By agreement with the publisher, this book is accessible by the search feature, but cannot be browsed.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Cooper GM. The Cell: A Molecular Approach. 2nd edition. Sunderland (MA): Sinauer Associates; 2000.

The Cell: A Molecular Approach. 2nd edition.
Show detailsMany tasks that a cell must perform, such as movement and the synthesis of macromolecules, require energy. A large portion of the cell's activities are therefore devoted to obtaining energy from the environment and using that energy to drive energy-requiring reactions. Although enzymes control the rates of virtually all chemical reactions within cells, the equilibrium position of chemical reactions is not affected by enzymatic catalysis. The laws of thermodynamics govern chemical equilibria and determine the energetically favorable direction of all chemical reactions. Many of the reactions that must take place within cells are energetically unfavorable, and are therefore able to proceed only at the cost of additional energy input. Consequently, cells must constantly expend energy derived from the environment. The generation and utilization of metabolic energy is thus fundamental to all of cell biology.
Free Energy and ATP
The energetics of biochemical reactions are best described in terms of the thermodynamic function called Gibbs free energy (G), named for Josiah Willard Gibbs. The change in free energy (ΔG) of a reaction combines the effects of changes in enthalpy (the heat that is released or absorbed during a chemical reaction) and entropy (the degree of disorder resulting from a reaction) to predict whether or not a reaction is energetically favorable. All chemical reactions spontaneously proceed in the energetically favorable direction, accompanied by a decrease in free energy (ΔG < 0). For example, consider a hypothetical reaction in which A is converted to B:

If ΔG < 0, this reaction will proceed in the forward direction, as written. If ΔG > 0, however, the reaction will proceed in the reverse direction and B will be converted to A.
The ΔG of a reaction is determined not only by the intrinsic properties of reactants and products, but also by their concentrations and other reaction conditions (e.g., temperature). It is thus useful to define the free-energy change of a reaction under standard conditions. (Standard conditions are considered to be a 1-M concentration of all reactants and products, and 1 atm of pressure). The standard free-energy change (ΔG°) of a reaction is directly related to its equilibrium position because the actual ΔG is a function of both ΔG° and the concentrations of reactants and products. For example, consider the reaction
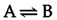
The free-energy change can be written as follows:
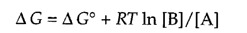
where R is the gas constant and T is the absolute temperature.
At equilibrium, ΔG= 0 and the reaction does not proceed in either direction. The equilibrium constant for the reaction (K= [B]/[A] at equilibrium) is thus directly related to ΔG° by the above equation, which can be expressed as follows:
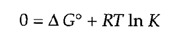
or
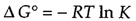
If the actual ratio [B]/[A] is greater than the equilibrium ratio (K), ΔG > 0 and the reaction proceeds in the reverse direction (conversion of B to A). On the other hand, if the ratio [B]/[A] is less than the equilibrium ratio, ΔG < 0 and A is converted to B.
The standard free-energy change (ΔG°) of a reaction therefore determines its chemical equilibrium and predicts in which direction the reaction will proceed under any given set of conditions. For biochemical reactions, the standard free-energy change is usually expressed as ΔG°′, which is the standard free-energy change of a reaction in aqueous solution at pH= 7, approximately the conditions within a cell.
Many biological reactions (such as the synthesis of macromolecules) are thermodynamically unfavorable (ΔG > 0) under cellular conditions. In order for such reactions to proceed, an additional source of energy is required. For example, consider the reaction
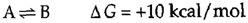
The conversion of A to B is energetically unfavorable, so the reaction proceeds in the reverse rather than the forward direction. However, the reaction can be driven in the forward direction by coupling the conversion of A to B with an energetically favorable reaction, such as:
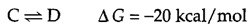
If these two reactions are combined, the coupled reaction can be written as follows:
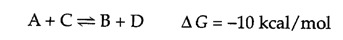
The ΔG of the combined reaction is the sum of the free-energy changes of its individual components, so the coupled reaction is energetically favorable and will proceed as written. Thus, the energetically unfavorable conversion of A to B is driven by coupling it to a second reaction associated with a large decrease in free energy. Enzymes are responsible for carrying out such coupled reactions in a coordinated manner.
The cell uses this basic mechanism to drive the many energetically unfavorable reactions that must take place in biological systems. Adenosine 5′-triphosphate (ATP) plays a central role in this process by acting as a store of free energy within the cell (Figure 2.31). The bonds between the phosphates in ATP are known as high-energy bonds because their hydrolysis is accompanied by a relatively large decrease in free energy. There is nothing special about the chemical bonds themselves; they are called high-energy bonds only because a large amount of free energy is released when they are hydrolyzed within the cell. In the hydrolysis of ATP to ADP plus phosphate (Pi), ΔG°′= -7.3 kcal/mol. Recall, however, that ΔG°′ refers to “standard conditions,” in which the concentrations of all products and reactants are 1 M. Actual intracellular concentrations of Pi are approximately 10-2M, and intracellular concentrations of ATP are higher than those of ADP. These differences between intracellular concentrations and those of the standard state favor ATP hydrolysis, so for ATP hydrolysis within a cell, ΔG is approximately -12 kcal/mol.
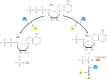
Figure 2.31
ATP as a store of free energy. The bonds between the phosphate groups of ATP are called high-energy bonds because their hydrolysis results in a large decrease in free energy. ATP can be hydrolyzed either to ADP plus a phosphate group (HPO42-) or to AMP (more...)
Alternatively, ATP can be hydrolyzed to AMP plus pyrophosphate (PPi). This reaction yields about the same amount of free energy as the hydrolysis of ATP to ADP does. However, the pyrophosphate produced by this reaction is then itself rapidly hydrolyzed, with a ΔG similar to that of ATP hydrolysis. Thus, the total free-energy change resulting from the hydrolysis of ATP to AMP is approximately twice that obtained by the hydrolysis of ATP to ADP. For comparison, the bond between the sugar and phosphate group of AMP, rather than having high energy, is typical of covalent bonds; for the hydrolysis of AMP, ΔG°′= -3.3 kcal/mol.
Because of the accompanying decrease in free energy, the hydrolysis of ATP can be used to drive other energy-requiring reactions within the cell. For example, the first reaction in glycolysis (discussed in the next section) is the conversion of glucose to glucose-6-phosphate. The reaction can be written as follows:
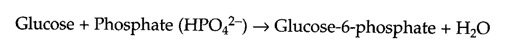
Because this reaction is energetically unfavorable as written (ΔG°′= +3.3 kcal/mol), it must be driven in the forward direction by being coupled to ATP hydrolysis (ΔG°′= -7.3 kcal/mol):
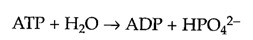
The combined reaction can be written as follows:
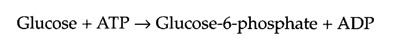
The free-energy change for this reaction is the sum of the free-energy changes for the individual reactions, so for the coupled reaction ΔG°′= -4.0 kcal/mol, favoring glucose-6-phosphate formation.
Other molecules, including other nucleoside triphosphates (e.g., GTP), also have high-energy bonds and can be used as ATP is to drive energy-requiring reactions. For most reactions, however, ATP provides the free energy. The energy-yielding reactions within the cell are therefore coupled to ATP synthesis, while the energy-requiring reactions are coupled to ATP hydrolysis. The high-energy bonds of ATP thus play a central role in cell metabolism by serving as a usable storage form of free energy.
The Generation of ATP from Glucose
The breakdown of carbohydrates, particularly glucose, is a major source of cellular energy. The complete oxidative breakdown of glucose to CO2 and H2O can be written as follows:
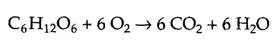
The reaction yields a large amount of free energy: ΔG°′= -686 kcal/mol. To harness this free energy in usable form, glucose is oxidized within cells in a series of steps coupled to the synthesis of ATP.
Glycolysis, the initial stage in the breakdown of glucose, is common to virtually all cells. Glycolysis occurs in the absence of oxygen and can provide all the metabolic energy of anaerobic organisms. In aerobic cells, however, glycolysis is only the first stage in glucose degradation.
The reactions of glycolysis result in the breakdown of glucose into pyruvate, with the net gain of two molecules of ATP (Figure 2.32). The initial reactions in the pathway actually consume energy, using ATP to phosphorylate glucose to glucose-6-phosphate and then fructose-6-phosphate to fructose-1,6-bisphosphate. The enzymes that catalyze these two reactions—hexokinase and phosphofructokinase, respectively—are important regulatory points of the glycolytic pathway. The key control element is phosphofructokinase, which is inhibited by high levels of ATP. Inhibition of phosphofructokinase results in an accumulation of glucose-6-phosphate, which in turn inhibits hexokinase. Thus, when the cell has an adequate supply of metabolic energy available in the form of ATP, the breakdown of glucose is inhibited.
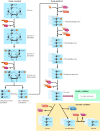
Figure 2.32
Reactions of glycolysis. Glucose is broken down to pyruvate, with the net formation of two molecules each of ATP and NADH. Under anaerobic conditions, the NADH is reoxidized by the conversion of pyruvate to ethanol or lactate. Under aerobic conditions, (more...)
The reactions following the formation of fructose-1,6-bisphosphate constitute the energy-producing part of the glycolytic pathway. Cleavage of fructose-1,6-bisphosphate yields two molecules of the three-carbon sugar glyceraldehyde-3-phosphate, which is oxidized to 1,3-bisphosphoglycerate. The phosphate group of this compound has a very high free energy of hydrolysis (ΔG°′= -11.5 kcal/mol), so it is used in the next reaction of glycolysis to drive the synthesis of ATP from ADP. The product of this reaction, 3-phosphoglycerate, is then converted to phosphoenolpyruvate, the second high-energy intermediate in glycolysis. In the hydrolysis of the high-energy phosphate of phosphoenolpyruvate, ΔG°′= -14.6 kcal/mol, its conversion to pyruvate is coupled to the synthesis of ATP. Each molecule of glyceraldehyde-3-phosphate converted to pyruvate is thus coupled to the generation of two molecules of ATP; in total, four ATPs are synthesized from each starting molecule of glucose. Since two ATPs were required to prime the initial reactions, the net gain from glycolysis is two ATP molecules.
In addition to producing ATP, glycolysis converts two molecules of the coenzyme NAD+ to NADH. In this reaction, NAD+ acts as an oxidizing agent that accepts electrons from glyceraldehyde-3-phosphate. The NADH formed as a product must be recycled by serving as a donor of electrons for other oxidation-reduction reactions within the cell. In anaerobic conditions, the NADH formed during glycolysis is reoxidized to NAD+ by the conversion of pyruvate to lactate or ethanol. In aerobic organisms, however, the NADH serves as an additional source of energy by donating its electrons to the electron transport chain, where they are ultimately used to reduce O2 to H2O, coupled to the generation of additional ATP.
In eukaryotic cells, glycolysis takes place in the cytosol. Pyruvate is then transported into mitochondria, where its complete oxidation to CO2 and H2O yields most of the ATP derived from glucose breakdown. The next step in the metabolism of pyruvate is its oxidative decarboxylation in the presence of coenzyme A (CoA), which serves as a carrier of acyl groups in various metabolic reactions (Figure 2.33). One carbon of pyruvate is released as CO2, and the remaining two carbons are added to CoA to form acetyl CoA. In the process, one molecule of NAD+ is reduced to NADH.
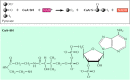
Figure 2.33
Oxidative decarboxylation of pyruvate. Pyruvate is converted to CO2 and acetyl CoA, and one molecule of NADH is produced in the process. Coenzyme A (CoA-SH) is a general carrier of activated acyl groups in a variety of reactions.
The acetyl CoA formed by this reaction enters the citric acid cycle or Krebs cycle (Figure 2.34), which is the central pathway in oxidative metabolism. The two-carbon acetyl group combines with oxaloacetate (four carbons) to yield citrate (six carbons). Through eight further reactions, two carbons of citrate are completely oxidized to CO2 and oxaloacetate is regenerated. During the cycle, one high-energy phosphate bond is formed in GTP, which is used directly to drive the synthesis of one ATP molecule. In addition, each turn of the cycle yields three molecules of NADH and one molecule of reduced flavin adenine dinucleotide (FADH2), which is another carrier of electrons in oxidation-reduction reactions.
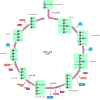
Figure 2.34
The citric acid cycle. A two-carbon acetyl group is transferred from acetyl CoA to oxaloacetate, forming citrate. Two carbons of citrate are then oxidized to CO2 and oxaloacetate is regenerated. Each turn of the cycle yields one molecule of GTP, three (more...)
The citric acid cycle completes the oxidation of glucose to six molecules of CO2. Four molecules of ATP are obtained directly from each glucose molecule—two from glycolysis and two from the citric acid cycle (one for each molecule of pyruvate). In addition, ten molecules of NADH (two from glycolysis, two from the conversion of pyruvate to acetyl CoA, and six from the citric acid cycle) and two molecules of FADH2 are formed. The remaining energy derived from the breakdown of glucose comes from the reoxidation of NADH and FADH2, with their electrons being transferred through the electron transport chain to (eventually) reduce O2 to H2O.
During oxidative phosphorylation, the electrons of NADH and FADH2 combine with O2, and the energy released from the process drives the synthesis of ATP from ADP. The transfer of electrons from NADH to O2 releases a large amount of free energy: ΔG°′= -52.5 kcal/mol for each pair of electrons transferred. So that this energy can be harvested in usable form, the process takes place gradually by the passage of electrons through a series of carriers, which constitute the electron transport chain (Figure 2.35). The components of the electron transport chain are located in the inner mitochondrial membrane of eukaryotic cells, and oxidative phosphorylation is considered in more detail when mitochondria are discussed in Chapter 10. In aerobic bacteria, which use a comparable system, components of the electron transport chain are located in the plasma membrane. In either case, the transfer of electrons from NADH to O2 yields sufficient energy to drive the synthesis of approximately three molecules of ATP. Electrons from FADH2 enter the electron transport chain at a lower energy level, so their transfer to O2 yields less usable free energy, only two ATP molecules.
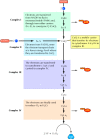
Figure 2.35
The electron transport chain. Electrons from NADH and FADH2 are transferred to O2 through a series of carriers organized into four protein complexes in the mitochondrial membrane. The free energy derived from electron transport reactions at complexes (more...)
It is now possible to calculate the total yield of ATP from the oxidation of glucose. The net gain from glycolysis is two molecules of ATP and two molecules of NADH. The conversion of pyruvate to acetyl CoA and its metabolism via the citric acid cycle yields two additional molecules of ATP, eight of NADH, and two of FADH2. Assuming that three molecules of ATP are derived from the oxidation of each NADH and two from each FADH2, the total yield is 38 molecules of ATP per molecule of glucose. However, this yield is lower in some cells because the two molecules of NADH generated by glycolysis in the cytosol are unable to enter mitochondria directly. Instead, their electrons must be transferred into the mitochondrion via a shuttle system. Depending on the system used, this transfer may result in these electrons entering the electron transport chain at the level of FADH2. In such cases, the two molecules of NADH derived from glycolysis give rise to two rather than three molecules of ATP, reducing the total yield to 36 rather than 38 ATPs per molecule of glucose.
The Derivation of Energy from Other Organic Molecules
Energy in the form of ATP can be derived from the breakdown of other organic molecules, with the pathways involved in glucose degradation again playing a central role. Nucleotides, for example, can be broken down to sugars, which then enter the glycolytic pathway, and amino acids are degraded via the citric acid cycle. The two principal storage forms of energy within cells, polysaccharides and lipids, can also be broken down to produce ATP. Polysaccharides are broken down into free sugars, which are then metabolized as discussed in the previous section. Lipids, however, are an even more efficient energy storage molecule. Because lipids are more reduced than carbohydrates, consisting primarily of hydrocarbon chains, their oxidation yields substantially more energy per weight of starting material.
Fats (triacylglycerols) are the major storage form of lipids. The first step in their utilization is their breakdown to glycerol and free fatty acids. Each fatty acid is joined to coenzyme A, yielding a fatty acyl-CoA at the cost of one molecule of ATP (Figure 2.36). The fatty acids are then degraded in a stepwise oxidative process, two carbons at a time, yielding acetyl CoA plus a fatty acyl-CoA shorter by one two-carbon unit. Each round of oxidation also yields one molecule of NADH and one of FADH2. The acetyl CoA then enters the citric acid cycle, and degradation of the remainder of the fatty acid continues in the same manner.
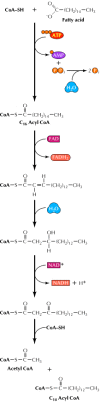
Figure 2.36
Oxidation of fatty acids. The fatty acid (e.g., the 16-carbon saturated fatty acid palmitate) is initially joined to coenzyme A at the cost of one molecule of ATP. Oxidation of the fatty acid then proceeds by stepwise removal of two-carbon units as acetyl (more...)
The breakdown of a 16-carbon fatty acid thus yields seven molecules of NADH, seven of FADH2, and eight of acetyl CoA. In terms of ATP generation, this yield corresponds to 21 molecules of ATP derived from NADH (3 × 7), 14 ATPs from FADH2 (2 × 7), and 96 from acetyl CoA (8 × 12). Since one ATP was used to start the process, the net gain is 130 ATPs per molecule of a 16-carbon fatty acid. Compare this yield with the net gain of 38 ATPs per molecule of glucose. Since the molecular weight of a saturated 16-carbon fatty acid is 256 and that of glucose is 180, the yield of ATP is approximately 2.5 times greater per gram of the fatty acid—hence the advantage of lipids over polysaccharides as energy storage molecules.
Photosynthesis
The generation of energy from oxidation of carbohydrates and lipids relies on the degradation of preformed organic compounds. The energy required for the synthesis of these compounds is ultimately derived from sunlight, which is harvested and used by plants and photosynthetic bacteria to drive the synthesis of carbohydrates. By converting the energy of sunlight to a usable form of chemical energy, photosynthesis is the source of virtually all metabolic energy in biological systems.
The overall equation of photosynthesis can be written as follows:
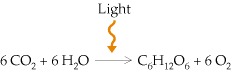
The process is much more complex, however, and takes place in two distinct stages. In the first, called the light reactions, energy absorbed from sunlight drives the synthesis of ATP and NADPH (a coenzyme similar to NADH), coupled to the oxidation of H2O to O2. The ATP and NADPH generated by the light reactions drive the synthesis of carbohydrates from CO2 and H2O in a second set of reactions, called the dark reactions because they do not require sunlight. In eukaryotic cells, both the light and dark reactions occur in chloroplasts.
Photosynthetic pigments capture energy from sunlight by absorbing photons. Absorption of light by these pigments causes an electron to move from its normal molecular orbital to one of higher energy, thus converting energy from sunlight into chemical energy. In plants the most abundant photosynthetic pigments are the chlorophylls (Figure 2.37), which together absorb visible light of all wavelengths other than green. Additional pigments absorb light of other wavelengths, so essentially the entire spectrum of visible light can be captured and utilized for photosynthesis.
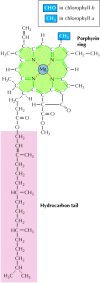
Figure 2.37
The structure of chlorophyll. Chlorophylls consist of porphyrin ring structures linked to hydrocarbon tails. Chlorophylls a and b differ by a single functional group in the porphyrin ring.
The energy captured by the absorption of light is used to convert H2O to O2 (Figure 2.38). The high-energy electrons derived from this process then enter an electron transport chain, in which their transfer through a series of carriers is coupled to the synthesis of ATP. In addition, these high energy electrons reduce NADP+ to NADPH.

Figure 2.38
The light reactions of photosynthesis. Energy from sunlight is used to split H2O to O2. The high-energy electrons derived from this process are then transported through a series of carriers and used to convert NADP+ to NADPH. Energy derived from the electron (more...)
In the dark reactions, the ATP and NADPH produced from the light reactions drive the synthesis of carbohydrates from CO2 and H2O. One molecule of CO2 at a time is added to a cycle of reactions—known as the Calvin cycle after its discoverer, Melvin Calvin—that leads to the formation of carbohydrates (Figure 2.39). Overall, the Calvin cycle consumes 18 molecules of ATP and 12 of NADPH for each molecule of glucose synthesized. Two electrons are needed to convert each molecule of NADP+ to NADPH, so 24 electrons must pass through the electron transport chain to generate sufficient NADPH to synthesize one molecule of glucose. These electrons are obtained by the conversion of 12 molecules of H2O to six molecules of O2, consistent with the formation of six molecules of O2 for each molecule of glucose. It is not clear, however, whether the passage of the same 24 electrons through the electron transport chain is also sufficient to generate the 18 ATPs that are required by the Calvin cycle. Some of these ATP molecules may instead be generated by alternative electron transport chains that use the energy derived from sunlight to synthesize ATP without the synthesis of NADPH (see Chapter 10).
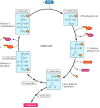
Figure 2.39
The Calvin cycle. Shown here is the synthesis of one molecule of glucose from six molecules of CO2. Each molecule of CO2 is added to ribulose-1,5-bisphosphate to yield two molecules of 3-phosphoglycerate. Six molecules of CO2 thus lead to the formation (more...)
- Metabolic Energy - The CellMetabolic Energy - The Cell
Your browsing activity is empty.
Activity recording is turned off.
See more...