and Antigenic Types
Nontuberculous mycobacteria are classified by pigmentation in the light or dark and by growth rate. Several species contain many serotypes, based upon lipooligosaccharides or peptidoglycolipids.
Pathogenesis
Pathogenesis is similar to that of other mycobacteria. There may be granuloma formation and delayed hypersensitivity.
Host Defenses
Host defenses are similar to those of other mycobacteria. Cell-mediated resistance is important.
Epidemiology
The infection is not transmissible between humans, but is acquired from natural sources (e.g., soil and water). Nontuberculous mycobacteria are important opportunistic pathogens in immunocompromised patients (e.g., patients infected with HIV).
Diagnosis
Diagnosis requires culture and identification.
Treatment
Many of these mycobacteria are resistant to the usual antituberculosis drugs. Therefore, different drugs are often necessary. Surgical resection is sometimes required. No vaccine is available.
Mycobacterium leprae
Clinical Manifestations
Leprosy is an infection of the skin, peripheral nerves, and mucous membranes, leading to lesions, hypopigmentation, and loss of sensation (anesthesia), particularly in the cooler areas of the body.
Structure
Mycobacterium leprae is similar to other mycobacteria; the cell wall contains unique phenolic glycolipids. It cannot be cultivated in vitro: it multiplies very slowly in vivo (12-day doubling time).
Classification and Antigenic Types
All isolates of M leprae, both human and sylvatic, appear to be the same by DNA homology.
Pathogenesis
The spectrum of leprosy (Hansen's disease) ranges from lepromatous (disseminated, multibacillary, with loss of specific cell-mediated immunity) to tuberculoid (localized, paucibacillary, with strong cell-mediated immunity).
Host Defenses
Host defenses are similar to those against other mycobacteria.
Epidemiology
Transmission requires prolonged contact and occurs directly through intact skin, mucous membranes, or penetrating wounds. Armadillos in Louisiana and Texas are naturally infected.
Diagnosis
Diagnosis is based on acid-fast stain and cytologic examination of affected skin and response to the lepromin skin test; M leprae cannot be cultured.
Treatment
Treatment (including prophylaxis in close contacts) with multi-drug therapy or MDT (dapsone, rifampin, and clofazimine) is performed on an outpatient basis for 3 to 5 years; vaccination with M bovis BCG has been effective in some endemic areas.
Nocardia
Clinical Manifestations
The most common manifestation of nocardial infection is pneumonia: fever, weight loss, cough, pleuritic chest pain, and dyspnea. In about 20 percent of patients there are granulomatous skin lesions and/or central nervous system abnormalities.
Structure
The bacteria are Gram-positive, partially acid-fast rods, which grow slowly in branching chains resembling fungal hyphae.
Classification and Antigenic Types
Three species cause nearly all human infections: N asteroides, N brasiliensis, and N caviae. These are distinguished by proteolytic and fermentation patterns in culture.
Pathogenesis
Infection is by inhalation of airborne bacilli from an environmental source (soil or organic material); the disease is not contagious. Skin lesions caused by N brasiliensis often result from direct inoculation. Nocardia subverts antimicrobial mechanisms of phagocytes, causing abscess or rarely granuloma formation with hematogenous or lymphatic dissemination to the skin or central nervous system. Mortality is up to 45 percent even with therapy.
Host Defenses
The natural resistance to infection is high in normal individuals, and the disease is usually associated with cellular immune dysfunction, immunoglobulin deficiencies, or leukocyte defects. Acquired resistance is complex and involves activated macrophages, cytotoxic T cells, and neutrophil inhibition.
Epidemiology
Nocardiosis is rare in normal persons. It usually occurs in recipients of organ transplants; in patients with leukemia, lymphoma, humoral, or leukocyte defects; or after prolonged steroid therapy.
Diagnosis
Diagnosis is by Gram stain, modified acid-fast stain, and culturing of organisms from sputum, bronchoscopic specimens (washing, brushing), aspirates of abscesses, or by biopsy.
Treatment
Nocardiosis is treated by prolonged (up to 1 year) therapy with trimethoprim-sulfamethoxazole.
Introduction
The genera Mycobacterium and Nocardia have been grouped into the family Mycobacteriaceae within the order Actinomycetales based upon similarities in staining and motility, lack of spore formation, and catalase production. These genera are characterized by the presence of long-chain fatty acids, called mycolic aids, which have the following general structures in their cell walls:
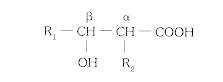
The side chains (R1 and R2) vary in length according to the genus; C60 to C90 in Mycobacterium; C40 to C56 in Nocardia. Several species produce disease in humans.
Mycobacterium tuberculosis Complex
Clinical Manifestations
The first sign of a new infection is often conversion of the intradermal skin test with purified protein derivative (PPD) to positive or detection of a lesion by chance on a chest x-ray in an otherwise asymptomatic individual (see Diagnosis below). Clinical signs and symptoms develop in only a small proportion (5–10 percent) of infected healthy people. These patients usually present with pulmonary disease; prominent symptoms are chronic, productive cough, low-grade fever, night sweats, easy fatigability, and weight loss. Tuberculosis may present with or also exhibit extrapulmonary manifestations including lymphadenitis; kidney, bone, or joint involvement; meningitis; or disseminated (miliary) disease. Lymphadenitis and meningitis are more common among normal infants with tuberculosis, and all extrapulmonary manifestations are increased in frequency among immunocompromised individuals such as patients on chronic renal dialysis and elderly, malnourished, or HIV-infected individuals.
Structure
Mycobacteria are slender, curved rods in stained clinical specimens. The cell wall is composed of mycolic acids, complex waxes, and unique glycolipids. Figure 33-1 depicts the typical cell wall structure for M tuberculosis and other mycobacteria. The mycolic acids containing extremely long (C60 to C90) side chains are joined to the muramic acid moiety of the peptidoglycan by phosphodiester bridges and to arabinogalactan by esterified glycolipid linkages. Species variations are characterized by variation in sugar substitutions in the glycolipids or peptidoglycolipids. The mycobacterial cell wall is acid-fast (i.e., it retains carbolfuchsin dye when decolorized with acid-ethanol). This important property allows differential staining in contaminated clinical specimens such as sputum. Other important wall components are trehalose dimycolate (so-called cord factor, as it is thought to induce growth in serpentine cords on artificial medium) and mycobacterial sulfolipids, which may play a role in virulence. Another unique constituent which may contribute to pathogenesis is lipoarabinomannan (LAM). Purified LAM from virulent and attenuated strains of mycobacteria may differ structurally, and these differences may contribute to their varying abilities to stimulate cytokine production in mononuclear cell cultures.
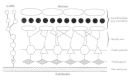
Figure 33-1
Complex cell wall structure of mycobacteria.
This unusual cell wall structure endows mycobacteria with resistance to dehydration, acids, and alkalis. The resistance to acids and alkalis is useful in the isolation of mycobacteria from contaminated clinical specimens such as sputum. Treatment of sputum with dilute solutions of sulfuric acid or sodium hydroxide will allow mycobacteria to survive and grow on culture medium in the absence of the members of the respiratory flora.
Another important consequence of the unique cell wall structure of mycobacteria is the adjuvant action of whole cells when mixed with a wetting agent (e.g., Tween) in an oil-water emulsion. Such a mixture is called Freund's complete adjuvant. Researchers have identified muramyl dipeptide as the peptidoglycan component that mediates adjuvanticity (Fig. 33-2).
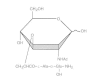
Figure 33-2
Structure of muramyl dipeptide from mycobacteria, which mediates adjuvanticity.
Although mycobacteria are normally cultured from clinical material by inoculation onto enriched agar media containing bovine serum albumin, they can grow on a chemically defined medium containing asparagine, glycerol, and micronutrients. Even under ideal culture conditions M tuberculosis and M bovis grow very slowly, with doubling times on the order of 18 to 24 hours. This extremely slow growth, even in vivo, has two consequences of clinical significance: (1) the infection is an insidious, chronic process, which may take several weeks or months to become clinically patent, and (2) on solid media inoculated with clinical material, identifiable mycobacterial colonies may not appear for 4 to 6 weeks. When they do appear, the colonies are irregular, waxy, and buff colored, with bacteria piled up into clumps or ridges.
Classification and Antigenic Types
With the exception of M leprae, the mycobacteria are classified into two broad categories—members of the M tuberculosis complex ( M tuberculosis, M bovis, M microtii, M africanum) and nontuberculous mycobacteria (virtually all other species), which often are described based on their growth rate and pigmentation with and without exposure to light. Although there are antigenic differences among species on the basis of serologic reactions to carbohydrate moieties in the glycolipids, such determinations are not clinically useful. Modern molecular biologic techniques have revealed a remarkable conservation of genes coding for the immunodominant antigens of all mycobacteria, including M leprae.
Pathogenesis
In this country, virtually all M tuberculosis infections occur by airborne transmission of droplet nuclei containing a few viable, virulent organisms produced by a sputum-positive individual (Fig. 33-3). The bacilli are deposited in the alveolar spaces of the lungs, where they are engulfed by alveolar macrophages. A portion of the infectious inoculum resists intracellular destruction and persists, eventually multiplying and killing the macrophage. The ability of virulent mycobacteria to survive within phagocytes justifies their designation as facultative intracellular pathogens. The mechanisms of intracellular survival are not clear and may vary from species to species. There is some evidence that M tuberculosis can prevent phagosome-lysosome fusion. Other studies have demonstrated that virulent mycobacteria can prevent acidification of the phagolysosome, perhaps by modulating the activity of a membrane proton pump. In addition, some of the components of the mycobacterial cell wall (e.g., cord factor) may be directly cytotoxic to macrophages. Although hemolysins and lipases are produced by M tuberculosis, their role in escape of tubercle bacilli from the phagosome, and the importance of extravacuolar organisms in pathogenesis are unknown. Most of the tissue destruction associated with tuberculosis results from cell-mediated hypersensitivity, however, rather than direct microbial aggression.
Figure 33-3
Pathogenesis of tuberculosis.
Eventually, the accumulating mycobacteria stimulate an inflammatory focus which matures into a granulomatous lesion characterized by a mononuclear cell infiltrate surrounding a core of degenerating epithelioid and multinucleated giant (Langhans) cells. This lesion (called a tubercle) may become enveloped by fibroblasts, and its center often progresses to caseous necrosis. Liquefaction of the caseous material and erosion of the tubercle into an adjacent airway may result in cavitation and the release of massive numbers of bacilli into the sputum. In the resistant host, the tubercle eventually becomes calcified.
Early in infection, mycobacteria may spread distally either indirectly through the lymphatics to the hilar or mediastinal lymph nodes and thence via the thoracic duct into the blood stream, or directly into the circulation by erosion of the developing tubercle into a pulmonary vessel. Extrapulmonary hematogenous dissemination results in the seeding of other organs (e.g., spleen, liver, and kidneys) and, eventually, reinoculation of the lungs. The resulting secondary lung lesions (as opposed to the initial site of implantation) may serve as the origin of reactivation of clinical disease years or decades later owing to the persistence of viable tubercle bacilli. Figure 33-4 illustrates the radiologic differences between primary and secondary tuberculosis. Primary disease is usually characterized by a single lesion in the middle or lower right lobe with enlargement of the draining lymph nodes. Endogenous reactivation is often accompanied by a single (cavitary) lesion in the apical region, with unremarkable lymph nodes and multiple secondary tubercles.
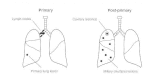
Figure 33-4
Radiologic differences between primary and post-primary tuberculosis. Miliary lesions, which are small granulomas, resemble millet seeds spread throughout the lung fields.
In parts of the world where bovine tuberculosis has not been eliminated and where dairy products are not properly treated, direct infection of the gastrointestinal tract may occur by ingestion of virulent M bovis organisms. The gut is also exposed occasionally in pulmonary tuberculosis when large numbers of viable M tuberculosis cells are coughed up and swallowed. In either case, the principal site of involvement is the mesenteric lymph nodes, with subsequent dissemination.
Host Defenses
Innate susceptibility to pulmonary infection with M tuberculosis is clearly influenced by genetic and/or ethnic variables that have not been defined. Studies of mono- and dizygotic twins and siblings indicate a significant relationship between genotype and resistance to tuberculosis. Studies of inbred experimental animals point to genes both within and outside the major histocompatibility complex that apparently contribute to resistance. In the mouse, at least two genes ( Bcg and Tbc) have been implicated in the expression of innate resistance to mycobacteria. A search for human homologues is currently under way. The mechanism for this genetic effect may reflect the ability of macrophages to process and present mycobacterial antigens to the immune system.
Acquired immunity following mycobacterial infection usually develops within 4 to 6 weeks and is associated temporally with the onset of delayed hypersensitivity to mycobacterial antigens such as PPD. Successful acquired resistance is mediated by T lymphocytes. Antimycobacterial antibodies, although present in many patients, do not play a protective role in tuberculosis. Figure 33-5 depicts two of the principal mechanisms by which T lymphocytes activated by specific mycobacterial antigens can limit the replication of tubercle bacilli. Cells of the helper/inducer phenotype (CD4+) up-regulate populations of antigen-specific effector T cells (CD4+ ) and cytotoxic T cells (CD8+). CD4 cells produce factors (e.g., gamma interferon) that activate macrophages and endow them with enhanced mycobacteriostatic or mycobactericidal capabilities. Unlike normal macrophages, these activated cells can limit the replication of intracellular M tuberculosis and may kill tubercle bacilli. CD8 cells attack infected macrophages expressing mycobacterial antigens and lyse the cells, releasing the mycobacteria from their protective niche and exposing them to activated macrophages.
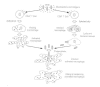
Figure 33-5
Principal mechanisms of T-lymphocyte activation or destruction of macrophages following stimulation by mycobacterial antigens. Activation of macrophages can result in bacterial killing while cytotoxicity may release bacteria from phagocytes and allow (more...)
Recently, CD4+ (and perhaps CD8+) T cells have been dichotomized into functional subsets in the murine system based upon their cytokine profiles. So-called “Th1” cells produce interleukin-2 (IL-2) and interferon gamma and promote inflammatory reactions and cell-mediated immunity. “Th2” cells produce interleukin-4, -5, and -10 and drive the immune response toward antibody production. The two subsets apparently cross-regulate principally via opposing actions of interferon gamma and interleukin-4. Although evidence for this clean functional separation is not as compelling in humans, it is likely that disease resistance in tuberculosis depends upon the predominance of a Th1-like cytokine response to mycobacterial antigens.
Since the vast majority (90–95 percent) of otherwise healthy individuals who are infected with M tuberculosis never develop clinically apparent disease, acquired resistance must be quite effective. However, the immune response to mycobacteria is a double-edged sword: the intense cell-mediated hypersensitivity that usually accompanies infection is responsible for much of the pathology associated with clinical tuberculosis. Some clinical studies suggest that inappropriately high levels of circulating cytokines, such as tumor necrosis factor alpha, may be responsible for some of the clinical features of tuberculosis (e.g. fever, weight loss). Therapy with cytokine-blocking drugs, such as pentoxyfilline and thalidomide, may prove to be an important adjunct to standard chemotherapy in some tuberculosis patients. Experimental evidence suggests that protection and hypersensitivity may be mediated by distinct subsets of T lymphocytes and may be directed against different mycobacterial antigens. Much progress has been made in recent years in defining the important antigens of M tuberculosis and other mycobacteria by using gene cloning and monoclonal antibody technology. Interestingly, some of these immunodominant mycobacterial antigens show remarkable homology with a family of stress (or heat shock) proteins that are widely conserved in both prokaryotes and eukaryotes, including humans. This observation may have relevance for the autoimmune phenomena (e.g., arthritis) associated with mycobacterial infection in some people. Some highly purified or recombinant antigens are currently being tested for their usefulness in diagnosis or in the development of subunit vaccines to prevent tuberculosis.
Epidemiology
Numerous studies of tuberculosis epidemics in closed populations (e.g., on naval vessels and in nursing homes) document the contagious nature of this infection. Fortunately, overt clinical disease actually develops in only a small percentage of those infected. Identification of recently infected individuals is still important, however, because viable mycobacteria persisting in tissues may lead to endogenous reactivation of tuberculosis later in life (see Treatment and Control, below). Reactivation is usually associated with deterioration of the cell-mediated immune response due to aging or to some associated clinical condition. Exogenous reinfection also has been documented, but most cases of so-called “post-primary” tuberculosis in this country are thought to be the result of endogenous reactivation.
Tuberculosis epidemiology has been clarified significantly by the development of molecular biological techniques which allow the relatively unambiguous identification of a particular clinical isolate. Recent, active transmission of a single strain of M tuberculosis would result in clinical isolates from several patients which exhibited identical DNA patterns or “fingerprints.” In contrast, endogenous reactivation in a group of patients would likely result in isolates which exhibited unique patterns. The most commonly used procedure for comparing isolates involves the use of molecular probes which bind to segments of DNA called insertion sequences (IS) in the mycobacterial genome. Such a sequence, IS6110, has been employed very successfully to track outbreaks of tuberculosis within institutions (e.g. hospitals) associated with recent transmission. This “fingerprinting” procedure, referred to as restriction fragment length polymorphism (RFLP) analysis, is currently being used to detect previously unsuspected transmission between apparently unrelated patients in the community.
Tuberculosis is particularly common in groups such as the elderly, the chronically malnourished, alcoholics, and the poor. The prevalence of clinical tuberculosis among the homeless in the United States may be up to 300 times higher than the national average rate. In recent years, the incidence of disease in racial minorities in the United States has been more than five times that observed in whites. Of particular concern is the very high incidence of tuberculosis among recent immigrants.
Perhaps the most significant factor influencing the incidence of mycobacterial disease in the United States since 1984 has been the HIV epidemic. HIV-infected individuals have a high incidence of tuberculosis, characterized by frequent extrapulmonary disease. Both primary, pulmonary infection and endogenous reactivation are seen in HIV-positive individuals. Owing to the loss of T-cell function in these patients, the tuberculin skin test may not be reliable and the chest radiograph may not show the classic well-defined primary tubercle. Both of these observations make the diagnosis of tuberculosis in HIV-infected patients more challenging. Furthermore, tuberculosis often occurs early in the course of HIV infection, before a significant decline in CD4+ T cells has occurred. In this sense, tuberculosis in an apparently healthy young adult may “signal” the presence of underlying HIV infection.
Diagnosis
Infection in an asymptomatic individual can be diagnosed with the help of the intradermal PPD skin test. Intradermal introduction of PPD into a previously infected, hypersensitive person results in the delayed (48–72 hr) appearance of an indurated (raised, hard) reaction with or without erythema. It is impossible to distinguish between present and past infection on the basis of a positive tuberculin test. Recent conversion of the reaction from negative to positive warrants clinical attention. Although multiple-puncture (or tine) tests once were popular for screening for tuberculin hypersensitivity, they are not as accurate as the Mantoux test and should not be used. The Mantoux test requires the intradermal injection of a measured volume (0.1 ml) containing a specified quantity (5 tuberculin units) of PPD. The transverse diameter of induration is measured 48 to 72 hours later. Interpretation varies, as shown in Table 33-1. In a person with symptoms suggestive of tuberculosis, clinical specimens (sputum, bronchial or gastric washings, pleural fluid, urine, or cerebrospinal fluid) should be stained and cultured for acid-fast bacilli. Culture and identification of mycobacteria in such specimens are mandatory for diagnosis. Two types of stains are used specifically for detection of mycobacteria: fluorochrome (recommended) and carbol fuchsin. In smears stained with carbol fuchsin, mycobacteria typically appear as red rods (1–10 μm long and 0.2–0.6 μm wide) and often are beaded or banded, but also may appear coccoid or filamentous. In general, the microscopic appearance of the mycobacteria as slightly curved rods does not provide a species identification but may be suggestive for some species.
Table 33-1
Guidelines for Interpretation of the Mantoux Test.
The specificity of stains for AFB typically is ≥ 99% and the sensitivity 25% to 75%. A positive stain and negative culture may be caused by nonviable organisms, such as might occur in persons receiving antituberculosis medication. Higher sensitivity of smear results occurs with cavitary lesions, respiratory specimens, increased number of specimens, increased number of mycobacteria present in the sample (above the minimum of 5,000 organisms/ml), the presence of M tuberculosis or M kansasii species, observer experience, and stain used.
Culture for mycobacteria involves inoculation of solid and broth media. For specimens such as sputum that are contaminated with normal bacterial flora, a selective medium containing antimicrobial agents should be inoculated. Sterile body fluids should be inoculated to solid media and a broth medium. Cultures are incubated at 35° to 37° C in an atmosphere of 5 to 10% CO2. For specimens from cutaneous sites a second set of cultures should be incubated at 30°C. All cultures should be examined weekly for 8 weeks.
The major advantage of culture on solid media is that it allows visualization of colony morphology and pigmentation, which is useful diagnostically for distinguishing colonies of M tuberculosis from those of some nontuberculous mycobacteria. However, they require 3 or 4 weeks. The more rapid broth systems (e.g. Bactec) require only 5 to 12 days, and rely upon the detection of 14C-labeled CO2 produced by growing mycobacteria.
Commercial chemiluminescent DNA probes, gas-liquid chromatography, high-performance liquid chromatography, and thin-layer chromatography allow identification of a few species of mycobacteria within hours after sufficient growth is present on solid or in a liquid medium.
In the future, nucleic acid amplification methods may prove useful for detection of mycobacteria directly in clinical material within 24 hours or less of specimen receipt. Currently, standardized guidelines for susceptibility testing of mycobacteria have been developed only for isolates of M tuberculosis a positive BACTEC TB vial (indirect test), or on sputum specimens that are smear-positive (direct test). Using a broth system, results are available 5–7 days after bottles are inoculated.
Treatment and Control
In the United States, tuberculosis in the general population is controlled by intensive case finding (by means of PPD skin testing) and aggressive prophylactic chemotherapy in tuberculin converters. In individuals with clinical disease, short term (6–9 month) ambulatory therapy with so-called first-line anti-mycobacterial drugs, such as isoniazid, rifampin, pyrazinamide, and ethambutol, results in disappearance of viable tubercle bacilli from the sputum, rendering the patient noninfectious. Prompt therapy, even in the absence of other signs or symptoms, is thought to sterilize the tissues and prevent endogenous reactivation of tuberculosis later in life. Patient compliance is probably the single most important variable affecting treatment outcome. Directly observed therapy (DOT) has been instituted in high prevalence areas, especially among recalcitrant patients, as the only reliable means of ensuring that patients complete their treatment successfully.
Multiple-drug resistance (MDR) has become a particularly threatening aspect of the current tuberculosis epidemic in this country. Although confined at present predominantly to large metropolitan areas (e.g. New York City) MDR strains of M tuberculosis have been associated with several outbreaks characterized by rapid progression and high mortality (50–75%). Some progress has been made in the research laboratory in elucidating the mechanisms of drug resistance in mycobacteria. Multiple-drug resistance in mycobacteria is apparently the result of the step-wise accumulation of resistance to individual drugs. For example, mutations in the catG and inhA genes are associated with isoniazid resistance, while the rpoB gene responsible for RNA polymerase is altered in many clinical isolates resistant to rifampin. The threat of drug-resistant tuberculosis has made susceptibility testing mandatory for all initial isolates. Unfortunately, more rapid techniques are needed to provide this information in a timely manner to physicians. Such assays are presently being developed. One of the most exciting involves the use of a luciferase reporter gene which is introduced into the clinical isolate on a mycobacteriophage. Light production in the presence of the drug reveals resistance, and can be detected very quickly. When resistance to two or more of the first line drugs is detected, additional drugs (ethionamide, streptomycin, ciprofloxacin) may be added to the regimen.
A viable, attenuated strain of M bovis, called bacille Calmette-Guérin (BCG), after the French microbiologists who developed the strain, has been used in more than 120 countries for many years as a vaccine to prevent clinical tuberculosis. Vaccination is the only feasible approach to controlling this disease in much of the developing world. The efficacy of BCG has varied in field trials from 0 to 85 percent, indicating an influence of unknown local environmental or host factors. BCG is not used in the United States because it results in PPD conversion, thereby interfering with the epidemiological and diagnostic value of the skin test. In the past 5 years, researchers have turned their efforts to the development and animal model testing of new tuberculosis vaccines. Promising results have been obtained in animal experiments with purified subunit vaccines, recombinant BCG strains, and auxotrophic BCG mutants. Excellent animal models for vaccine testing are available using mice, guinea pigs or rabbits infected with M tuberculosis by the pulmonary route.
Nontuberculous Mycobacteria
Clinical Manifestations
Nontuberculous mycobacteria, previously referred to as “atypical” mycobacteria, comprise several species, which may produce a wide range of clinical conditions involving several organ systems (Table 33-2). Clinically, pulmonary disease caused by these organisms is virtually indistinguishable from tuberculosis. Disseminated infection is usually limited to immunocompromised patients, particularly HIV-infected individuals, in whom the M avium-intracellulare complex is responsible for more than 90 percent of cases. Cervical lymphadenitis due to infection with M scrofulaceum is seen especially in children younger than 5 years. Granulomatous skin lesions and soft tissue infections are usually associated with M marinum (swimming pool granuloma) or M ulcerans.
Table 33-2
Clinical Presentation of Nontuberculous Mycobacterial Infections.
Classification and Antigenic Types
Table 33-3 lists the nontuberculous mycobacteria associated with human disease and their classification within the Runyon scheme, in which the species in groups I to III are slow growers and those in group IV are rapid growers. The Runyon groups are further characterized by pigment production: nonchromogens (group III) are rarely pigmented; photochromogens (group I) are pigmented only when exposed to light; scotochromogens (group II) form pigment in the dark. Some of these species have been grouped into complexes based on similarities in the clinical condition which they cause. Other associations may be based upon biochemical similarities (e.g., the inclusion of M scrofulaceum and M avium-intracellulare to form the MAIS complex). Further distinction of multiple serotypes within some species (e.g., the MAIS complex) is based upon variations in the sugar residues on the lipooligosaccharides or the peptidoglycolipids.
Table 33-3
Classification of Nontuberculous Mycobacteria.
Epidemiology
A crucial difference between M tuberculosis and nontuberculous mycobacteria is the lack of transmission of the latter from patient to patient (Fig. 33-3). There is no evidence that infections caused by nontuberculous mycobacteria are contagious. Rather, the organisms exist saprophytically in the soil or water, occasionally in association with some infected-animal reservoir (e.g., poultry infected with M avium). Inhalation or ingestion of viable mycobacteria or introduction of bacilli through skin abrasions initiates the infection. Evidence of geographic concentrations of nontuberculous mycobacteria in the southeastern United States comes from skin test surveys with “tuberculins” made from specific nontuberculous mycobacteria (e.g., PPD-Y for M kansasii; PPD-A for M avium). In endemic areas many subclinical infections may occur. The ubiquity of nontuberculous mycobacteria makes them ideal opportunists for immunocompromised hosts. Up to 30 percent of patients with acquired immune deficiency syndrome (AIDS) may suffer disseminated mycobacterial infections, most of which are caused by members of the M avium-intracellulare complex. Such infections, which are associated with shortened survival, result from environmental exposure. In fact, nontuberculous mycobacteria have been cultured directly from tap water in several hospitals.
Treatment and Control
Many nontuberculous mycobacteria are resistant to the drugs commonly used successfully in the treatment of tuberculosis (e.g., isoniazid, pyrazinamide, and streptomycin). Antibiotic regimens may require several (five or six) drugs including rifampin, which is quite effective against M kansasii, or clarithromycin, which has marked activity against the M avium-intracellulare complex. Surgical resection is occasionally recommended with or without chemotherapy. In treating disseminated infections in AIDS patients, a regimen of five or six drugs, including clarithromycin, ethambutol and perhaps rifabutin, should be considered.
Mycobacterium leprae
Clinical Manifestations
The clinical spectrum of leprosy (Hansen's disease) reflects variations in three aspects of the illness: bacterial proliferation and accumulation, immunologic responses to the bacillus, and the resulting peripheral neuritis. The disease affects peripheral nerves, skin, and mucous membranes. Skin lesions, areas of anesthesia, and enlarged nerves are the principal signs of leprosy. The disease manifestations fall on a continuum from lepromatous leprosy to tuberculoid leprosy. The polar lepromatous leprosy patient presents with diffuse or nodular lesions (lepromas) containing many acid-fast M leprae bacilli cells (multibacillary lesions). These lesions are found predominantly on the cooler surfaces of the body, such as the nasal mucosa and the peripheral nerve trunks at the elbow, wrist, knee, and ankle. Sensory loss results from damage to nerve fibers. On the other hand, polar tuberculoid leprosy consists of a few well-defined anesthetized lesions containing only a few acid-fast bacilli (paucibacillary lesions). Borderline forms of the disease are unstable conditions, presenting with intermediate signs and symptoms.
Pathogenesis
Since M leprae has never been cultured in vitro, it appears to be an obligate intracellular pathogen that requires the environment of the host macrophage for survival and propagation. Estimates of the replication rate in vivo are on the order of 10 to 12 days. The bacilli resist intracellular degradation by macrophages, perhaps by escaping from the phagosome into the cytoplasm, and accumulate to high levels (1010 bacilli/g of tissue) in lepromatous leprosy. The peripheral nerve damage appears to be mediated principally by the host immune response to bacillary antigens. Tuberculoid leprosy is characterized by self-healing granulomas containing only a few, if any, acid-fast bacilli.
Host Defenses
The successful host response in tuberculoid leprosy involves macrophage activation and recruitment by T lymphocytes that recognize M leprae antigens. Very little circulating antibody against the bacillus is present in tuberculoid leprosy. In contrast, lepromatous leprosy is associated with profound specific anergy (lack of T cell-mediated immunity against M leprae antigens) and high levels of circulating antibodies. These antibodies play no protective role and may actually interfere with effective cell-mediated immunity. There is experimental evidence that components of the leprosy bacillus may induce suppressor T cells or interfere with macrophage function in the lesions. Figure 33-6 summarizes the immunologic and pathologic spectrum of leprosy. Much of the pathology is caused by the host immune response.

Figure 33-6
Pathologic (bacillary load), immunologic, and clinical spectrum of leprosy.
The dichotomy between Th1 and Th2 CD4+ T cell cytokine profiles mentioned earlier is even more clear-cut in leprosy. Elegant studies of cytokine production in cells from lepromatous and tuberculoid lesions have revealed a marked propensity for a Th2-like pattern in lepromatous leprosy and a Th1-like pattern in the tuberculoid form. These results imply that the production of IL-2 and IFNγ in response to mycobacterial antigens is associated with successful bacillary control. The conclusion has been strengthened by clinical observations of upgrading lesion status in patients receiving IL-2 therapy by direct intralesional injection.
Epidemiology
More than 10 million cases of leprosy are estimated to exist worldwide, predominantly in Asia (two-thirds) and Africa (one-third). Human-to-human transmission requires prolonged contact and is thought to occur via intact skin, penetrating wounds or insect bites, or by inhalation of M leprae and deposition on respiratory mucosa (Fig. 33-7). The source of the organism in nature is unknown. Recently, the development of PCR techniques for the detection of M leprae DNA in environmental and clinical specimens has allowed investigators to begin to study natural distribution as well as the contribution of asymptomatic human “carriers” to the epidemiology of leprosy. Natural infections have been documented in mangabey monkeys, and in wild armadillos in Texas and Louisiana. Several human infections have been reported following contact with armadillos, but their role in the epidemiology of this disease is controversial. Armadillos experimentally infected with M leprae serve as an important source of bacilli for researchers.
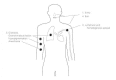
Figure 33-7
Pathogenesis of leprosy.
Diagnosis
The diagnosis of leprosy is based on the clinical signs previously discussed, and histologic examination of biopsy specimens taken from lepromas or other skin lesions. A consistent pattern of inflammation plus the presence of acid-fast bacilli is presumptive evidence of infection with M leprae. Although M leprae cannot be grown in vitro, bacteriologic cultures of clinical material should be done to rule out the presence of other mycobacteria. The lepromin skin test, in which a heat-killed suspension of armadillo-derived M leprae is injected into the skin of the patient, has little diagnostic value but will provide information of prognostic importance about the immune status of the individual. The PCR technique mentioned above, by which very small amounts of M leprae DNA can be detected directly in clinical specimens, may prove to be a useful diagnostic tool.
Treatment and Control
A variety of combinations of the following drugs (so-called multidrug therapy or MDT) are used to treat leprosy: dapsone, rifampin, clofazimine, and either ethionamide or prothionamide. Paucibacillary cases (tuberculoid and borderline tuberculoid) can be treated in 6 months, although dapsone alone is usually given for up to 3 years after disease inactivity. Therapy for patients with lepromatous or borderline lepromatous leprosy may require primary treatment for 3 years, with dapsone alone continued for the rest of the patient's life. Although some public health officials believe that MDT alone will result in eradication of leprosy in the near future, other experts are much more cautious. Drug resistance has been documented in M leprae. In many cases patient management must include anti-inflammatory therapy to alleviate the immunologic sequelae. Irreversible nerve damage leading to loss of sensation may result in paralysis or occult wounds and deformities. Wound prevention techniques and proper wound care are important.
Nocardia
Clinical Manifestations
Nocardia rarely causes clinical disease except in immunocompromised individuals, especially organ transplant recipients. Ninety percent of such patients present with pulmonary involvement, including cough, pleuritic chest pain, dyspnea, and radiologic abnormalities such as nodules and nodular infiltrates. Other clinical findings include weight loss, malaise, fever, and night sweats. About 20 percent of patients with nocardiosis present with cutaneous lesions, either localized or disseminated, and/or central nervous system involvement. About 50 percent of patients have an associated disease process (another infection or a tumor). Cutaneous infection with N brasiliensis results in localized development of granulomata and abscesses with soft tissue and bone involvement (Fig. 33-8).
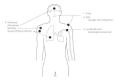
Figure 33-8
Pathogenesis of nocardiosis.
Structure
Nocardia organisms are Gram-positive rods, which in old cultures or clinical specimens may appear as branching chains resembling fungal hyphae. Figure 33-9 illustrates a typical colony of N asteroides and shows these organisms infecting rabbit alveolar macrophages. The filamentous morphology is evident. Nocardia are weakly acid-fast following staining with the modified Ziehl-Neelsen or Kinyoun stain. Cultures may grow in a few days, but typically require 2 to 3 weeks of incubation. The colony in Figure 33-9 is 3 weeks old.
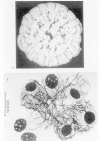
Figure 33-9
(A) Colony of N asteroides after 3 weeks of growth at 37° C on brain heart infusion agar (X11). (B) Interaction of N asteroides, virulent strain 14795, with seven (rabbit) alveolar macrophages (some in the process of fusing) 24 hours postinfection. (more...)
Classification and Antigenic Types
Three species of Nocardia are responsible for most human infections. Nocardia asteroides causes most nocardial pulmonary infections in this country (80 to 90 percent), with N brasiliensis (5 to 6 percent) and N caviae (3 percent) being recovered from only a few patients with nocardiosis. In the southern United States and in the tropics, N brasiliensis is an important agent of cutaneous nocardiosis. The three species can be distinguished by their patterns of proteolytic hydrolysis or of acid fermentation of several substrates.
Pathogenesis
Nocardia cells have been isolated from soil and organic material throughout the world. Natural infections occur in domestic animals. Human infection usually results from the inhalation of airborne bacilli or the traumatic inoculation of organisms into the skin. The infection is not transmissible between individuals. Natural resistance, mediated by intact mucous membranes and alveolar and tissue phagocytes, is quite strong.
In immunocompromised hosts, pulmonary infection results in the formation of abscesses and, rarely, granulomas with hematogenous or lymphatic dissemination to the skin or central nervous system. Nocardia can subvert the antimicrobial mechanisms of phagocytes by inhibiting phagosome-lysosome fusion. Owing to the debilitated nature of the infected patients, mortality is high (up to 45 percent), even with appropriate therapy.
Host Defenses
Nocardiosis is usually associated with T-cell dysfunctions, immunoglobulin deficiencies, or leukocyte abnormalities. Acquired resistance to Nocardia is complex, involving antibody-dependent phagocytosis by neutrophils, macrophage activation by the products of immune T cells, and the development of cytotoxic T lymphocytes. Neutrophils ingest opsonized bacteria but may not kill them. Macrophage activation is associated with containment and clearance of Nocardia organisms from the lungs. In a murine model, resistance to nocardiosis can be transferred with whole spleen cells or splenic T cells from immune mice.
Epidemiology
Although nocardiosis has been diagnosed in individuals with no detectable deficiency of humoral or cell-mediated immunity, it usually occurs in patients whose immune status has been compromised by post-transplant immunosuppressive therapy, leukemia, lymphoma, dysgammaglobulinemia, pancytopenia, humoral defects, chronic granulomatous disease, or steroid therapy. The male/female ratio in nocardiosis is approximately 2:1, and infections occur from infancy to old age. There is no apparent geographic clustering of cases in the United States, except for cutaneous infection with N brasiliensis, which is more common in the south.
Diagnosis
Nocardia can be identified presumptively by Gram and acid-fast stains and definitively by culture from appropriate clinical specimens. Sputum culture is useful for patients with a productive cough. The presence of branching, weakly acid-fast organisms in histologic sections, pus, or sputum suggests the clinical diagnosis. In one series, nearly 40 percent of cases required more invasive procedures (e.g., thoracentesis, transtracheal aspiration, bronchial washing, or biopsy) to obtain useful material for stain and culture of Nocardia.
Treatment and Control
Antimicrobial therapy with sulfa drugs (e.g., trimethoprim-sulfamethoxazole) is the treatment of choice. The duration of therapy ranges from 2–3 months for minor infections to 1 year for major infections.
References
- Beaman BL, Beaman L. Nocardia species: host-parasite relationships. Clin Microbiol Rev. 1994;7:213. [PMC free article: PMC358319] [PubMed: 8055469]
- Bloom BR (ed): Tuberculosis - Pathogenesis, Protection and Control. ASM Press, Washington, DC, 1994 .
- Johnson JL, Elner JJ, Shiratsuchi H. Monocyte-Mycobacterium avium complex interactions: studies of potential virulence factors for humans. Immunol Ser. 1994;60:263. [PubMed: 8251573]
- Krahenbuhl JL, Adams, LB The role of the macrophage in resistance to the leprosy bacillus. Immunol Ser. 1994;60:281. [PubMed: 8251574]
- Modlin RL. Th1-Th2 paradigm: insights from leprosy. J Invest Dermatol. 1994;102:828. [PubMed: 8006444]
- Reichman LB, Hershfield ES (eds): Tuberculosis - A Comprehensive International Approach. Marcel Dekker, New York, 1993 .
- Rigsby MO, Curis, AM Pulmonary disease from nontuberculous mycobacteria in patients with human immunodeficiency virus. Chest. 1994;106:913. [PubMed: 8082377]
- Rom WN, Garay S (eds): Tuberculosis. Little, Brown and Co, New York, 1995 .
- Shinnick T (ed): Tuberculosis. Curr Top Microbiol Immunol, Springer-Verlag, Heidelberg, 1995 .
- Thin-layer chromatographic analysis of mycolic acid and other long-chain components in whole-organism methanolysates of coryneform and related taxa.[J Gen Microbiol. 1976]Thin-layer chromatographic analysis of mycolic acid and other long-chain components in whole-organism methanolysates of coryneform and related taxa.Goodfellow M, Collins MD, Minnikin DE. J Gen Microbiol. 1976 Oct; 96(2):351-8.
- Phylogenetic analysis of mycolic acid-containing wall-chemotype IV actinomycetes and allied taxa by partial sequencing of ribosomal protein AT-L30.[Int J Syst Bacteriol. 1995]Phylogenetic analysis of mycolic acid-containing wall-chemotype IV actinomycetes and allied taxa by partial sequencing of ribosomal protein AT-L30.Ochi K. Int J Syst Bacteriol. 1995 Oct; 45(4):653-60.
- A simple chemical test to distinguish mycobacteria from other mycolic-acid-containing actinomycetes.[J Gen Microbiol. 1993]A simple chemical test to distinguish mycobacteria from other mycolic-acid-containing actinomycetes.Hamid ME, Minnikin DE, Goodfellow M. J Gen Microbiol. 1993 Sep; 139(9):2203-13.
- Review [Structural and biogenetic correlations of mycolic acids in relation to the phylogenesis of various genera of Actinomycetales].[Bull Soc Chim Biol (Paris). 1967]Review [Structural and biogenetic correlations of mycolic acids in relation to the phylogenesis of various genera of Actinomycetales].Etemadi AH. Bull Soc Chim Biol (Paris). 1967; 49(6):695-706.
- Review The envelope of mycobacteria.[Annu Rev Biochem. 1995]Review The envelope of mycobacteria.Brennan PJ, Nikaido H. Annu Rev Biochem. 1995; 64:29-63.
- Mycobacteria and Nocardia - Medical MicrobiologyMycobacteria and Nocardia - Medical Microbiology
Your browsing activity is empty.
Activity recording is turned off.
See more...
