NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Institute of Medicine (US) Forum on Microbial Threats. The Science and Applications of Synthetic and Systems Biology: Workshop Summary. Washington (DC): National Academies Press (US); 2011.

The Science and Applications of Synthetic and Systems Biology: Workshop Summary.
Show detailsThe Emergence of Sociomicrobiology as an Interest Area in Microbiology
Up until the later part of the past century, microbiologists believed that with rare exceptions bacteria did not practice sociality; this was in spite of the fact that growing bacteria as colonies on agar plates was and still remains a cornerstone technology for the discipline. Just the terminology “colonial” or “colony growth” implies some sort of social interactions. But with the few exceptional scientists working on myxobacteria, a special group of social bacteria, the eyes of microbiology did not see social interactions among individual bacteria. The longstanding view that bacteria were by-and-large asocial creatures began to crumble in the 1990s with rapid developments in the areas of quorum sensing and biofilm research. Now it seems apparent that the same selective pressures that led to the evolution of sociality in animals are forces for evolution of sociality in bacteria, and we see and appreciate social behavior in bacteria (Camilli and Bassler, 2006; Parsek and Greenberg, 2005). I have no proof but I believe that contributing to our past resistance to sociomicrobiology is the fact that bacteria are at once single cells and single individuals. They have served biology as wonderful models for cellular activities. We have not focused as much on the bacterial cell as an individual member of a species as we have on their existence as single-celled organisms.
My laboratory has focused on three areas of sociomicrobiology. The first is quorum sensing, which can be defined as a cell-to-cell communication system that enables individuals to sense the local population density and regulate expression of specific genes in response to population density. This is the primary topic of this paper. The second is biofilm biology. Biofilms are organized assemblages of bacteria embedded in a self-produced extracellular matrix. As a consequence of the biofilm lifestyle, the assembled bacteria exist in a heterogeneous environment and bacteria in different regions of a biofilm have different functions, which confer different traits to the group. As a consequence of the biofilm lifestyle, at least a subpopulation of the assemblage is protected from environmental stresses and in fact biofilm infections are difficult or impossible to cure by antibiotic treatment (Costerton et al., 1999). Because biofilm infections are very prevalent medical problems there is considerable interest in developing novel therapies that are effective in killing biofilm bacteria. To name just a few, infections of any implanted medical device are biofilm infections, cystic fibrosis lung infections are biofilm infections, heart valve infections are biofilms, and at least some chronic middle ear infections are biofilms (Costerton et al., 1999). Third, we have also worked on conspecific territoriality exhibited by swarms of bacteria moving over surfaces (Gibbs and Greenberg, 2011; Gibbs et al., 2008).
The emergence of sociomicrobiology as a new subdiscipline is important for several reasons. First, there is an idea that we might be able to develop novel antivirulence therapeutics, which target quorum sensing in bacterial species that control virulence gene expression by cell-to-cell signaling. In fact there are many investigators working to identify potent quorum-sensing and biofilm inhibitors, and we ourselves have undertaken such programs together with collaborators in the pharmaceutical industry (Banin et al., 2008; Muh et al., 2006a,b), but this is not the primary motivation of our work. This is in part because it is my belief that we do not yet understand enough about the biology of quorum sensing to predict how, when, or where its inhibition might be of therapeutic value. Second, it is now clear that if one strives to really understand bacteria, social aspects of their biology cannot be ignored. Third, now that we understand that bacteria are social and we understand sociality at an unprecedented level of molecular detail in bacteria like Pseudomonas aeruginosa, these model organisms have become wonderful tools to study fundamental questions about the costs and benefits of cooperation, the selective pressures that lead to cooperative behaviors, and the advantages of controlling cooperative behaviors by communication. Finally, our detailed understanding of quorum-sensing signal synthesis and signal reception, as is discussed below, has led to the widespread use of quorum-sensing regulatory circuits in synthetic biology (Marguet et al., 2010, and references therein).
Quorum Sensing in Proteobacteria
In the late 1960s and early 1970s there was a very modest literature describing pheromone production and activity in bacteria (Eberhard, 1972; Tomasz, 1965). It was not until the 1980s that work on quorum sensing in marine luminescent bacteria led to the idea that these sorts of gene regulatory activities function as intercellular communication systems that coordinate group activities. Not until the 1990s did we begin to understand the prevalence of quorum sensing in bacteria. Although there are many different types of bacterial cell-to-cell signaling systems considered as quorum-sensing systems, our work focuses on acyl-homoserine lactone quorum-sensing systems prevalent but not universal among the Proteobacteria. Our original model system was a marine bacterium, Vibrio fischeri, which controls a small set of about 25 or fewer genes, including genes for light production, by a transcriptional activator called LuxR, and 3-oxo-hexanoyl-homoserine lactone (3OC6-HSL), which is produced by the luxI gene product (Antunes et al., 2007; Engebrecht et al., 1983). This quorum-sensing system allows V. fischeri to discriminate between its free-living low-population-density lifestyle and its high-density host-associated lifestyle (Figure A7-1). It exists in the light organs of specific marine animals where it produces light, which serves the mutualistic symbiosis. Of note, 3OC6-HSL moves in and out of cells by passive diffusion. In this way the environmental signal concentration is a reflection of cell density. The luxI gene itself is controlled by quorum sensing—it is activated by LuxR and 3OC6-HSL. In terms of biology, this provides hysteresis to the system. The population density required to activate quorum-controlled genes is much higher than the density required to shut down an activated system.
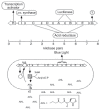
FIGURE A7-1
Quorum sensing in Vibrio fischeri. (Top) The lux gene cluster. The luxR gene encodes a 3OC6-HSL (AHL)-dependent transcriptional activator of the luxI-G operon. The luxI product is the AHL synthase; luxC, D, and E form a complex responsible for generation (more...)
We later turned our attention to the opportunistic pathogen P. aeruginosa. We learned that there were two acyl-HSL circuits, the C4-HSL-RhlI-RhlR circuit and the 3OC12-HSL-LasI-LasR circuits, which together are required for activation of about 325 genes (Figure A7-2) (Pearson et al., 1994, 1995; Schuster et al., 2003). Other investigators showed that at least under certain experimental conditions quorum-sensing mutants were impaired in virulence (Pearson et al., 2000; Tang et al., 1996), and thus the belief was that, as for V. fischeri quorum sensing, P. aeruginosa quorum sensing allowed discrimination between host and free-living states. However, it is not clear from the evidence that this is in fact the case. Among the 300-plus quorum-controlled genes, those coding for extracellular products like exoenzymes or coding for functions required for the production of exoproducts like hydrogen cyanide or pyocyanin are grossly overrepresented.
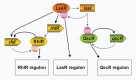
FIGURE A7-2
Diagram of the acyl-HSL quorum-sensing regulatory circuit in P. aeruginosa. The lasI and R genes are linked on the chromosome, as are the rhlR and I genes. The qscR gene is not linked tightly to any other quorum-sensing regulator. LasI-R sit atop a quorum-sensing (more...)
In a social context these sorts of extracellular products can be considered public goods or resources produced by individuals and shared by all members of the group. Thus we believe quorum sensing serves to coordinate cooperative behaviors. Investigators have devised experiments where growth of P. aeruginosa requires quorum sensing. This can be accomplished by providing protein as the sole source of carbon and energy. Growth requires quorum-sensing-induced production of the exoprotease elastase. In this setting, LasR quorum-sensing mutants will emerge and become a stable, significant minority of the overall population. These cheaters or freeloaders do not bear the cost of contributing to the public good (elastase in this case), but they benefit from use of the public goods (Sandoz et al., 2007). It is obvious that light production by V. fischeri is a shared behavior and we believe this also represents quorum control of cooperation. As far as I am aware, there are no biological systems sensitive enough to detect the light produced by one or a few V. fischeri cells (maximum emission is about 1,000 photons per second per cell). But the light produced by large groups of cells can easily be observed. For example, we can see the light produced by colonies of V. fischeri growing on a Petri plate. So here are two examples where it seems apparent that quorum-sensing functions control and coordinate cooperative behaviors. Is this universal for LuxR-LuxI-acyl-HSL-type systems? Of course we do not know the answer to this question and one might imagine that such regulatory elements have been adapted by different bacteria for different purposes.
As a general principle, the LuxR-LuxI type of regulatory circuits appears to represent elements of true communication. That is, the signal and the signal receptor are coevolved (Keller and Surette, 2006). One finds that a disturbing anthropomorphic trend has crept into the field and the term “bacterial language” is sometimes used to describe quorum-sensing systems. I do not believe any linguist would find evidence for syntax and grammar in the bacterial world.
With the discovery that quorum-sensing-controlled virulence of bacteria like P. aeruginosa, the number of scientists studying acyl-HSL signaling grew exponentially. Generally speaking, studies of quorum sensing and control of social activity in bacteria are important for the several reasons listed earlier in this paper. We might be able to develop novel antivirulence therapeutics that target quorum sensing in bacteria like P. aeruginosa. Sociality is an important aspect of bacterial biology. Bacteria are now viewed as experimental models for fundamental studies about the costs and benefits of cooperation, the selective pressures that lead to cooperative behaviors, and the advantages of controlling cooperative behaviors by communication. In addition, our detailed understanding of signal synthesis by Lux-type proteins and signal reception by LuxR-type transcription factors has led to their widespread use in the area of synthetic biology (Marguet et al., 2010). This was a largely unintended consequence of our work, and I believe there are three reasons for the popularity of these systems in building synthetic regulatory circuits. First, the acyl-HSLs are diffusible signaling ligands (Kaplan and Greenberg, 1985). Thus, the complication of transport systems for the ligand need not concern the synthetic biologist. Second, the V. fischeri system (Engebrecht et al., 1983), the P. aeruginosa systems, and many other acyl-HSL quorum-sensing circuits have positive autoregulatory loops. Signal production proceeds at a low level until it has accumulated sufficiently to activate quorum-controlled genes, one of which is the gene for its own synthesis. The signal concentration then increases very rapidly. Work in the area of synthetic biology has focused on this phenomenon as a method to reduce noise in regulatory circuits or build gene expression oscillators. Finally, we have identified many different systems and we know of dozens of acyl-HSL signals—the signal specificity of a given LasR homolog resides in the nature of the acyl side group. Thus, multiple systems can be strung together in a single bacterium with each controlling, for example, expression of fluorescent proteins that emit at different wavelengths. Examples showing the diversity of acyl-HSL signals identified in different bacterial species are shown in Figure A7-3.
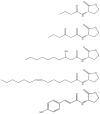
FIGURE A7-3
Some examples of acyl-HSL quorum-sensing signals. From top to bottom; the P. aeruginosa RhlI signal butanoyl-HSL, the V. fischeri LuxI signal, 3-oxo-hexanoyl-HSL, The B. mallei BmaI signal, 3-hydroxy-decanoyl-HSL, and the Rhodopseudomonas palustris RpaI (more...)
New Twists to the Acyl-Homoserine Lactone Signaling Story
The P. aeruginosa quorum-sensing circuitry described in Figure A7-2 shows, in addition to LasR-LasI and RhlR-RhlI, a LuxR homolog, QscR. There is no cognate acyl-HSL synthase gene for QscR and it has been termed an orphan quorum-sensing signal receptor (Chugani et al., 2001). These orphans, which have also been called solos (Subramoni and Venturi, 2009), are quite commonly found in sequenced proteobacterial genomes. We know that QscR responds to the LasI-produced 3OC12-HSL, and it controls a set of genes that partially overlaps with the genes controlled by LasR and RhlR (Lee et al., 2006; Lequette et al., 2006). There has been recent interest in studying orphans (Subramoni and Venturi, 2009). There are orphan LuxR homologs in Salmonella and in pathogenic E. coli, neither of which have any luxI homologs. Evidence indicates that the E. coli and Salmonella LuxR orphans respond to acyl-HSLs made by other bacterial species in mixed microbial communities (Soares and Ahmer, 2011; Sperandio, 2010).
Most of the acyl-HSL signals that have been identified are fatty acyl-HSLs with fatty acyl groups of varying carbon length and with a limited number of substitutions on the fatty acyl carbon chain. We recently described examples of two bacteria that use LuxI-LuxR homologs to produce and respond to aryl-HSLs. Both are photosynthetic; Rhodopseudomonas palustris uses p-coumaroyl-HSL as a quorum-sensing signal and photosynthetic baradyrhizobia uses cinnamoyl-HSL (Ahlgren et al., 2011; Schaefer et al., 2008). These discoveries broaden the possibilities in terms of signal molecules. Furthermore, the photosynthetic bradyrhizobia produce nanomolar amounts of cinnamoyl-HSL and respond to picomolar amounts. This is of interest because the fatty acyl-HSL systems that have been described involve responses to nanomolar signal levels and the signals are usually produced at levels in the micromolar range. The bradyrhizobium system is shifted to much lower levels for production and detection. The significance of the low production and ultrasensitivity is not clearly understood. However, it is clear that, by assuming acyl-HSLs will be produced at higher levels, we likely have overlooked at least some acyl-HSL signaling systems.
Concluding Comments
This paper seeks to introduce an emerging field, sociomicrobiology, and to point out opportunities for synthetic biology and therapeutic development in the area of sociomicrobiology. The paper emphasizes quorum sensing in Proteobacteria and provides examples where quorum sensing serves to allow individuals of a species to communicate. This rudimentary form of chemical communication serves to coordinate certain cooperative behaviors. Whether this is a universal characteristic of acyl-HSL signaling systems remains to be seen. There are also a variety of other small-molecule signals produced by bacteria and many of these are considered quorum-sensing signals (Camilli and Bassler, 2006). If and how these intercellular signals are controlling cooperation also remains to be seen. Finally, this Forum has covered topics related to synthetic and systems biology, and opportunities in the area of human health and disease. We need to remain cognizant that bacterial cells are individual organisms involved in complex social interactions. So not only do we need to think about systems and synthetic biology from a cellular perspective, but we need to think about systems and synthetic ecology too.
References
- Ahlgren NA, Harwood CS, Schaefer AL, Giraud E, Greenberg EP. Aryl-homoserine lactone quorum sensing in stem-nodulating photosynthetic bradyrhizobia. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:7183–7188. [PMC free article: PMC3084126] [PubMed: 21471459]
- Antunes LC, Schaefer AL, Ferreira RBR, Qin N, Stevens AM, Ruby EG, Greenberg EP. Transcriptome analysis of the Vibrio fischeri LuxR-LuxI regulon. Journal of Bacteriology. 2007;189:8387–8391. [PMC free article: PMC2168698] [PubMed: 17827287]
- Banin E, Lozinski A, et al. The potential of desferrioxamine-gallium as an anti-Pseudomonas therapeutic agent. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:16761–16766. [PMC free article: PMC2575493] [PubMed: 18931304]
- Camilli A, Bassler BL. Bacterial small-molecule signaling pathways. Science. 2006;311:1113–1116. [PMC free article: PMC2776824] [PubMed: 16497924]
- Chugani SA, Whiteley M, Lee KM, D’Argenio D, Manoil C, Greenberg EP. QscR, a modulator of quorum-sensing signal synthesis and virulence in Pseudomonas aeruginosa. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:2752–2757. [PMC free article: PMC30211] [PubMed: 11226312]
- Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: A common cause of persistent infections. Science. 1999;284:1318–1322. [PubMed: 10334980]
- Eberhard A. Inhibition and activation of bacterial luciferase synthesis. Journal of Bacteriology. 1972;109:1101–1105. [PMC free article: PMC247330] [PubMed: 5011244]
- Engebrecht J, Nealson KH, Silverman M. Bacterial bioluminescence: Isolation and genetic analysis of functions from Vibrio fischeri. Cell. 1983;32:773–781. [PubMed: 6831560]
- Gibbs KA, Greenberg EP. Territoriality in Proteus: Advertisement and aggression. Chemical Reviews. 2011;111(1):188–194. [PMC free article: PMC3024916] [PubMed: 21162556]
- Gibbs KA, Urbanowski ML, Greenberg EP. Genetic determinants of self identity and social recognition in bacteria. Science. 2008;321:256–259. [PMC free article: PMC2567286] [PubMed: 18621670]
- Kaplan HB, Greenberg EP. Diffusion of autoinducer is involved in regulation of the Vibrio fischeri luminescence system. Journal of Bacteriology. 1985;163:1210–1214. [PMC free article: PMC219261] [PubMed: 3897188]
- Keller L, Surette MG. Communication in bacteria: An ecological and evolutionary perspective. Nature Reviews Microbiology. 2006;4:249–258. [PubMed: 16501584]
- Lee JH, Lequette Y, Greenberg EP. Activity of purified QscR, a Pseudomonas aeruginosa orphan quorum-sensing transcription factor. Molecular Microbiology. 2006;59:602–609. [PubMed: 16390453]
- Lequette Y, Lee JH, Ledgham F, Lazdunski A, Greenberg EP. A distinct QscR regulon in the Pseudomonas aeruginosa quorum-sensing circuit. Journal of Bacteriology. 2006;188:3365–3370. [PMC free article: PMC1447466] [PubMed: 16621831]
- Marguet P, Tanouchi Y, Spitz E, Smith C, You LC. Oscillations by minimal bacterial suicide circuits reveal hidden facets of host-circuit physiology. PLoS One. 2010;5:e11909. [PMC free article: PMC2912849] [PubMed: 20689598]
- Muh U, Hare BJ, Duerkop BA, Schuster M, Hanzelka BL, Heim R, Olson ER, Greenberg EP. A structurally unrelated mimic of a Pseudomonas aeruginosa acyl-homoserine lactone quorum-sensing signal. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:16948–16952. [PMC free article: PMC1636559] [PubMed: 17075036]
- Muh U, Schuster M, Heim R, Singh A, Olson ER, Greenberg EP. Novel Pseudomonas aeruginosa quorum-sensing inhibitors identified in an ultra-high-throughput screen. Antimicrobial Agents and Chemotherapy. 2006;50:3674–3679. [PMC free article: PMC1635174] [PubMed: 16966394]
- Parsek MR, Greenberg EP. Sociomicrobiology: The connections between quorum sensing and biofilms. Trends in Microbiology. 2005;13:27–33. [PubMed: 15639629]
- Pearson JP, Gray KM, Passador L, Tucker KD, Eberhard A, Iglewski BH, Greenberg EP. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:197–201. [PMC free article: PMC42913] [PubMed: 8278364]
- Pearson JP, Passador L, Iglewski BH, Greenberg EP. A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:1490–1494. [PMC free article: PMC42545] [PubMed: 7878006]
- Pearson JP, Feldman M, Iglewski BH, Prince A. Pseudomonas aeruginosa cell-to-cell signaling is required for virulence in a model of acute pulmonary infection. Infection and Immunity. 2000;68:4331–4334. [PMC free article: PMC101761] [PubMed: 10858254]
- Sandoz KM, Mitzimberg SM, Schuster M. Social cheating in Pseudomonas aeruginosa quorum sensing. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:15876–15881. [PMC free article: PMC2000394] [PubMed: 17898171]
- Schaefer AL, Greenberg EP, Oliver CM, Oda Y, Huang JJ, Bittan-Banin G, Peres CM, Schmidt S, Juhaszova K, Sufrin JR, Harwood CS. A new class of homoserine lactone quorum-sensing signals. Nature. 2008;454:595–599. [PubMed: 18563084]
- Schuster M, Lostroh CP, Ogi T, Greenberg EP. Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: A transcriptome analysis. Journal of Bacteriology. 2003;185:2066–2079. [PMC free article: PMC151497] [PubMed: 12644476]
- Soares JA, Ahmer BM. Detection of acyl-homoserine lactones by Escherichia and Salmonella. Current Opinions in Microbiology. 2011;14:188–193. [PMC free article: PMC3078957] [PubMed: 21353625]
- Sperandio V. SdiA sensing of acyl-homoserine lactones by enterohemorrhagic E. coli (EHEC) serotype O157:H7 in the bovine rumen. Gut Microbes. 2010;1:432–435. [PMC free article: PMC3056111] [PubMed: 21468228]
- Subramoni S, Venturi V. LuxR-family ‘solos’: Bachelor sensors/regulators of signalling molecules. Microbiology. 2009;155:1377–1385. [PubMed: 19383698]
- Tang HB, DiMango E, Bryan R, Gambello M, Iglewski BH, Goldberg JB, Prince A. Contribution of specific Pseudomonas aeruginosa virulence factors to pathogenesis of pneumonia in a neonatal mouse model of infection. Infection and Immunity. 1996;64:37–43. [PMC free article: PMC173724] [PubMed: 8557368]
- Tomasz A. Control of the competent state in Pneumococcus by a hormone-like cell product: An example for a new type of regulatory mechanism in bacteria. Nature. 1965;208:155–159. [PubMed: 5884251]
- 44
Department of Microbiology, University of Washington School of Medicine.
- THE NEW SCIENCE OF SOCIOMICROBIOLOGY AND THE REALM OF SYNTHETIC AND SYSTEMS ECOL...THE NEW SCIENCE OF SOCIOMICROBIOLOGY AND THE REALM OF SYNTHETIC AND SYSTEMS ECOLOGY - The Science and Applications of Synthetic and Systems Biology
Your browsing activity is empty.
Activity recording is turned off.
See more...