Primary respiratory tract tumors in animals have a lower incidence compared to other systems and tissues, as well as compared to humans. In contrast, the lung is the preferential site of most metastases, in all animals.
The presentation of respiratory tract tumors requires their systematization depending on anatomical parts and histological structures. The following will be presented:
- – nasopharyngeal, paranasal sinus, laryngeal and tracheal tumors;
- – lung tumors;
- – pleural cavity tumors;
- – experimental induction of nasal and pulmonary tumors.
Histological Classification of the Respiratory System of Domestic Animals (Dungworth et al. 1999)
Tumors of the Nasal and Paranasal Regions
- Epithelial Tumors
- 1.1. Benign
- 1.1.1 Papilloma
- 1.1.2 Adenoma
- 1.2 Malignant
- 1.2.1 Squamous cell (epidermoid) carcinoma
- 1.2.2 Transitional carcinoma
- 1.2.3 Adenocarcinoma
- 1.2.4 Adenosquamous (mucoepidermoid) carcinoma
- 1.2.5 Adenoid cystic carcinoma
- 1.2.6 Acinic cell carcinoma
- 1.2.7 Undifferentiated (anaplastic) carcinoma
- 1.2.8 Olfactory neuroblastoma (esthesioneuroblastoma)
- 1.2.9 Neuroendocrine carcinoma
- Mesenchymal Tumors
- Miscellaneous Tumors
- Tumorlike Lesions
- 4.1 Polyps
- 4.2 Equine progressive ethmoid hematoma
- 4.3 Chronic proliferative rhinitis
Tumors of the Larynx and Trachea
- Epithelial Tumors
- 1.1 Benign
- 1.1.1 Adenoma
- 1.1.2 Papilloma
- 1.2 Malignant
- 1.2.1 Adenocarcinoma
- 1.2.2 Squamous cell carcinoma
- 1.2.3 Undifferentiated carcinoma
- Mesenchymal Tumors
- 2.1 Rhabdomyoma
- 2.2 Others
- Miscellaneous Tumors
- 3.1 Oncocytoma
- 3.2 Others
- Secondary Tumors
- 4.1 Thyroid carcinoma
- Tumorlike Lesions
Tumors of the Lung
- Epithelial Tumors
- 1.1 Benign
- 1.1.1 Papilloma
- 1.1.2 Adenoma
- 1.1.1.1 Papillary adenoma
- 1.1.1.2 Bronchioloalveolar adenoma
- 1.2 Malignant
- 1.2.1 Bronchial gland carcinoma
- 1.2.2 Squamous cell carcinoma
- 1.2.3 Adenocarcinoma
- 1.2.3.1 Adenocarcinoma, papillary, acinar, solid, or mixed
- 1.2.3.2 Bronchioloalveolar carcinoma
- 1.2.4 Adenosquamous carcinoma
- 1.2.5 Small cell carcinoma
- 1.2.6 Large cell carcinoma
- 1.2.7 Neuroendocrine tumor
- 1.2.8 Pulmonary blastoma
- 1.2.9 Combined carcinoma
- Mesenchymal Tumors
- Mixed Epithelial and Mesenchymal Tumors
- 3.1 Carcinosarcoma
- Miscellaneous Tumors
- 4.1 Granular cell tumor
- 4.2 Lymphomatoid granulomatosis
- 4.3 Malignant histiocytosis
- 4.4 Others
- Secondary Tumors
- Tumorlike Lesions
- 6.1 Bronchioloalveolar hyperplasia
- 6.2 Others
- 7. Pleural Tumors
- 7.1 Mesothelioma
7.1. NASOPHARYNGEAL, PARANASAL SINUS, LARYNGEAL AND TRACHEAL TUMORS
The tumors of the nasal cavity and paranasal sinuses generally have a low incidence, being frequently reported in dogs. The morphological forms of these neoplasms in animals are similar to human forms. The tumors of the nasal cavity and paranasal sinuses are associated with chronic irritations produced by the polluted environment, and in incipient lesions, metaplasias of nasal mucosal epithelium occur [12].
The complex structure of the nasal cavity and paranasal sinuses (bone, cartilage, vascular connective tissue, covering epithelium and glandular epithelium) determines the appearance and development of various tumor forms. In addition to this, domestic animal species live under different habitat, nutrition and management conditions, with particular longevity for companion animals and a short economic life for production animals. Under the given circumstances, a strictly morphological classification is less practical; consequently, nasal and paranasal sinus tumors will be presented for each species.
Morphological classification, according to STÜNZI and HANSER (1976), updated by DUNGWORTH (1993) includes the following tumor forms:
- tumors of the surface and glandular epithelium: papilloma; adenoma; squamous cell carcinoma; spindle cell carcinoma; intermediate or transitional carcinoma; adenocarcinoma; anaplastic carcinoma;
- mesenchymal tumors;
- bone and cartilage tumors;
- unclassified tumors;
- olfactory neuroblastoma.
7.1.1. Nasal and paranasal sinus tumors in dogs
The incidence of benign tumors (papillomas and adenomas) is much lower compared to malignant neoplasms. The literature reports, depending on the tumor type, similar figures and percentages, for nasal and paranasal carcinomas about 1 % [11]; other authors place adenocarcinoma in the first position, followed by squamous cell carcinoma and undifferentiated carcinoma, and olfactory gland carcinoma is mentioned by ZAKI and LIU (1974). Malignant forms, in the first place carcinomas (epithelial carcinoma; adenocarcinoma; squamous cell carcinoma; mucoepidermoid carcinoma and undifferentiated carcinoma) are more numerous, compared to sarcomas (chondrosarcoma and undifferentiated sarcoma) [17]. Of a total of 78 connective tissue tumors, MOULTON (1978) diagnosed: 28 fibrosarcomas, 18 chondrosarcomas, 13 osteosarcomas, 3 reticular cell sarcomas, 3 mastocytomas, 6 undifferentiated sarcomas, 3 angiosarcomas, and 1 leiomyoma.
Nasal and paranasal sinus tumors in dogs are associated with chronic irritations produced by the polluted environment, presenting as a first morphological change the metaplasia of the mucosal epithelium. An additional argument for this supposition is the advanced age at which neoplasms appear in this species, at a mean age of 9.7 years for epithelial neoplasms and 8.1 years for mesenchymal tissue neoplasms [101].
The mean age of dogs with nasal and paranasal sinus neoplasms is 9 years, with extremely wide variations, from 1 year to 16 years; however, adult or old subjects are frequently affected [75].
Dog breeds with long noses, medium and large size breeds, such as German Shepherd, Collie and Cocker Spaniel, are most frequently affected [30, 75]. Breed peculiarities and the length of the nose could be explained by the higher possibility of filtration of irritating substances, noxious pollutants, which induce changes, metaplasias and initiate the neoplastic phenomenon.
Sex in both dogs and cats influences the incidence of nasal and sinus tumors, males being more affected than females; a 45:25 ratio is estimated [64,101].
Clinical signs are not specific, and the following may appear: sneezing, epistaxis, mucopus, tumefactions or other manifestations that are found in rhinitis, sinusitis, osteomyelitis, foreign bodies, traumas, etc. Additional data in the making of diagnosis are provided by radiological examination and especially tomographic evaluation [75]. The cited authors mention the presence of metastases in regional lymph nodes in 41% of cases and sometimes at distance.
MOULTON (1990) states that benign tumors are much more rarely found, compared to malignant tumors. The author cites the observations of ZAKI and LIU (1974), who report olfactory gland adenocarcinomas, extremely rare neoplasms in dogs. The epidemiological data provided by GEORGE and FUHRER (1988) show a 1% incidence of nasal tumors, of all tumors in dogs. Malignant forms represent 80%, the tumors of epithelial origin being the most frequent (67%). PATNAIK (1989) established the following incidence of histological types, of a total of 285 sinonasal tumors: lymphomas 26.6%, fibrosarcomas 22%, hemangiosarcomas 16%, fibrous histiocytomas 11%, myosarcomas 11%, malignant nerve sheath tumors 7%. The rate of metastases was 17.6%, lower than in other dog neoplasms.
Nasal neoplasms, especially malignant ones, have infiltrative growth and tend to extend, possibly up to the sinuses. Concomitant osteolytic and deforming bone changes occur. On necropsy, the atrophy or even disappearance of the nasal conchae is found, as well as the thinning and deviation of the nasal septum. Complications also occur in the mucosa, under the form of extended necrosis, ulcerations with hemorrhagic purulent exudate [102,103].
Nasal and sinus carcinomas rarely produce distant metastases; locally, they have invasive growth, with serious bone lesions, reaching more rarely the orbit. Neoplastic cells have been identified in mandibular, retropharyngeal and lower cervical ganglia. Cases of metastases in the lungs, liver, exceptionally in the brain, adrenal gland, testicle, ischium and femur are reported (MORGAN et al., 1972) [66].
Nasal papilloma appears as a single or multiple exophytic tumor, more frequently unilateral, sometimes with a polypous aspect. The polyp is smooth, pedunculated, dense, sometimes with bleeding ulcers, of white, pink or red color. The incidence of papilloma in dogs and cats is moderate, being higher in horses. Occasionally, it recurs after removal.
In the etiology of this tumor, chronic inflammations of the nasal mucosa or/and allergic reactions may be incriminated [12,17].
Histopathologically, structure suggests a soft fibroma aspect, sometimes with islands of bone metaplasia, covered on the surface by cuboid or prismatic epithelium, with the frequent presence of inflammatory cells (Fig. 7.1.).

Fig. 7.1
Nasal papilloma, with focal slight keratinization.
Nasal adenoma is a rare benign tumor, being localized and well circumscribed, frequently small sized. Histologically, it is formed by exophytic surface epithelium and glandular structures, with collagenous stroma, which sometimes may be abundant [101].
Squamous cell carcinoma is relatively frequent in dogs, it appears as an irregular white formation, and in section, the connective tissue provides a lobular aspect. Secondary infections, necroses and hemorrhages confer a low consistency to both the tumor and the adjacent tissues.
Histological, structure is characterized by hyperkeratosis on the surface and the presence of keratotic globes in the depth, but in many cases keratinization is reduced, and the characteristics of increased malignancy are obvious. Tumor cells form islands of compact carcinoma or ramified bands. Cells are large, non-uniform, with the presence of intercellular bridges, abundant, red, finely granulated or foamy cytoplasm. Nuclei are heterogeneous, pale, with 1 or 2 nucleoli. In general, anaplasia and the high number of mitoses is remarked. The connective stroma separates groups and islands of neoplastic cells, sometimes being infiltrated with monocytes (Fig. 7.2. and 7.3.).
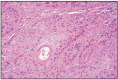
Fig. 7.2
Nasal squamos carcinoma, slight keratinization.
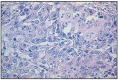
Fig. 7.3
Nasal epidermoid - squamos carcinoma, without keratinization.
Spindle cell carcinoma is considered by STUNZI and HUSER (1976) as a variant, a type of squamous cell carcinoma, since forms of transition of neoplastic cells to squamous or polyhedral cells have been noted. The tumor is more rarely found in animals. Macroscopically, the tumor appears as poorly delimited masses, of white-gray color, in general similar to squamous cell carcinoma. Fusiform cells are arranged in layers, nests or ramified bundles. In the center of the tumor, cells form spirals, and at the periphery they are perpendicular to the neoplasm margin. Cellular limits are little obvious and keratinization is usually absent. The stroma, which is not particularly abundant, delimits tumor islands and may be infiltrated with monocytes (Fig. 7.4.).

Fig. 7.4
Nasal spindle- cell carcinoma.
Transitional or intermediate carcinoma is formed by cuboid cells, arranged in layers, similarly to transitional epithelium and respiratory epithelium neoplasm, and in humans to nasal epithelium tumor. It is classified as a non-keratinized squamous cell tumor. Characteristic of this neoplasm is the arrangement of cuboid cells in thick layers, nuclei being round, without border cells, with an obvious basal membrane. Fine connective vascular septa are noted in the structure of carcinoma, which delimit neoplastic cell groups. Microcysts can be sometimes found in the epithelial mass. Squamous metaplasia is absent, and necrotic microfoci appear occasionally [35,101] (Fig. 7.5. and 7.6.).

Fig. 7.5
Nasal transitional carcinoma.

Fig. 7.6
Nasal transitional carcinoma.
Adenocarcinoma is formed by acini and canaliculi of serous and mucous glands. The histological aspect is an acinar or papillary neoplasm, with mucus retention, sometimes taking the aspect of mucinous carcinoma.
Papillary adenocarcinoma is the most frequent, in which cylindrical epithelium is arranged on a basal membrane that lies on connective filaments. Massive connective tissue proliferation is rare (Fig. 7.7.).
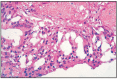
Fig. 7.7
Nasal adenocarcinoma.
Mixed carcinomas are composed of miscellaneous cells, some of which with compact arrangement, others in bundles or acini or even squamous metaplasias.
Adenoid cystic carcinoma is more rarely diagnosed, having a multilobular aspect, and it may be derived from the salivary gland tissue in the soft palate, rather than from the nasal epithelial structure [35].
Anaplastic or undifferentiated carcinoma has been diagnosed in both the nasal cavities and the paranasal sinuses of dogs. Microscopic structure is characterized by large islands of round or polygonal cells, without the possibility of establishing their origin. Sometimes, by special methodologies, carcinoma types such as olfactory neuroblastomas, amelanotic melanomas, lymphoreticular tumors and poorly differentiated mastocytomas may be distinguished.
The poorly differentiated cells are arranged in thick compact cords or bundles of cells with minimal glandular appearance, separated by fine septa of connective tissue. No glandular structures appear in these neoplastic forms. Some tumor cells resemble reticular sarcoma cells or histiocytic lymphosarcoma. Mitotic forms are numerous, and squamous metaplasias may be found extremely sporadically in the structures of the undifferentiated carcinoma (Fig. 7.8.).

Fig. 7.8
Nasal anaplastic carcinoma.
Neuroendocrine carcinoma, a highly malignant tumor consisting of small-to-medium-sized cells arranged in sheets or packets and with phenotypic characteristics of neuroendocrine cells. These tumors are extremely rare; report of their occurrence in dogs. The sheets, nests or cords of cells are separated by mostly delicate fibrovascular stroma to give an endocrinelike packeting. Differential diagnosis is made from olfactory neuroblastomas [123].
Megavoltage radiation therapy of nasal and paranasal tumors was used by THÉON et al. (1993), in 58 dogs with carcinomas and 19 subjects with sarcomas. The authors obtained a 1-year survival time in 60.3% of cases and a 2-year survival time in 25% of cases. In cases without recurrences, 1-year survival was 38.2% and 2-year survival 17.6%.
Surgical treatment, sometimes quite laborious, provides good results in the case of benign forms. By performing surgical excision alone in 37 dogs with nasal tumors, MORRISON et al. (1989) did not obtain significant results in increasing survival time. In the case of malignant tumors, the surgical act is supplemented by cobalt therapy, the results of which are satisfactory, according to MC ENTEE et al. (1991). The authors obtained a survival time between 2.5 months and 46 months, with a mean of 20.7 months.
7.1.2. Nasal and paranasal sinus tumors in cats
The incidence of nasal and paranasal tumors in cats is much lower compared to the incidence in dogs. Thus, the data mentioned by MOULTON (1978), in a study performed on 100 000 cats, show a 2.8% incidence rate of malignant tumors. The same author mentions that of 571 nasal tumors in cats, only 5 had a nasal location, and of these, 4 were carcinomas. In another study performed on 31 nasal neoplasms, the following were diagnosed: 22 squamous cell carcinomas, most of them located on the external side of the nostrils; 4 adenocarcinomas, of which one was mucinous carcinoma; 2 carcinomas of undetermined type and 3 sarcomas. Incidence is estimated at 90% of all nasal tumors in cats, being of malignant nature [67].
Adult or old cats have a higher incidence of nasal neoplasms [30]. Clinical signs consist of nervous and respiratory manifestations, sometimes with epistaxis and facial bone deformity [95].
The treatment of nasal neoplasms includes surgery, followed by additional therapy, especially that total excision is difficult to perform. The results obtained by THEON et al. (1994) in 16 cats with nasal and paranasal neoplasms recommend the use of radiotherapy. Thus, 14 cats were treated by radiation alone, and 2 cats were treated by radiation following incomplete surgery. Survival, after treatment, was between 1 and 36 months. The survival rate at 1 year was 44.3%, and at 2 years 16.6%. The authors emphasize the good tolerance to radiation in cats, especially compared to dogs. PEASTON et al. (1993) treated 19 squamous cell carcinomas using aluminium phthalocyanine tetrasulfonate as a photosensitizer; the result obtained in 10 tumors after one treatment and in more than 2 tumors was a complete response after 1 or 2 additional treatments, and in 5 tumors, a partial response. Therapy was well tolerated by all cats.
Olfactory neuroblastoma in cats is a rare neoplasm. Its histogenetic origin is controversial, hence the various designations of these tumors, such as: olfactory esthesioneuroblastoma; esthesioneurocytoma; esthesioneuroepithelioma; olfactory neuroepithelioma; intranasal neuroblastoma [13]. A malignant tumor composed primarily of neuroblasts that originate from the olfactory membrane.
Spontaneous cases of olfactory neuroblastoma have been reported in dogs, horses and young cows [5, 53]. It has been experimentally induced in rats, hamsters and Cynomolgus monkeys. The growth of these nasal tumors may be complicated by the invasion of paranasal sinuses, orbits and nasal bones, accidentally and intracranially. Frequently, metastases are found in the regional lymph nodes, lungs and bones.
Histologically, the structure of the neoplasm is difficult to differentiate from other nasal tumors with small, round cells, such as anaplastic carcinoma, poorly differentiated carcinoma, malignant lymphoma, melanoma and poorly differentiated rhabdomyosarcoma. Histologically, olfactory neuroblastoma may have a stratified cell arrangement or a lobular aspect, under the form of nests or cords of cells separated by fibrovascular stroma. Cells are frequently arranged in palisades around the vessels. Cells have a uniform appearance; they are poorly differentiated, with round or oval nuclei and scarce cytoplasm. The arrangement in rosettes or pseudorosettes is extremely sporadical, but there are also cases in which these aspects may be present in a high number. Occasionally, cells are apparently bipolar and have a positive reaction to silver impregnation [19]. The presence of rosettes is a useful, but not absolute, diagnostic aid for neuroblastomas, and is mostly applicable to the cat. Ultrastructural demonstration of neural processes containing microtubules is the most specific criterion. Immunohistochemical identification of neuron-specific enolase, S-100 protein, and possibly other neural and glial markers adds to the weight of the evidence [123].
7.1.3. Nasal and paranasal sinus tumors in horses
Nasal and paranasal tumors in horses have a medium frequency, incidence increasing with age. The tumors diagnosed in horses are: carcinomas of the covering and glandular epithelium; squamous cell carcinomas; myxomas; chondromas and osteomas; melanomas; sarcomas and osteosarcomas [31, 43, 67].
Regarding the histogenesis of nasal tumors in horses, NOACK (1956) [67] suggests three possibilities: 1.chronic inflammations of the nasal mucosa, with metaplasia; 2.tumors of the oral mucosa that invade the sinuses. Concerning this hypothesis, the author himself makes some remarks, such as the fact that the primary tumors of the oral epithelium are intensely keratinized, unlike those of the horse maxillary sinus. Sinus tumors may be present, although there are no lesions in the oral cavity and, finally, gingival tumors also occur in other species, e.g. in dogs, but only exceptionally do they invade maxillary sinuses; 3.maxillary sinus tumors in horses may originate from tooth germ residues, but it is known that adamantinoma occurs in young horses, while maxillary sinus tumors appear in old subjects, and adamantinoma usually involves the mandible, not the maxillary.
Nasal mucosal polyps have a high incidence; this tumor is reported to appear on a chronic inflammation background. No age, breed or sex preference has been found.
Histologically, they have a dense structure, with abundant fibrous connective tissue, without a spiral arrangement, sometimes with bone tissue islands, and on the surface, cuboid or prismatic epithelium, the structure being frequently infiltrated with inflammatory cells.
The therapeutic approach consists of surgical removal, either through the nasal cavity or by trepanation.
Nasal and paranasal sinus fibrosarcoma is a neoplasm found in adult and old horses [60], while in young subjects, congenital nasal tumors occur in particular, but they are extremely rare [1, 58]. Congenital nasal tumors have been diagnosed under the form of spindle cell fibrosarcomas [45]. The cited authors diagnosed a congenital ethmoid carcinoma and a paranasal sinus osteoma in a newborn foal. In 2 young horses, they reported a fibrosarcoma of the nasal and maxillary sinuses, and in other subjects, a spindle cell sarcoma. Electron microscopy showed irregular fusiform cells, with irregularly shaped elongated nuclei that contained prominent, frequently multiple nucleoli. The endoplasmic reticulum was prominent, frequently vesiculated and dilated; the presence of rough endoplasmic reticulum was mentioned, as well as of polyribosomes, the Golgi complex and occasionally, mitochondria. Numerous mitotic forms were present.
In general, the tumor is not frequently diagnosed and it seems to be a variant of squamous cell carcinoma. Neoplastic cells are arranged in layers, in nests or ramified bundles, at the tumor periphery they are arranged in palisades, and in the center they form spirals.
The fibroblastic tumors of the skin and the anterior cavities in the horse are suspected of viral etiology [9].
Nasal cavity and paranasal sinus adenocarcinomas are rare, especially congenital ones.
In old subjects, adenocarcinomas are reported in the frontal sinus, extending to the cribriform plate, the cranial cavity and the orbit. Purulent discharge, hemorrhage and exophthalmos are clinically found [43, 84]. A congenital ethmoid carcinoma in a foal was described by ACLAND et al. (1984).
Squamous cell carcinoma has been diagnosed in both the nasal cavities and the paranasal sinuses, with a relatively high frequency, compared to other neoplasms [57, 66]. Of a total of 141 horses with squamous cell carcinomas, SCHUH (1986) showed the following locations: tongue mucosa, 3 cases; gums, 1 case; oral mucosa, 1 case; soft palate, 1 case; pharynx, 1 case; pharynx and epiglottis, 1 case; maxillary sinus, 1 case; and nasal septum, 1 case. The macroscopic characteristics proved, in some cases, the presence of keratin pearls, and at other times, poor keratinization, with the presence of intercellular connections.
Histologically, neoplastic cells are grouped in islands or ramified cords, with necrotic foci and/or keratotic pearls. Intercellular bridges and the presence of mitoses can be seen. Due to the occasional presence of mucus drops, this tumor has been compared to human mucoepidermoid carcinoma [101].
Progressive ethmoid hematoma is only mentioned in horses. The tumor usually has a unilateral, occasionally bilateral, development. Age may vary between 3 and 18 years, without any sex influence [49]. Macroscopically, prominent formations appear, 1–2 cm in diameter, surrounded by smaller, red or brownish tumors, bordered by a capsule. Neoplasms frequently ulcerate and bleed. The more frequent location is in the posterior third, sometimes with pharyngeal extension.
Histologically, old and recent hemorrhages appear, with fibrous stroma, with numerous microphages that have phagocytosed hemosiderin, with the presence of giant cells grouped in nests. Necrotic foci appear in the bone tissue.
Progressive ethmoid hematomas have raised controversies, whether they are true neoplasms or not. The lesion has been considered as the result of a chronic infection or repeated hemorrhage, while the pleomorphic aspect suggests its tumor al nature.
Surgical treatment, by total removal, is followed in more than 30% of cases by recurrences or/and the appearance of the tumor on the other side, which would be an additional argument for its neoplastic nature [49].
7.1.4. Nasal and paranasal sinus tumors in cattle
The incidence of nasal and paranasal tumors in cattle is low, only reaching 0.1% of all respiratory tract neoplasms [88, 93]. By exfoliative cytology, in 18 cattle suspected of ethmoid carcinoma that subsequently underwent necropsy examination, MURRALIMANOHAR and SUNDARARAY (1991) diagnosed 16 neoplasms: 7 adenocarcinomas, 6 squamous cell carcinomas, and 3 undifferentiated carcinomas.
The osteoma of the nasal cavity and/or paranasal sinuses has been described in cattle as a sporadic tumor, whose etiology can be discussed in terms of a metaplasia of chronically inflamed connective tissues. The neoplastic nature of bone and cartilaginous tissues of the nasal cavities in cattle is placed under question by COTCHIN (1967) [105].
RUMBAUCH et al. (1978) reported a calcified tumor in the nasal cavities and paranasal sinuses of a 4-year-old Holstein bull. Histologically, the tumor tissue was formed by fusiform cells, similar to fibroblasts, which made up a fibrous bone matrix. The microscopic image showed a benign process or at most, low malignancy. The diagnosis was of atypical osteoma.
Sporadic nasal and paranasal sinus tumors have been diagnosed in cattle as: adenocarcinomas; squamous cell carcinomas and undifferentiated carcinomas.
In 1984, BABA and ROTARU described ethmoid tumors in 2 Holstein cows aged 7 and 8 years, respectively, which presented dyspnea and suffocation signs. On necropsy, the nasal cavities and the paranasal sinuses, bilaterally and unilaterally, respectively, presented large tumor formations that filled the cavities and compressed the nasal conchae and septum. Histologically, adenocarcinoma was established in one subject, and in the other, myxosarcoma with islands of bone metaplasia.
In small ruminants, sheep and goats, sporadic neoplasms, similar to those found in cattle, have been reported. STEEN et al. (1985) described in a 10.5-year-old female goat a tumor developed at the base of the nasal cavities, penetrating in the retrobulbar area and the cranial cavity, and compressing the left cerebral hemisphere. Macroscopically, soft, gray tumor masses appeared, with necrotic and hemorrhagic foci. Microscopically, the authors described two types of tumors with invasive growth. A structure was formed by fusiform cells, with palisade arrangement and rosette disposition and "fishbone" images, the diagnosis of malignant schwannoma being established. Within the same formations, epithelial proliferations were present, with poorly differentiated cells and vascular connective stroma, with papilliferous aspect, the carcinoma diagnosis being made.
7.2. ENDEMIC ETHMOID TUMORS
Endemic ethmoid tumors were reported in Sweden, by STENSTROM in 1909, then MAGNUSON, in 1916, as epitheliomas and sarcomas in cattle, and in 1913, FORSEL presented some cases of ethmoid lymphosarcomas and round cell sarcomas in horses [109].
Nasal mucosal adenocarcinomas, with endemic character, have been described in sows [20], sheep and cattle [69, 73, 79, 121]. In the following years, endemic ethmoid tumors have been reported on all continents.
Endemic ethmoid tumors in horses have been reported in Sweden (BERGMAN, 1914) and Norway (HORNE and STENERSEN, 1916), located in the posterior third of the nasal cavities and in the sinuses. COTCHIN (1967), who cites the mentioned authors, emphasizes the fact that the attempts to experimentally transmit these tumors have failed.
Histologically, endemic ethmoid tumors are squamous cell carcinomas, adenocarcinomas and myxomas.
Clinically, mucous, mucopurulent or even sanguinolent secretion from one or both nostrils is found. Then, the deformation of the area, atrophy and bone fibrosis occur.
As it has been shown, endemic ethmoid tumors in cattle were reported at the beginning of the century in Sweden, Norway and Denmark, extending subsequently to the great majority of the world countries.
Macroscopically, ethmoid mucosal tumors have been found to invade the paranasal sinuses, the orbits and cranial blood vessels, with metastases in the nerve substance. Evolution is slow, affecting a number of cattle from the same stable.
The cattle breeds that are frequently mentioned in the literature with these endemic neoplasms are the Jersey Dutch milk breed and its cross-breeds [109], tumors being less frequently reported in other breeds, such as the Holstein breed [8]. The authors mention in all cases ages over 7 years.
Histologically, endemic ethmoid tumors have been diagnosed as malignant forms, carcinomas and adenocarcinomas having a higher incidence (adenocarcinomas; epidermoid carcinomas; transitional carcinomas; squamous cell carcinomas); osteomas and myxosarcomas have been extremely sporadically mentioned.
Electron microscopically, POSPISCHIL et al. (1979) described a transitional or undifferentiated carcinoma, which was formed by dark cells, similar to olfactory mucosal cells, being identical to the dark cells of the Bowman's gland. Ultrastructurally, the lymphoid cells of the undifferentiated bovine ethmoid carcinoma were similar to lymphoid nasopharyngeal cells that are associated with the Epstein-Barr virus. These morphological similarities, as well as epidemiological data, suggest a viral etiology of bovine endemic ethmoid carcinoma.
Endemic ethmoid tumors in sheep and goats have been studied with particular interest, due to the supposed or possible viral etiology and/or irritations due to pollution, as well as to the permanent character of neoplasm reporting in certain countries.
Ethmoid tumors in sheep were reported for the first time in 1939, by NIEBERLE, who suggested that the inhalation of gases and arsenic powder would predispose to these neoplasms [20].
COHRS (1953) successfully transmitted ethmoid tumors by acellular filtrate instillations, in the nasal cavities of healthy sheep. DRIEUX et al. (1952) and VOHRADSKY (1974) did not succeed in reproducing the neoplasms, by using similar methods [69].
In the etiology of these tumors, DUNCAN et al. (1967) admit an infectious cause, associated with a hereditary predisposition. According to COTCHIN (1967), endemic ethmoid tumors in sheep could be considered as mucosal hyperplasias induced by viral infections.
Endemic ethmoid tumors may occur at a very young age, between 4–14 months, up to advanced ages, over 7–8 years. No sex or breed preference has been noted [56, 73].
Clinical signs in affected sheep are of respiratory type: snoring, mucopurulent discharge and dyspnea; the general state is characterized by anorexia, sometimes the asymmetry of the nasal area and exophthalmos [24, 118, 121].
Macroscopic examination shows the uni- or bilateral presence of tumor masses, under the form of nodules or extensive proliferations up to the pharyngeal, laryngeal or sinus areas. The tumor surface is irregular or cauliflower-like, with necrotic hemorrhagic foci, of pink color, soft, elastic consistency, and sometimes hard foci appear in the fleshy section. The nasal conchae and septum gradually undergo compression atrophy, deformations and necroses. Metastases in the regional lymph nodes have been observed [17, 41].
In most cases, the histological structures of endemic ethmoid tumors in sheep have corresponded to the diagnosis of adenoma and adenocarcinoma.
Adenoma has a glandular structure, with papilliferous proliferations, starting from the submucous layer. Glandular structures are similar to tubuloacinar glands, Bowman's glands, under the form of well differentiated acini, with cuboid or prismatic cells, with oval vesicular nuclei. In general, the tumor epithelium is non-ciliated, and pseudostratified ciliated epithelial areas may be noted. Sometimes, mucoid metaplasia areas occur. The microscopic structure of adenomas has the characteristics of a benign tumor [66, 79].
Adenocarcinomas have a pleomorphic structure, cellular heterogeneity, they may have a cystic or cystadenomatous appearance, with compact cell islands, separated by a fine connective structure. Neoplastic cells have infiltrative growth, with the presence of frequent mitoses. Horny metaplasias may be sometimes noted, even keratotic pearl structures [121,122].
The electron microscopic structure of adenocarcinoma, except for changes due to malignancy, is similar to that of adenoma. The tumor mass is infiltrated with plasmocytes and lymphocytes, fibroblast proliferations. In the nuclei of neoplastic cells, nucleoli are prominent, chromatin blocks being found adjacent to the nuclear membrane. On the cell surface, microvilli are in a low number. Secretion drops are almost constantly present in the cell cytoplasm, which have a non-uniform distribution and variable sizes, being delimited by a single membrane. In almost all tumor cells, the smooth endoplasmic reticulum presents a high number of small vesicles, some of which are distributed in the cytoplasm. The Golgi apparatus is well developed, as well as the rough endoplasmic reticulum, situated at the cellular base.
The first authors who reported the disease already supposed the role of an infectious agent in the etiology of ethmoid tumors. The investigations performed by YONEMICHI et al. (1978) evidenced viral particles similar to the visna-maedi virus. The virus was situated on the apical surface of the epithelium and in the intracytoplasmic vacuoles of the leukocytes from the tumor mass. Viral particles, similar to the visna-maedi virus, were identified in 3 of 4 tumor tissue cultures.
Endemic ethmoid tumors in goats have been less cited by the literature, being reported by LOMBARD et al. (1966) and FONTAINE et al. (1983) in France, while in Spain the first cases were described by DE LAS HERAS et al. (1985), and in India by RAJAN et al., in 1980 [26]. In 1993, VITELLOZZI et al. reported the disease in Italy. Incidence was 2–3%.
The study performed by DE LAS HERAS et al. (1991) on a group of 34 female and 4 male goats with endemic ethmoid neoplasms, aged between 7 months and 8 years, showed unilateral tumors in 12 subjects, and bilateral tumors in 26 subjects.
Clinical manifestations were: seromucous discharge, stertorous respiration, cough and dyspnea, sometimes exophthalmos. Animals gradually lost weight, became cachectic, and finally died from exhaustion.
Necropsy shows the compression and atrophy of tissues adjacent to tumors, with the total obliteration of nasal cavities. Neoplasms are soft, of gray or red-whitish color, with granular surface covered with mucus. The invasion of paranasal sinuses is frequent.
The microscopic structure is characterized by hyperplastic changes, acinar or papilliferous proliferations, glandular cysts and compact proliferations of neoplastic cells. The neoplasms studied by GAZQUEZ et al. (1992) were adenocarcinomas, in which cystic proliferations could be detected, as well as compact proliferations, with abundant lax connective tissue and monocyte infiltration. Neoplastic cells have cuboid or prismatic shapes, round or oval nuclei, with basal arrangement. The mitotic index is low. Reduced lymphoplasmacytic and macrophage infiltration may be found in the connective stroma. Metastases are extremely rare. Papilliferous proliferations are covered with hyperplastic aciliated prismatic cells. Compared to what is known of endemic ethmoid tumors in other species, neoplasms are benign or with low malignancy. Tumor cells originate in the serous glands, not in the Bowman's glands.
On the electron microscopic examination, particles similar to retroviruses appear at the apical pole of cells in extracellular spaces, between the microvilli. Viral particles are similar to the visna-maedi virus, with an eccentric electron dense nucleus, 90 nm in diameter, surrounded by numerous spicules [39]. Electron microscopically, the epithelial nature of endemic ethmoid tumors is confirmed. Cells are cuboid or columnar, forming acini and tubules. Cells have a compact arrangement, as well as numerous cytoplasmic interdigitations and well developed desmosomes and tight junctions between adjacent cells. The authors consider that the virus could be the primary cause of the tumor, which is supported by epidemiological aspects, the presence of viral particles and experimental transmission.
The attempts to experimentally transmit endemic ethmoid tumors have been successful, both in the case of intranasal inoculation of nasal fluid from subjects with neoplasms, and the concentrated virus from the fluid. The neoplasm appeared in 3 of 10 2-day old kids, 12–14 months after inoculation.
Endemic ethmoid tumors have also been reported in other domestic or experimental species.
Nasopharyngeal tumors are rare in domestic animals, except for tonsillar squamous cell carcinoma in dogs. In other species, this carcinoma is rarer: COTCHIN (1967) describes a squamous cell carcinoma of the pharyngeal mucosa and tongue base; a pharyngeal melanoma in an 11 -year-old dog and a pharyngeal squamous cell carcinoma in an 11 -year-old cat. Nasopharyngeal locations of melanoma in dogs are not rare.
Guttural pouch tumors are extremely rare: MOULTON (1978) mentions the presence of squamous cell carcinoma in an 11 -year-old stallion and a 20-year-old castrated horse.
Laryngeal tumors have been reported in 6-month-old cats, and in 14-year-old subjects squamous cell carcinomas are predominant. Some subjects present metastases in the cervical lymph nodes, lungs, spleen, adrenal glands and liver. One adenocarcinoma located in the laryngeal wall has been reported.
Tracheal mucosal tumors are reported as round cell carcinomas in horses, adenocarcinomas in the lower trachea of dogs. A primary intratracheal squamous cell carcinoma has been diagnosed in a cat [51]. Secondary tracheal neoplasms are frequently malignant lymphomas, in cats [66].
Laryngopharyngeal rhabdomyoma in dogs. Primary malignant neoplasms of the larynx and pharynx are considered as rare, in dogs, and benign forms are extremely rare. LEIGGETT et al. (1985) emphasize the rarity of laryngopharyngeal rhabdomyomas in dogs. In humans, extracardiac rhabdomyomas are rare and are especially found in the head and neck areas. Mature rhabdomyoma is easy to confuse with oncocytoma, since both tumors are formed by large cells, with granular eosinophilic cytoplasm. The same authors mention the necessity and possibility of differentiation. The differentiation of granular tumor cells in the head and neck areas in humans is based on the identification of granule producing material. The granular aspect of the cell may be due to either numerous lysosomes, like in the case of granular cell myeloblastoma, or the high number of mitochondria, like in the case of oncocytomas. More rarely, the granular aspect may be determined by microcrystals, similarly to alveolar sarcoma; sometimes, cytoplasmic granulations may be due to the abundant secretory bodies, as it happens in the case of acinar cell carcinomas and paragangliomas.
The cases of laryngopharyngeal rhabdomyoma in dogs, reported by LIGGETT et al. (1985) and MEUTEN et al. (1985) demonstrate, on the one hand, the existence of this neoplasm in dogs and, on the other hand, the need for a differential diagnosis with oncocytoma. The authors define the following histopathological structure of rhabdomyoma: large polygonal eosinophilic cells, intermingled with small dark cells. The large tumor cells have granular cytoplasm with clear peripheral vacuoles that are intensely eosinophilic. Few elongated cells are dispersed along the tumor, with multiple nuclei and transverse cytoplasmic striations. The tumor cells contain numerous mitochondria and myofibrillar bundles with electron dense Z lines, typical of striated muscle cells. The presence of myoglobin and desmin can be detected by immunocytochemistry. The presence of nuclear pseudoinclusions is mentioned in large tumor cells. Pseudoinclusions are delimited by a double membrane and are invaginations of cytoplasmic organelles.
Oncocytoma, a tumor formed by lobulated masses of large pleomorphic cells with abundant, highly eosinophlic, granular or foamy cytoplasm that is packed with mitochondria and intermitochondrial accumulations of rough endoplasmic reticulum and glycogen granules. Some cells contain large, clear cytoplasmic vacuoles; nuclei are round to oval and have one distinct nucleolus or several small ones; cytoplasmic invaginations into nuclei are common. Ultrastructural and immunohistochemical features diagnostic for rhabdomyomas or granular cell tumors should also be lacking [123].
7.3. TUMORS OF THE LUNG
Lung tumors in animals are not as frequent as in humans; in spite of this, a similarity has been found between the biological behavior of different tumor types in animals and in man.
The high incidence of lung neoplasms in humans, with an increasing frequency from one year to another, especially of epidermoid carcinoma and small cell anaplastic carcinoma cases, has raised particular interest in the study of lung cancer in animals, in terms of incidence, behavior and morphology. The increased interest in lung cancer of companion animals has been stimulated by the fact that these live in the same environment as man, breathe the same polluted air, feed almost on the same food and are even exposed to common social stress. STUNZI et al. (1974) remark an increase in the mean life time of dogs, from 3.8 years in 1953 to 7.8 years in 1970, and over the past decades, the mean life duration in this species has certainly extended, which favors the appearance of lung neoplasms.
Comparative oncology studies will lead to the clarification of epidemiological aspects, risk factors, but also to the interpretation of observations made following experiments perfomed in animals.
In production animals, the frequency of lung tumors is significantly lower, which is explained by their much shorter life.
Statistics demonstrate an increase in the incidence of lung tumors, in time. Thus, until 1970, STUNZI et al. reported a 0.9% incidence of primary lung neoplasms in a total number of 544 tumors from 405 dogs. Incidence has gradually increased until recent years, in some areas the number of cases reaching over 3%.
In dogs, lung carcinomas appear almost constantly around the age of 10 years, extremely rarely under 6 years, and anaplastic carcinoma occurs around the age of 8.5 years. The mean age in cats with lung carcinomas is about 12 years, and in cattle 5 years or more, lung neoplasms being diagnosed in this species even at the age of 1 year.
Incidence does not seem to be influenced by breed, in dogs, although there are observations supporting a higher sensitivity of the Boxer and German Shepherd breeds. Sex does not influence the development of lung neoplasms in any species.
The general frequency of lung tumors in dogs examined post mortem is 1.24%, distributed as it follows: squamous cell carcinomas, 7%; anaplastic carcinomas, 3%; adenomas, 88%; and other carcinomas, 2%. In cats, the incidence of lung tumors diagnosed by necropsy is 0.38%, and the different tumor types are similar to those of dogs. In cattle, the majority of neoplasms are adenocarcinomas, and incidence is 2.8% of all neoplasms in this species. In sheep and goats, lung tumors are extremely rare. Mucoepidermoid carcinomas with abundant keratinization have been identified. The same situation is found in swine, in which early killing may occur.
Histological researches on lung tumors have shown cellular heterogeneity in various areas of the same neoplasm, which makes difficult diagnosing and the assessment of the malignancy grade.
The classification of lung neoplasms is slightly more difficult due to the possibilities of metaplasia of the epithelium and mesenchymal tissues; in addition, intermediate or mixed forms occur, and the histogenetic origin cannot be established with certainty.
According to STUNZI, HEAD and NIELSEN (1974), histological classification also includes the nomenclature of lung neoplasms in animals:
- Epidermoid or squamous cell carcinoma.
- Anaplastic carcinoma:
- – small cell anaplastic carcinoma: lymphocyte-like type; spindle cell type; polygonal type;
- – large cell anaplastic carcinoma: giant cell type.
- Adenocarcinoma:
- – papillary adenoma;
- – bronchoalveolar adenocarcinoma, including ovine pulmonary adenomatosis.
- Epidermoid (squamous cell) carcinoma combined with adenocarcinoma.
- Carcinoid tumors.
- Bronchial gland tumors.
- Mixed tumors.
- Benign tumors.
- Sarcomas.
- Unclassified tumors.
A similar, adapted and more pragmatic classification, is proposed by DUNGWORTH(1993):
- – primary epithelial lung tumors;
- – bronchial papilloma;
- – bronchial gland adenoma;
- – bronchogenic carcinoma: squamous cell (epidermoid) carcinoma; adenocarcinoma; adenosquamous carcinoma; undifferentiated (aplastic) small cell or large cell carcinoma;
- – bronchoalveolar tumors: adenoma; carcinoma;
- – carcinoids.
Some general considerations on lung neoplasms in animals are required, regarding epidemiological, clinical and pathomorphological aspects.
The epidemiology of these tumors depending on the species shows, according to MOULTON (1978), that of a total of 150 lung tumors, 64 were found in dogs, 33 in cattle, 12 in sheep, 8 in cats and 1 in donkeys.
The most frequently diagnosed lung tumors in domestic animals are bronchogenic or bronchoalveolar adenocarcinomas. In dogs and cats, lung adenocarcinomas are the neoplasms with the highest incidence, while anaplastic carcinomas and squamous cell carcinomas are rare, and the latter have been diagnosed in cats. These aspects are completely different from humans, in whom anaplastic carcinoma and squamous cell carcinoma with pulmonary location are the most frequent.
Clinical signs, regardless of the histological type of the neoplasm that manifests in dogs as well as in other species, are characterized by cough, dyspnea, gradual weight loss resulting in cachexia, heart failure and exitus by pulmonary edema. In dogs, lung neoplasms are associated with hypertrophic osteoarthropathy. The lesion has been found both in the case of primary lung neoplasms and non-pulmonary tumors metastasized in the lung, or even after tumor excision and the appearance of recurrences and pulmonary metastasis.
The anatomopathological picture is characterized by frequent location at the periphery of diaphragmatic lobes, subpleurally, the right lung being involved more than the left lung. Bronchopulmonary neoplasms seem to have a unicentric onset, sometimes with early and rapid dissemination, so that apparently they are multicentric. The primary lung tumor may start with a pneumonia aspect, gray hepatization, with small nodular foci.
Histopathologically, the tumor structure varies depending on the neoplastic type, to which the peculiarities of each tumor type are added. The definition of the neoplastic type and malignancy grade will take into consideration some peculiarities of lung tumors. Different histological types may occur in the structure of a lung tumor, which requires the investigation of different portions of that tumor. The differentiation grade will be estimated, as it is known that this is indicative of tumor malignancy. Various differentiation degrees may coexist within the same tumor. The name of the neoplasm will reflect the most highly differentiated type, while the characteristics of the less differentiated part is also mentioned [66]. Histological structure suggests that most lung tumors in dogs originate from the bronchial glands [72].
7.3.1. Papillary adenoma
A benign tumor consisting of mucus-secreting glandular structures with a predominant papillary pattern. The literature considers this tumor to be extremely rare in domestic animals. Adenoma has been diagnosed in cattle and dogs. The majority of the affected subjects are adult, without any breed or sex predisposition.
The tumor may be located in one or both lungs, it can have a unicentric or multicentric onset. Its origin is bronchial, under the form of papillary excrescences on the mucosal surface. The tumor may develop both at the surface of the epithelium and on the bronchial mucosal glands.
Histologically, bronchial papillary adenoma has fine, central, sligthly vascularized connective tissue, covered with one or more layers of columnar or cuboid epithelial cells. These cells secrete mucin. Bronchial papillary adenoma does not manifest invasive characteristics and malignant transformation has not been evidenced.
Pulmonary papillary adenomas have a globoid aspect, a yellow to gray color, a diameter that varies from several millimeters to 4–5 cm, and are encapsulated. Histologically, the presence of cuboid to columnar cells is noted, which are pale and foamy. Cells have an orderly arrangement, papilliferous growth and secrete abundant mucin; they may form cysts lined by flat epithelium (Fig. 7.14).

Fig. 7.14
Lung adenoma.
7.3.2. Bronchioloalveolar adenoma
Usually, a solitary nodule located peripherally in the pulmonary parenchyma and consisting of cuboidal or low-columnar cells arranged in a regular alveolar or perhaps papillary pattern. The use of the designation bronchioloalveolar implies a presumed origin from bronchiolar Clara cells and/or alveolar type II epithelial cells. The neoplastic cells are cuboidal to columnar, and sometimes clear cytoplasmic vacuoles are present; the epithelial cells are uniform and lack significant atypia, and mitotic figures are rare or absent. Separation of benign and malignant bronchioloalveolar tumors is not absolute. Differentiation between a bronchioloalveolar adenoma and papillary adenoma can also be difficult. When an adenoma is present within the pulmonary parenchyma, a papillary adenoma is distinguished from a bronchioloalveolar adenoma by the presence of a nodular growth that replaces normal pulmonary parenchyma and has a coarsely irregular papillary pattern formed by cuboidal to columnar, often mucus-secreting cells. Identification of a bronchioloalveolar adenoma can also be confirmed by positive immunostaining for Clara cell antigen (CC-10) or surfactant apoprotein [123].
7.3.3. Epidermoid or squamous cell carcinoma
Squamous cell carcinoma originates almost always from the bronchial epithelium, being located more frequently in the pulmonary hilum.
Histologically, it is characterized by compact masses of large, stratified cells, with different degrees of flattening and keratinization. Intercellular desmosomes and/or keratinizations can be identified, which facilitates diagnosis. Necrotic foci sometimes appear in the neoplastic cell islands, and around the neoplastic islands, fibrous stroma may develop. Metastases occur in bronchial lymph nodes, but they can also develop at distance [100,104] (Fig. 7.10, 7.12).

Fig. 7.10
Lung epidermoid - squamous carcinoma.
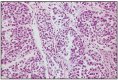
Fig. 7.12
Lung anaplastic carcinoma, lymphocyte - like type.
7.3.4. Adenocarcinoma
Adenocarcinoma is characterized by a structure similar to a gland, with the specific peculiarities of a neoplasm. The histological origin of this neoplasm can be bronchial epithelium, in the bronchial or bronchiolar glands [66] (Fig. 7.21).
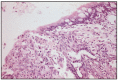
Fig. 7.21
Bronchial gland adenocarcinoma.
The growth of adenocarcinoma is infiltrative, which makes it appear as pneumonia. These pulmonary neoplasms are frequent, they metastasize easily in the lymph nodes, the pleura or parenchymatous organs.
Microscopically, adenocarcinoma may develop as papillary, acinar, solid or mixed adenocarcinoma and bronchioloalveolar adenocarcinoma.
Papillary and acinar adenocarcinomas are the most frequent type of primary lung tumor found in domestic animals; usually highly destructive and invasive; formation of satellite nodules by intra-airspace spread is common, as is more widespread dissemination through lymphatics; metastases to other organs are not common. Frequently, it is not possible to determine the site of origin.
Histologically, malignant cells are usually columnar and have large, ovoid basal nuclei; occasionally, vacuoles with mucous content and abundant secretion appear in the cytoplasm; frequently, large tumors have regional variations in the size, height, and nuclear and cytoplasmic appearance of the cells. Necroses, cholesterol and/or calcareous salt deposits and inflammatory reaction are present; cartilaginous or even bone metaplasias are sometimes found [100,123] (Fig. 7.15).

Fig. 7.15
Lung papillary adenocarcinoma.
Bronchioloalveolar carcinoma occurs mostly in dogs, occasionally in horses. The main distinguishing structural feature of the bronchioloalveolar carcinoma is its regular alveolar pattern and general preservation of pulmonary architecture, although the alveolar structures frequently contain sloughed cells. There might be a delicate papillary or tubulopapillary pattern superimposed on the alveolar appearance. The cells are cuboidal to low columnar, and lie on a fibrous septum.
Clara cells are positive for Clara-cell antigen (CC-10), and alveolar type II cells are more strongly positive for surfactant apoprotein A. Histologically, architecture maintains tumor cell lines from both the alveoli and the terminal bronchioles. Moderate cellular pleomorphism is found, with rare binucleated or multinucleated cells.
Differentiation of bronchioloalveolar carcinomas from adenomas can be difficult in the absence of stromal and/or lymphatic invasion; also, differentiation between bronchioloalveolar carcinomas and papillary adenocarcinomas can be a problem [6,123] (Fig. 7.16).
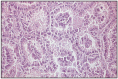
Fig. 7.16
Bronchioloalveolar adenocarcinoma.
7.3.5. Ovine pulmonary carcinomatosis
Ovine pulmonary carcinomatosis or carcinoma, a neoplasm known under the older designation of ovine pulmonary adenomatosis, is a bronchoalveolar tumor, its viral etiology being demonstrated by numerous investigations and experiments. The disease is considered as a transmissible pulmonary tumor [117], which is also demonstrated by the possibility of reproduction by cells from pulmonary exudate, as well as from the neoplastic focus.
Ovine pulmonary carcinoma is produced by a retrovirus, type B and/or D, and experiments have proved that the lymphoid interstitial pneumonia lentivirus significantly shortens the incubation period. In fact, this lentivirus is almost constantly isolated, concomitantly with the retrovirus, from ovine pulmonary carcinoma. The lentivirus replicates in alveolar macrophages, and macrophages regulate the remodeling and repair of the connective tissue from pulmonary lesions, by the production of soluble mediators. Macrophages can also promote, by similar mechanisms, the proliferation of type II alveolar cells, increasing in this way the potential of target-cells for the ovine pulmonary carcinoma retrovirus [28, 88, 92]. Interdependence between the pathogenicity of the two viruses may be supposed.
Ovine pulmonary carcinoma, produced by a retrovirus in the presence of the ovine lentivirus of lymphoid interstitial pneumonia, is of special interest for human pathology, since the association of HIV retroviruses and lymphoid interstitial pneumonia is known [28]. The neoplasm has been reproduced in goats, by intratracheal inoculation with partially purified virus [112], goats developing both macroscopic and microscopic lesions characteristic of ovine pulmonary carcinoma.
Macroscopically, small, well-delimited, white-gray, dense nodules appear subpleurally, which gradually become confluent, reaching 1–3 cm in diameter, and form large nodules or include large portions of the lung, having a diffuse growth. The extended lesion becomes dense, cartilaginous, and may coexist with verminous pneumonia or chronic lymphoid interstitial pneumonia (Maedi). Differential diagnosis is performed microscopically. Metastases [2, 7, 74] have been found in the lymph nodes of the respiratory tract, more rarely in muscles or parenchymatous organs.
Clinically, signs appear late, when neoplastic lesions affect extensive areas of the pulmonary mass. Natural disease occurs in 1–4-year-old sheep. Non-specific signs appear, such as tachypnea, dyspnea, especially following exercise, occasionally cough, weight loss and mucous discharge, which is eliminated in high amounts in forced head position. Signs are similar to those of pneumonia, but subjects do not manifest fever.
Histologically, characteristic of pulmonary carcinoma is the proliferation in foci of cuboid or columnar cells in alveolar septa, with epithelialization aspects and under the form of papillary projections in the alveolar lumen, frequently with palisade proliferation images (Fig. 7.16., 7.17–7.20). In advanced forms, epithelial proliferation is accompanied by fibrosis or even a sarcomatous aspect of the mesenchyma. Epithelial proliferations extend to the bronchioles. Columnar or cuboid cells lie against a supporting stromal connective tissue, with the occasional presence of multinucleated cells [87]. The presence of intranuclear inclusions has been reported in pulmonary histiocytes or even in proliferated epithelium [92]. In the lumen of bronchioles and in alveoli, columnar epithelial cells are found, with secretory cytoplasmic granules and glycogen, which suggests their origin in the secretory epithelial (Clara) cells, from the bronchioles [35]. Electron microscopically, type II neoplastic cells present microvilli, lamellar formations and desmosomes [29,117].
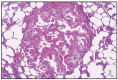
Fig. 7.17
Ovine pulmonary carcinoma.
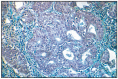
Fig. 7.18
Ovine pulmonary carcinoma.

Fig. 7.19
Ovine pulmonary carcinoma.
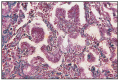
Fig. 7.20
Ovine pulmonary carcinoma.
The lesions of ovine pulmonary carcinoma are very similar to those of human bronchoalveolar carcinoma, which has a 3% incidence of all pulmonary neoplasms, and are not associated with smoking.
7.3.6. Anaplastic carcinoma
Anaplastic carcinoma is characterized by poor differentiation and marked anaplasia, and depending on the shape and size of the cells that compose it, the following types may be distinguished: large cell, small cell, round cell, fusiform cell, and polygonal cell anaplastic carcinoma. This subclassification is used for humans, in whom incidence and variability are high.
Small cell anaplastic carcinoma is characterized by dispersed, isolated cells, with an extremely fine, discrete stroma, delimiting cell groups under the form of lobes. Optical microscopy shows cellular uniformity, but electron microscopically, diversity is wide. RONALD et al. (1987) remark the ultrastructural, cellular diversity of cell components, as well as architectural diversity, which suggest the possibility of differentiation towards epidermoid or glandular aspects. The authors support the unicist theory of bronchopulmonary carcinoma based on small cell carcinoma. Tumors with a predominance of small, dense, fusiform cells are sometimes referred to as oat cell carcinoma. Subsequently, by transitional forms, large cell carcinoma appears that may generate through a double differentiation neuroendocrine carcinoma and glandular carcinoma. The authors underscore the capacity of these carcinomas to elaborate hormone peptides. The invasive character of the neoplasm manifests by the presence of tumor cells in the lymphatic, blood capillaries and in alveoli. Metastasizing in regional lymph nodes is a rule (Fig. 7.12). The few reports of small cell tumors have been in dogs, and cows. Most tumors diagnosed as small cell carcinomas have not been subjected to the detailed ultrastructural and immunohistochemical investigations necessary to establish either their epithelial origin (cytokeratin markers) or their phenotypic characteristics [123].
Large lymphocyte-like cell anaplastic carcinoma is extremely rare. Histologically, the tumor structure is characterized by islands formed by lymphocyte-like cells, with small, round, dense nuclei, and scarce, annular cytoplasm. The stroma is fine, and necroses and inflammatory areas may sometimes occur. Large cell anaplastic carcinoma is frequently diagnosed in humans. This neoplasm may contain pleomorphic, multinucleated giant cells. Tumor cells are poorly differentiated, they frequently form cords, and mitotic activity is less reduced than in small cell anaplastic carcinomas (Fig. 7.13).

Fig. 7.13
Lung squamous cell carcinoma with anaplastic areas.
Large cell carcinoma, a malignant epithelial tumor consisting of large, anaplastic cells with large nuclei, prominent nucleoli, abundant cytoplasm, and usually well-defined cell borders without identifying features of squamous cell carcinomas, adenocarcinomas, or small cell carcinomas. The tumors consist of large, rounded-to-polyhedral cells occupying and effacing alveolar parenchyma. The cells have ample eosinophilic, sometimes foamy cytoplasm and large, round, oval, or distorted nuclei. Accurately identified cases of large carcinoma, of giant cell or any other type, are therefore extremely rare. There is no current justification for separate listing of giant cell or clear cell types [123].
Small spindle cell anaplastic carcinoma can be considered, according to STUNZI et al. (1974) as a form or a stage in the development of epidermoid carcinoma.
Small polygonal cell anaplastic carcinoma is similar to lymphocyte-like cell carcinoma, only cells have a more abundant cytoplasm and an irregular shape.
Anaplastic carcinomas with either small cells or giant cells have been diagnosed in dogs [72]. The authors mention the malignancy grade, with marked pleomorphism, with metastases in the regional lymph nodes and in the central nervous system. Anaplastic carcinomas have been diagnosed in horses, sometimes as single carcinomas [32] or as adenocarcinomas and anaplastic bronchogenic carcinomas [91].
7.3.7. Adenosquamous carcinoma
Adenosquamous carcinoma is characterized by the presence of both structures within the same neoplasm. In general, one structure is dominant, and diagnosis will be attributed to that form, but the presence of the other structure will also be mentioned. This adenosquamous neoplasm or combined tumor is rarely reported by the literature, being mentioned in dogs and cats [100] (Fig. 7.19.). Adenosquamous carcinomas tend to be highly aggressive malignancies.
7.3.8. Neuroendocrine tumor
A tumor believed to be derived from the dispersed neuroendocrine system and consisting of polyhedral large cells with fine granular eosinophilic or clear cytoplasm, and a rounded nucleus. Neuroendocrine tumors have been diagnosed in dogs as large, pale, firm nodules located in the neighborhood of a main bronchus, with metastases in bronchial lymph nodes and more rarely the brain [100].
Histologically, the cells form either cords a few cells wide or packets separated by delicate, well-vascularized stroma. The cytoplasm of the cells is slightly granular, and special stains reveal argentophilic and/or argyrophilic granules. Ultrastructurally, the cells contain small, dense-core "neurosecretory" granules in their cytoplasm. More accurate diagnosis is based on immunohistochemical demonstration of functional markers such as calcitonin gene-related peptide, neuron-specific enolase, chromogranin A, and synaptophysin.
Diagnosis must be based on the sum of morphologic and immunohistochemical features. The term carcinoid should be avoided until the situation in animals is clarified [123].
7.3.9. Granular cell tumor
Granular cell tumor is rarely diagnosed in animals, as well as in humans. In 1926, ABRIKOSSOFF described it for the first time in man.
The strictly pulmonary location seems to be found only in horses [4], species in which granular cell tumor has been most frequently diagnosed [62]. In dogs, lingual [120] and cutaneous [111] locations have been reported.
Macroscopically, multiple well delimited, nodules are found, with a 0.5–8 cm diameter, firm consistency, of a white, slightly yellowish color in section, situated in the proximity of the bronchi. Sometimes, nodules contain mucous cysts. Tumor formations cause the narrowing, even obliteration of the bronchi, and they are more frequently located in the right lung, exceptionally in the left lung [76, 46].
Regarding clinical symptomatology, there are few data reported by the literature. In an 8-year-old mare, INOUE et al. (1987) found rales and severe dyspnea, and radiological examination showed multiple formations, some of them reaching 25 cm in diameter. ALEXANDER et al. (1965) found hypertrophic osteoarthropathy in a stallion with granular cell myoblastoma.
Histologically, the tumor is formed by large, uniform, ovoid or polyhedral cells, arranged in compact areas, apparently dissociated by clear spaces or by fine collagen bands. The cell cytoplasm is abundant, with fine acidophilic and PAS-positive granulations [48].
Electron microscopy shows chromatin in moderate amounts, an irregular nuclear membrane, pleomorphic cytoplasmic granules with variable arrangements. Myelin figures occasionally appear in the cytoplasm.
The origin of these granular cells has raised numerous debates and hypotheses: ABRIKOSSOFF (1926) considers them to be derived from smooth muscle cells. The mesenchymal origin is supported, from fibroblasts, histiocytes or Schwann cells [35]; immunohistochemistry has demonstrated the presence of protein S-100, neuron-specific enolase antibodies, and vimentin, which suggests the neuronal origin [46, 48].
Bronchial gland tumors, originating from the bronchial mucosal glands, have been diagnosed in dogs and cats. The tumor has the character of mucous metaplasia, sometimes with keratinization images. In cats, tumor proliferations originating from the serous glands have been observed. These neoplasms frequently induce metastases in the regional lymph nodes.
Macroscopically, the bronchial mucosa, especially the pulmonary hilum, presents prominent, relatively well-delimited, soft, pink-red nodular formations, with local infiltration.
Microscopically, metaplastic changes are found, with flattened and less or more keratinized epithelium. The tumor base contains connective tissue that infiltrates between cartilage rings.
Pulmonary sarcomas are generally rare. GIESEL (1980) diagnosed pulmonary sarcomas in dogs. Histologically, the author identified: lipo- and angioblastic sarcoma; chondro- and osteoblastic sarcoma; polymorphic cell sarcoma. Metastases were found in the regional lymph nodes, kidneys, pancreas, diaphragm, breast and encephalon (Fig. 7.22–7.24).

Fig. 7.22
Lung osteosarcoma.
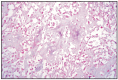
Fig. 7.23
Lung productive osteoblastic osteosarcoma.
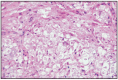
Fig. 7.24
Lung chondrosarcoma.
Carcinosarcomas have been diagnosed in adult Holstein cattle [47]. Macroscopically, they appear as circumscribed nodular masses, with pleural invasion and metastases in the lymph nodes of the respiratory tract. The cited authors microscopically identified two neoplastic cell populations, mesenchymal and epithelial cells. Epithelial cells were arranged in nests, tubules and formations similar to normal pulmonary bronchioles. Mesenchymal cells were fusiform, with oval nuclei and eosinophilic fibrillar cytoplasm, along with large round cells with multiple nuclei, eosinophilic granular or blast cell-like cytoplasm, with large hyperchromatic nuclei and amphophilic cytoplasm.
Following the studies performed, the authors subclassified pulmonary blastomas, defining sarcomatoid carcinoma or carcinosarcoma, supporting by the absence of epithelial markers and cytokeratin in all mesenchymal cells.
Pulmonary carcinosarcomas are also known in humans, in adult men.
Secondary tumors. The lung is the site of numerous neoplasms that metastasize, especially mammary carcinomas in dogs and cats, uterine adenocarcinomas in cattle and melanomas in horses. Another group of malignant tumors that metastasize in the lung is represented by endocrine gland carcinomas and skin carcinomas. Likewise, sarcomas, with their high variability, frequently metastasize in the lung.
Sometimes diagnosis is difficult, in order to establish the origin and especially the primary form of the tumor.
Usually, metastasis is microscopically similar to the primary tumor, but there are cases in which differences are significant. Microscopic examination of various tumor areas, as well as the presence of tumor cells in the arterioles, may help diagnosis.
7.4. TUMORLIKE LESIONS
The lesions most likely to be confused with tumors are granulomatous inflammation and bronchioloalveolar hyperplasia. Granulomatous inflammation, especially in the form of single or multiple granulomas, is more likely to pose a problem at clinical, radiologic, and gross pathologic examination. Histologically, its inflammatory basis is usually well evident.
Bronchioloalveolar hyperplasia, lining of terminal airways and alveoli by prominent epithelium derived from bronchiolar epithelium and/or alveolar type II epithelium. There is frequently an associated remodeling of bronchioloalveolar structure and interstitial fibrosis [123].
7.5. PLEURAL TUMORS
Primary pleural tumors are rare, pleural mesothelioma being found in cattle, dogs, horses, cats, and goats. The neoplasm extends to all the folds of the thoracic cavity. The main demonstrated cause of appearance of this neoplasm is asbestos exposure, but there is evidence supporting the incrimination of ferruginous particles.
Primary tumors exceptionally appear in the pleural cavity and the mediastinum; benign and malignant forms of bone tissue, cartilaginous tissue, nerves, thymus, lymph nodes have been diagnosed.
Secondary tumors are also rare, being found as transpulmonary or mediastinal extensions.
7.6. EXPERIMENTALLY INDUCED NASAL AND LUNG TUMORS
The exposure of man and animals to environmental pollutants, in particular gases and powders, has raised the interest of researchers in experimental investigations, in order to determine the oncogenic properties of polluting substances. It should be mentioned that in most cases, the breathed air contains a complex of potentially oncogenic noxious agents.
Formaldehyde as a potential inducer of nasal mucosal tumors
Experimental studies have demonstrated that formaldehyde induces nasal mucosal hyper- and metaplasias in rats, changes that persist long after the cessation of formaldehyde exposure.
The experiments performed by FERON et al. (1988) in rats established that hyper- and metaplastic changes induced by formaldehyde exposure in the nasal respiratory epithelium of rats did not always disappear after a long non-exposure time. In contrast, a number of rats that were exposed to 20ppm formaldehyde developed nasal tumors that were attributed to formaldehyde. This was demonstrated by the highest incidence of nasal tumors in rats exposed to 20ppm formaldehyde over a 13 week period. These rats developed squamous cell carcinoma. The appearance of polyploid adenomas has been reported in other experiments with formaldehydes. In conclusion, the nasal respiratory epithelium severely damaged by formaldehyde vapors no longer regenerates and in some cases develops tumors.
Rhesus monkeys exposed to formaldehyde in various concentrations, over a shorter or longer duration, have demonstrated changes in nasal mucosal epithelium, from moderate degeneration to squamous metaplasia [63]. These changes have been found in the epithelium of the nasal mucosa, tracheal mucosa, and large bronchi. Minimal histological changes have been detected in subjects exposed for a short time; changes worsen with the increase in the exposure duration, both in terms of intensity and surface of the mucosa. Thus, the proliferation rate of nasal mucosal epithelium is 18-fold higher in subjects exposed to formaldehyde for 6 weeks. The lower airways, the trachea, bronchi and sinuses are less affected. The authors mention the differences that appear depending on the species, regarding the most affected segments of the nasal mucosa. Thus, in rats, the lateral mucosa of the nasal conchae and the lateral walls of the nasal cavity are affected, while in monkeys, the middle conchal mucosa is more affected. Obvious lesions also occur in the anterior nasal mucosa. The explanation consists in interspecies differences regarding the direction of the airstream that enters the nasal cavity.
Animal experiments may represent predictions for the assessment of risk in respiratory cancer in humans exposed to formaldehyde. Epidemiological studies have attempted to answer the question whether there is a cancer risk for humans exposed to formaldehyde. Some observations estimate a risk by 10–14% higher for subjects exposed to formaldehyde [81].
Other carcinogens of the respiratory tract have also been experimentally tested. The direct carcinogenic action of beta-propiolactone, methylmethane sulfonate and dimethylcarbamyl chloride has been experimentally evaluated in the nasal mucosa of the rat [96]. The carcinogenic potential of these substances has revealed the action on nasal mucosal DNA. The ideal experimental model for the oncogenic capacity of some substances is the rat nasal mucosa, conclusion reached by HOCHWALT et al. (1988).
STONER et al. (1986) have studied the possibility of inducing pulmonary adenomas, using benzotrichloride, in order to verify the epidemiological data suggesting the carcinogenic effect of this chemical agent. Following intraperitoneal injection of benzotrichloride in rats, the authors conclude that the product only induces statistically significant lung tumors. So, benzotrichloride exposure involves a carcinogenic risk for humans.
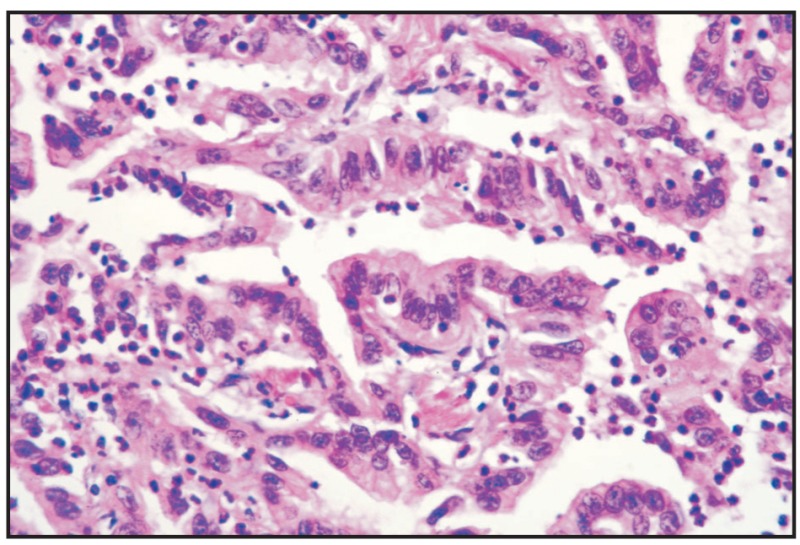
Fig. 7.9
Bronchial carcinoma.
BIBLIOGRAPHY
- 1.
- Acland HM, Orsini JA, Elkins S, Lee WI, Lein DH, Doris DD. Congenital ethmoid carcinoma in a foal. J. Am. Vet. Med. Assoc. 1984;184:979–981. [PubMed: 6715233]
- 2.
- Adameşteanu C, Rusu M, Baba AI, Vesa S. Cazuri de adenomatoză pulmonară la oaie. Rev. Zoot. Med. Vet. 1969;2:54–60.
- 3.
- Adamu D. Pathomorphological assessment and classification of spontaneous neoplasm in the dog. Med. Weternynaryina. 1992;48 (5):200–201.
- 4.
- Alexander JE, Keown GH, Palotay JL. Granular-cell myoblastoma with hypertrophic pulmonary osteoarthropathy in a mare. J. Am. Vet. Med. Assoc. 1965;146:703–708. [PubMed: 14317603]
- 5.
- Anderson BC, Cordy DR. Olfactory neuroblastoma in a heifer. Vet. Pathol. 1981;18:536–540. [PubMed: 7257093]
- 6.
- Anderson JD, Leonard JM, Zeliff JA, Garman RH. Primary pulmonary neoplasm in ahorse. J. Am. Vet. Med. Assoc. 1992;201 (9):1399–1401. [PubMed: 1331002]
- 7.
- Baba AI, Rotaru O, Gaboreanu M, Sissiko I. Observaţii morfopatologice în adenomatoza pulmonară la oi, Simpoz.,,Probleme de ameliorare, tehnologie, creştere şi patologie la taurine şi ovine". F.M.V. Cluj-Napoca. 1979:357–362.
- 8.
- Baba AI, Rotaru O. Neoplasme etmoidale la ovine şi bovine. Buletin IACN-ZMV. 1984;38:87–89.
- 9.
- Barber SM, Clark EG, Fretz PB. Fibroblastic tumor of the premaxilla in two horses. J. Am. Vet. Med. Assoc. 1983;182:700–702. [PubMed: 6573308]
- 10.
- Bostok DE, Owen N. Neoplasia in the cat, dog and horses. Wolfe Medical Publications Ltd; London: 1975. pp. 133–140.
- 11.
- Brodey RS. Canine and feline Neoplasia. Adv. Vet. Sci. 1970;14:311–354. [PubMed: 4922146]
- 12.
- Carbonell PL. Bovine Nasal Granuloma. Vet. Pathol. 1979;16:60–73. [PubMed: 462719]
- 13.
- Chaudhry AP, Haar JG, Koul A, Nickerson PA. Olfactory neuroblastoma (esthesioneuroblastoma). A light and ultrastructural study of two cases. Cancer. 1979;44:564–579. [PubMed: 383258]
- 14.
- Chen HC, Frame SR. Pulmonary Blastoma in a Rat. Vet. Pathol. 1991;28:255–257. [PubMed: 1858258]
- 15.
- Cho DY, Bahr RJ, Leipold HW. Adenocarcinoma in the nasal cavity and brain of a dog. J. Am. Vet. Med. Assoc. 1974;165:350–351. [PubMed: 4850435]
- 16.
- Cohrs P. Infectiöse Adenopapillome der Reichschleimahat beim Schaff. Berl. Munch. Tierarztl. Wochenschr. 1953;66:225–228.
- 17.
- Confer AW, De Paoli A. Primary Neoplasms of the Nasal Cavity, Paranasal Sinuses and Nasopharynx in the Dog. Vet. Pathol. 1978;15:18–30. [PubMed: 625866]
- 18.
- Cotchin E. Spontaneous Neoplasm of the Upper Respiratory Tract in Animals. In: Muir, Shanmugaratnam, editors. Cancer of the Nasopharynx. Intern Union Against Cancer Monogr. Series 1 Medical exam. Publ. Co; New York: 1967. pp. 203–259.
- 19.
- Cox NR, Powers RD. Olfactory Neuroblastomas in Two Cats. Vet. Pathol. 1989;26:341–343. [PubMed: 2763423]
- 20.
- Crespin GJ. Thèse. École Nationale Vétérinaire de Toulouse; 1968. Contribution à 1'étude des tumeurs chez les petits ruminants domestiques.
- 21.
- Cuba-CAPARO A, De Lavega E, Capaira M. Pulmonary adenomatosis of sheep. Am. J. Vet. Res. 1961;22:674–682. [PubMed: 13718767]
- 22.
- Cvjetanović V, Forsek Z, Nevjestic A. Sheep Pulmonary Adenomatosis (SPA) in CRNA GORA I. Veterinaria. 1970;19(2):315–319. Sarajevo.
- 23.
- De Las Heras M, Garcia De Jalon JA, Balaguer L, Garcia JF, Badiola JJ. Tumor intranasal enzootico en la cabra. Med. Vet. 1985;2:281–290.
- 24.
- De Las Heras M, Garcia De Jalon JA, Badiola JJ. Tumor intranasal en una oveja. Med. Vet. 1986;3:459–462.
- 25.
- De Las Heras M, Garcia De Jalon JA, Balaguer L, Badiola JJ. Retrovirus-like particles in enzootic intranasal tumours in Spanish goats. Vet. Rec. 1988;123:135. [PubMed: 2842931]
- 26.
- De Las Heras M, Garcia De Jalon JA, Sharp JM. Pathology of Enzootic Intranasal Tumor in Thirty-eight Goats. Vet. Pathol. 1991;28:474–482. [PubMed: 1771737]
- 27.
- De Las Heras M, Garcia De Jalon JA, Minguijon E, Gray EW, De War P, Sharp JM. Experimental Transmission of Enzootic Intranasal Tumors of Goats. Vet. Pathol. 1995;32:19–23. [PubMed: 7725594]
- 28.
- De Martini JC, Rosadio RH, Sharp JM, Russell HI, Lairmore MD. Experimental Coinduction of Type D Retrovirus-Associated Lymphoid Interstitial Pneumonia in Lambs. JNCI. 1987;79(1):167–177. [PubMed: 3474445]
- 29.
- De Martini JC, Rosandio RH, Lairmore MD. The Etiology and Pathogenesis of Ovine Pulmonary Carcinoma (Sheep Pulmonary Adenomatosis). Vet. Microb. 1988;17:219–236. [PubMed: 3055655]
- 30.
- Delmage DA. Some conditions of the nasal chambers of the dog and cat. Vet. Rec. 1973;92:437–442. [PubMed: 4722791]
- 31.
- Dletz O, Wlesner E. Handbuch der Pferdekrankheiten für Wissenschaft und Praxis. VEB Gustav Fisher Verlag; Jena: 1982 . pp. 430–432.
- 32.
- Dill S, Moise NS, Meschter CL. Cardiac failure in a stallion secondary to metastasis of an anaplastic pulmonary carcinoma. Equine Vet. J. 1986;18:414–417. [PubMed: 3769888]
- 33.
- Duncan JR, Tyler DE, Van Der Masten MJ. Enzootic nasal adenocarcinoma in sheep. J. Am. Vet. Med. Assoc. 1967;151:732–734. [PubMed: 6069985]
- 34.
- Dungal N. Experiments with jaagsiekte. Amer. J. Pathol. 1946;22:737–739. [PubMed: 20991974]
- 35.
- Dungworth DL. The Respiratory System. In: Jubb, Kennedy, Palmer, editors. Pathology of Domestic Animals. Academic Press; New York: 1993 . pp. 539–699.
- 36.
- Feron VJ, Bruyntjes JP, Woutersen RA, Immel HR, Appelman LM. Nasal Tumours in Rats after short-term exposure to a cytotoxic concentration of Formaldehyde. Cancer Letters. 1988;39:101–111. [PubMed: 3345504]
- 37.
- Fisher AF. A compact osteoma in the skull of a horse. J. Am. Vet. Med. Assoc. 1952;121:42–44. [PubMed: 14927530]
- 38.
- Fontaine JJ, Crespeau F, Guereaud JM, Parodi AL. Observation d'une enzootic d'adenome pituitaire de la chèvre. Rev. Med. Vet. Ec. Alfort. 1983;159:383–388.
- 39.
- Gazquez A, Roncero V, Redondo E, Duran E, Masot J, Gomez L. Adenocarcinoma of the ethmoid olfactory mucosa: a histopathological and ultrastructural study with evidence of virus like particles. J. Vet. Med., A. 1992;39 (8):609–615. [PubMed: 1455929]
- 40.
- Geisel O. Primäre Lungensarkome beim Hund. Berl. Münch. Tierärztl. Wschr. 1980;93:174–177. [PubMed: 7406855]
- 41.
- George C, Fuhrer L. Tumeur des cavités nasales. Rec. Méd. Vét. 1988;164 (12):975–978.
- 42.
- Goodger WJ, Carlson L. Surgical removal of a neoplasm from the nasal cavity of a dog. J. Am. Vet. Med. Assoc. 1972;160:726–728. [PubMed: 5010613]
- 43.
- Hill FWG, Moulton JE, Schiff PH. Exophthalmos in a horse resulting from an adenocarcinoma of the frontal sinus. J. South African Vet. Ass. 1989;60 (2):104–105. [PubMed: 2607529]
- 44.
- Hochwalt AE, Wlrgln L, Felber M, Currie DD, Garte SJ. Detection of Novel Non-ras Oncogenes in Rat Nasal Squamous Cell Carcinomas. Mol. Carcin. 1988;1:4–6. [PubMed: 2855601]
- 45.
- Hultgren BD, Schotzer WB, Watrous BJ, Hedstrom OR, Schmitz JA, Wagner PC, Kaneps AJ, Gallagher JA. Nasal-Maxillary Fibrosarcoma in Young Horses: A Light and Electron Microscopic Study. Vet. Pathol. 1987;24:194–196. [PubMed: 3576918]
- 46.
- Indue S, Okada N, Midoro K, Nakayama H, Takahashi R, Fujiwara K. An Equine Case of Granular Cell Tumor with Chondroplasia. Jpn. J. Vet. Sci. 1987;49(3):581–583. [PubMed: 3302437]
- 47.
- Kelley LC, Ouette M, Langheinrich KA, King B. Bovine Pulmonary Blastemas: Histomorphologic Description and Immunohistochemistry. Vet. Pathol. 1994;31:658–662. [PubMed: 7863581]
- 48.
- Kelley LC, Hill JE, Hafner S, Wortham KJ. Spontaneus equine pulmonary granular cell tumors: morphologic, histochemical and immunohistochemical characterization. Vet. Pathol. 1995;32 (2):101–106. [PubMed: 7771048]
- 49.
- Laing JA, Hutchins DR. Progressive ethmoidal haematoma in horses. Austr. Vet. J. 1992;69(3):57–58. [PubMed: 1586315]
- 50.
- Leiggett AD, Weiss R, Thomas KL. Canine Laryngopharyngeal Rhabdomyoma Resembling an Oncocytoma: Light Microscopic, Ultrastructural and Comparative Studies. Vet. Pathol. 1985;22:526–532. [PubMed: 4082377]
- 51.
- Lobetti RG, Williams MC. Anaplastic tracheal squamous cell carcinoma in a cat. J. South African Vet. Assoc. 1992;63 (3):132–133. [PubMed: 1404224]
- 52.
- Lombard CH, Cabanne P, Crespin J. Adénopapillome de la muqueuse pituitaire chez la chèvre. Bull. Acad. Vet. Fr. 1966;39:199–202. [PubMed: 6007080]
- 53.
- Loupal G, Mlkula M. Olfaktorius - neuroblastom bei einem Pferd. Pferdeheilkunde. 1985;1:65–69.
- 54.
- Meuten DJ, Calderwood Mays MB, Dillman RC, Cooper BJ, Valentine BA, Kuhatda FP, Pass DA. Canine Laryngeal Rhabdomyoma. Vet. Pathol. 1985;22:533–539. [PubMed: 4082378]
- 55.
- Mc Entre MC, Page RL, Heidner GL, Cline J, Thrall DE. A retrospective study of 27 dogs with intranasal neoplasms treated with cobalt radiation. Vet. Radiol. 1991;32 (3):135–139.
- 56.
- Mc Klnon AQ, Thorsen J, Hayes MA, Mlsener CR. Enzootic nasal adenocarcinoma of sheep in Canada. Can. Vet. J. 1982;23:88–94. [PMC free article: PMC1790125] [PubMed: 17422121]
- 57.
- Madewell BR, Priester WA, Gillete EL, Snyder SP. Neoplasm of the nasal passages and paranasal sinuses in domesticated animals as reported by 13 veterinary colleges. Am. J. Vet. Res. 1976;37:851–856. [PubMed: 937809]
- 58.
- Madewell BR, Theilen GH. Theilen and Madewell, Veterinary Cancer Medicine. Lea & Fibiger; Philalphia: 1987. Tumors of the Respiratory Tract and Thorax; pp. 535–565.
- 59.
- Markson LM, Terlechki S. The experimental transmission of ovine pulmonary adenomatosis. Pathol. Vet. 1964;1:269–288.
- 60.
- Mason BJE. Spindle-cell sarcoma of the equine paranasal sinuses and nasal chamber. Vet. Rec. 1975;96:287–288. [PubMed: 1129920]
- 61.
- Misdorp W, Van Der Heul RO. Tumors of bones and joints. Bull. World. Health Org. 1976;53:265–283. [PMC free article: PMC2366504] [PubMed: 1086157]
- 62.
- Misdorp W, Nauta-Van Gelder HL. Granular-cell myoblastoma in the horse. A report of 4 cases. Path. Vet. 1968;5:385–394. [PubMed: 4304683]
- 63.
- Monticello TM, Morgan KT, Everitt JI, Popp JA. Effects of Formaldehyde Gas on the Respiratory Tract of Rhesus Monkeys. Am. J. Pathol. 1989;134:515–527. [PMC free article: PMC1879517] [PubMed: 2923182]
- 64.
- Morgan JP, Suter PF, O'Brien TR, Park RD. Tumors of the nasal cavity of the dog: a radiologic study. J. Am. Vet. Radiol. Soc. 1972;13:18–26.
- 65.
- Morrison T, Read R, Eger C. A retrospective study of nasal tumours in 37 dogs. Austr. Vet. Pract. 1989;19 (3):130–134.
- 66.
- Moulton JE. Tumors in Domestic Animals. University of California Press; Berkeley: 1978. pp. 205–239.
- 67.
- Moulton JL. Tumors of the respiratory system. In: Moulton, editor. Tumors in Domestic Animals. 3rd ed. Berkeley, Calif: University of California Press; 1990. pp. 308–346.
- 68.
- Muraalimanohar B, Sundararay A. Exfoliative cytology using Papanicolau staining in the diagnosis of ethmoid carcinoma in cattle. Indians Vet. J. 1991;68(12):1113–1116.
- 69.
- Nascimento DoEF, Reis R, De Carvalho AU, Leite RC, Simplicio AA. Tumor etmoidal enzootic em ovinos. Arq. Esc. Vet. UFMG, Belo Horizonte. 1979;31 (3):337–342.
- 70.
- Nevjestic A, Rukavina Lj, Forsek Z, Cvjetanović V. Sheep Pulmonary Adenomatosis (SPA) in CRNA GORA II. Veterinaria. 1970;19(3):429–434. Sarajevo.
- 71.
- Nevjestic A, Forsek Z, Cvjetanović V, Rukavina Lj. Sheep Pulmonary Adenomatosis (SPA) in CRNA GORA IV. Veterinaria. 1971;20(1):31–35. Sarajevo.
- 72.
- Nii A, Nakayama H, Takahashi R, Fujiwara K. Pathology of Ten Cases of Canine Primary Lung Tumor. Jpn. J. Vet. Sci. 1985;47 (5):811–815. [PubMed: 4068442]
- 73.
- Njoku CO, Shannon D, Chineme CN, A-Bidam S. Ovine nasal adenopapilloma: incidence and clinicopathologic studies. A. J. Vet. Res. 1978;39 (11):1850–1852. [PubMed: 736344]
- 74.
- Nobel TA, Neuman F, Lopfer UK. Metastases in pulmonary adenomatosis of sheep. Ref. Vet. 1968;25:52–57.
- 75.
- Park RD, Beck ER, Lee RA. Comparison of computed tomography and radiography for detecting changes induced by malignant nasal neoplasia in dogs. J. Am. Vet. Med. Assoc. 1992;201 (11):1720–1724. [PubMed: 1293113]
- 76.
- Parodi AL, Tassin P, Rigoulet J. Mycoblastome à cellules granuleuses, trois nouvelles observations à localisation pulmonaire chez le cheval. Rec. Mèd. Vèt. 1974;6:489–494.
- 77.
- Patnaik AK. Canine sinonasal neoplasms: soft tissue tumors. J. Am. Anim. Hosp. Assoc. 1989;195 (5):491–497.
- 78.
- Peaston AE, Leach MW, Higgins RJ. Pathodynamic therapy for nasal and aural squamous cell carcinoma in cats. J. Am. Vet. Med. Assoc. 1993;202 (8):1261–1265. [PubMed: 8496082]
- 79.
- Perl S, Yokobson B, Orgad-Klopfer U, Abramson M, Nobel T. Enzootic nasal tumor of sheep. Vet. Quarterly. 1978;9:118–122. [PubMed: 3617417]
- 80.
- Pospischil A, Haenichen T, Schaeffler H. Histopathological and electronomicroscopic studies of endemic ethmoidal carcinomas in cattle. Vet. Path. 1979;16(2):180–190. [PubMed: 442448]
- 81.
- Purchase IFH, Paddle GM. Does formaldehyde cause nasopharyngeal cancer in man? Cancer Letters. 1989;46:79–85. [PubMed: 2665934]
- 82.
- Ramakrishna O. Osteoma on the left metatarsal bone of a bullock. Indian Vet. J. 1973;50:603–604.
- 83.
- Redondo E, Roncero V, Duran E, Moyano MC, Gazquez A. Étude structurale et morphométrique du parenchyme pulmonaire des ovines dans les infections par virus lents: adénomatose et maedi. Revue Méd. Vét. 1988;139:607–613.
- 84.
- Reynolds BL, Stedham MA, Lawrence JM, Heltsley JR. Adenocarcinoma of the frontal sinus with extension to the brain in a horse. J. Am. Vet. Med. Assoc. 1979;174:734–736. [PubMed: 429236]
- 85.
- Ronald J, Valade S, Chatelet F. Le carcinome anaplasique dit "à petites cellules" des branches: microscopie optique et ultrastructure. Bull. Cancer. 1987;74:491–494. [PubMed: 2825848]
- 86.
- Rosadio RH, Sharp JM, Lairmore MD, Dahlberg JE, De Martini JC. Lesions and Retroviruses Associated with Naturally Occurring Ovine Pulmonary Carcinoma (Sheep Pulmonary Adenomatosis). Vet. Pathol. 1988;25:58–66. [PubMed: 2830697]
- 87.
- Rosadio RH, Lairmore MD, Russell HI, De Martini JC. Retrovirus- associated Ovine Pulmonary Carcinoma (Sheep Pulmonary Adenomatosis) and Lymphoid Interstitial Pneumonia I. Lesion Development and Age Susceptibility. Vet. Pathol. 1988;25:475–483. [PubMed: 3212891]
- 88.
- Rumbaugh GE, Pool RR, Wheat JD. Atypical osteoma of the nasal passage and paranasal sinus in a bull. Cornell Vet. 1987;68(4):544–554. [PubMed: 710144]
- 89.
- Sagartz JW, Harris TW, Simon J. Carcinoma in the frontal sinus of a dog. J. Am. Vet. Med. Assoc. 1971;158:1945–1946. [PubMed: 5107323]
- 90.
- Schuh JCL. Squamous Cell Carcinoma of the Oral, Pharyngeal and Nasal Mucosa in the Horse. Vet. Pathol. 1986;23:205–207. [PubMed: 3962089]
- 91.
- Schultze AE, Sonea I, Bell IG. Primary malignant pulmonary neoplasia in two horses. J. Am. Vet. Med. Assoc. 1988;193:477–480. [PubMed: 2844708]
- 92.
- Sharp JM. Sheep pulmonary adenomatosis: a contagious tumour and its cause. Cancer Survey. 1987;6(1):73–78. [PubMed: 3319134]
- 93.
- Shortridge EH. Survey of bovine neoplasia. New Zeeland Vet. J. 1971;19:5–11. [PubMed: 5280753]
- 94.
- Smith W, Mackay JMK. Morphological observations on a virus associated with sheep pulmonary adenomatosis (jaagsiekte). J. Comp. Path. 1969;79:421–424. [PubMed: 5351412]
- 95.
- Smith MO, Turrel JM, Bailey CS, Cain GR. Neurologic abnormalities as the predominant signs of neoplasia of the nasal cavity in dogs and cats: seven cases (1973–1986). J. Am. Vet. Med. Assoc. 1986;195 (2):242–245. [PubMed: 2768045]
- 96.
- Snyder CA, Garte SJ, Sellakumar AR, Albert RE. Relation-ships between the Levels of Binding to DNA and the Carcinogenic potencies in Rat nasal mucosa for three Alkylating Agents. Cancer Letters. 1986;33:175–181. [PubMed: 3791188]
- 97.
- Steen M, Rehbinder C, Mörner T. Nasal tumor in a follow deer (Doma doma L.), case report. Acta vet. Scand. 1985;26:461–465. [PMC free article: PMC8202709] [PubMed: 3836566]
- 98.
- Stevens AJ. Respiratory diseases of sheep. Vet. Rec. 1957;69:1249–1254.
- 99.
- Stoner GD, You M, Morgan MA, Superczynski MJ. Lung Tumor Induction in Strain a Mice with Benzotrichloride. Cancer Letters. 1986;33:167–173. [PubMed: 3791187]
- 100.
- Stünzi H, Head KW, Nielson SW. Tumours of the lung. Bull. Wld. Hlth. Org. 1974;50:9–19. [PMC free article: PMC2481214] [PubMed: 4371738]
- 101.
- Stünzi H, Hauser Z. Tumours of the nasal cavity. Bull. World Health Organ. 1976;53:257–263. [PMC free article: PMC2366503] [PubMed: 1086156]
- 102.
- Sullivan M, Lee R, Jakovljevnic S, Sharp NJH. The radiological features of aspergillosis of the nasal cavity and frontal sinuses in the dog. J. Small Anim. Pract. 1986;27:167–180.
- 103.
- Sullivan M, Lee R, Skae CA. The radiological features of sixty cases of intranasal neoplasia in the dog. J. Small Anim. Pract. 1987;28:575–586.
- 104.
- Taylor GN, Shabestari L, Agnus W, Lloyd RD, Mays CW. Primary Pulmonic Tumors in Beagles. Am. J. Vet. Res. 1979;40 (9):1316–1318. [PubMed: 525940]
- 105.
- Theilen GH, Madewell BR. Veterinary Cancer Medicine. Lea & Fibiger; Philadelphia: 1987. pp. 535–565.
- 106.
- Théon AP, Madewell BR, Harb MF, Dungworth DL. Megavoltage irradiation of neoplasms of the nasal and paranasal cavities in 77 dogs. J. Am. Vet. Med. Assoc. 1993;202 (9):1469–1475. [PubMed: 8496103]
- 107.
- Théon AP, Peaston AE, Madewell BR, Dungworth DL. Irradiation of nonlymphoproliferative neoplasms of the nasal cavity and paranasal sinues in 16 cats. J. Am. Vet. Med. Assoc. 1994;204 (1):78–83. [PubMed: 8125825]
- 108.
- Thrall DE, Robertson ID, McLeod D, Heider GL, Hoopes PJ, Page RL. A comparison of radiographic and computed tomographic findings in 31 dogs with malignant nasal cavity tumors. Vet. Rad. 1989;30 (2):59–66.
- 109.
- Tokarnia CH, Döbereiner J, Canella CF. Tumor edmoidal enzootico em bovines no estado do Rio de Janeiro. Pesq. agropee. bras., Sér. Vet. 1972;7:41–46.
- 110.
- Tontis A, Bastetti G, König H, Luginbühl H. Enzootisches Auftreten von Lungenadenomatoses bei 13 Schafen in der Nähe von Berz. Scheiz. Arch. Tierheilk. 1979;121:151–262. [PubMed: 451519]
- 111.
- Troy MA. Granular-cell myoblastoma in a dog. J. Am. Vet. Med. Assoc. 1955;126:379. [PubMed: 14367200]
- 112.
- Tustin RC, Williamson AL, York DF, Verwoed DW. Experimental transmission of jaagsiekte (ovine pulmonary adenomatosis) to goats. Onderstepoort J. Vet. Res. 1988;55 (1):27–32. [PubMed: 3353097]
- 113.
- Verwoerd DW, De Villers EM, Tustin RC. Aetiology of Jaagsiekte: Experimental Transmission to Lambs by Means of Cultures Cells and Cell Homogenates, Ondestepoort. J. Vet. Res. 1980;47:13–18. [PubMed: 7454230]
- 114.
- Verwoerd DW, Williamson AL, De Villiers EM. Aetiology of Jaagsiekte: Transmission by Means of Subcellular Fractions and Evidence for the Involvement of a Retrovirus, Onderstepoort. J. Vet. Res. 1980;47:275–280. [PubMed: 6164973]
- 115.
- Zaki FA, Liu KS. Adenocarcinoma of the Olfactory Gland in the Dog. Vet. Pathol. 1974;11:138–143. [PubMed: 4450371]
- 116.
- Wandera JG. Clinical Pulmonary Adenomatosis of Sheep Produced Experimentally. Br. vet. J. 1970;126:185–193. [PubMed: 5448136]
- 117.
- Wandera JG, Krauss H. The ultrastructure of sheep pulmonary adenomatosis. Zbl. Vet. Med., A. 1970;18:325–334. [PubMed: 4999020]
- 118.
- Wendt M. Klinik und Diagnose von Siebbeintumoren (Adenopapillomatose) beim Schaf. Tierarztliche Umschau. 1989;44(9):540–547.
- 119.
- Wenger IE, Caron JP. Tracheal mastocytosis in a horse. Canad. Vet. J. 1988;29(7):563–565. [PMC free article: PMC1680810] [PubMed: 17423075]
- 120.
- Wyand DS, Wolke RE. Granular-cell myoblastoma of the canine tongue. Case reports. Am. J. Vet. Res. 1968;29:1309–1313. [PubMed: 4297859]
- 121.
- Yonemichi H, Ohgi T, Fujimoto Y, Okada K, Onuma M, Mokami T. Intranasal tumor of the ethmoid olfactory mucosa in sheep. Am. J. Vet. Res. 1978;39 (10):1599–1606. [PubMed: 213993]
- 122.
- Young S, Lovelace SA, Hawkins WW, Catlin JE. Neoplasm of the olfactory mucous membrane of sheep. Cornell Vet. 1961;51:96–116. [PubMed: 13787496]
- 123.
- Dungworth DL, Hauser B, Hahn FF, Wilson DW, Haenichen T, Harkema JR. Histological Classification of Tumors of the Respiratory System of Domestic Animals. Second Series. VI. WHO, Armed Forces Institute of Pathology; Washington, D.C: 1999.
List of Figures 7.1-7.24
- Fig. 7.2 Nasal squamos carcinoma, slight keratinization.
- Fig. 7.7 Nasal adenocarcinoma.
- Fig. 7.9 Bronchial carcinoma.
- Fig. 7.17 Ovine pulmonary carcinoma.
- Fig. 7.18 Ovine pulmonary carcinoma.
- Fig. 7.19 Ovine pulmonary carcinoma.
- Fig. 7.20 Ovine pulmonary carcinoma.
- Fig. 7.22 Lung osteosarcoma.
- Fig. 7.23 Lung productive osteoblastic osteosarcoma.
- Fig. 7.24 Lung chondrosarcoma.
Footnotes
- *)
Courtesy of W.H.O.
Publication Details
Copyright
Publisher
The Publishing House of the Romanian Academy, Bucharest (RO)
NLM Citation
Baba AI, Câtoi C. Comparative Oncology. Bucharest (RO): The Publishing House of the Romanian Academy; 2007. Chapter 7, TUMORS OF THE RESPIRATORY SYSTEM.






















