NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Baron S, editor. Medical Microbiology. 4th edition. Galveston (TX): University of Texas Medical Branch at Galveston; 1996.
General Concepts
Campylobacter Jejuni and other Enteric Campylobacters
Clinical Manifestations
Campylobacter species cause acute gastroenteritis with diarrhea, abdominal pain, fever, nausea, and vomiting. Recently, Campylobacter infections have been identified as the most common antecedent to an acute neurological disease, the Guillain-Barré syndrome.
Structure
Campylobacter species are Gram-negative, microaerophilic, non-fermenting, motile rods with a single polar flagellum; they are oxidase-positive and grow optimally at 37° or 42°C.
Classification and Antigenic Types
Campylobacter species have many serogroups, based on lipopolysaccharide (O) and protein (H) antigens. However, only a few serogroups account for most human isolates in a given geographic region. C jejuni possesses several common surface-exposed antigens, including porin protein and flagellin.
Pathogenesis
The bacteria colonize the small and large intestines, causing inflammatory diarrhea with fever. Stools contain leukocytes and blood. The role of toxins in pathogenesis is unclear. C jejuni antigens that cross-react with one or more neural structures may be responsible for triggering the Guillian-Barre syndrome.
Host Defenses
Nonspecific defenses such as gastric acidity and intestinal transit time are important. Specific immunity, involving intestinal immunoglobulin (IgA) and systemic antibodies, develops. Persons deficient in humoral immunity develop severe and prolonged illnesses.
Epidemiology
C jejuni and C coli infections are endemic worldwide and hyperendemic in developing countries. Infants and young adults are most often infected. Disease incidence peaks in the summer. Domestic and wild animals are the reservoirs for the organisms. Outbreaks are associated with contaminated animal products or water.
Diagnosis
Observation of darting motility in fresh fecal specimens or of vibrio forms on Gram stain permit presumptive diagnosis; definitive diagnosis is established by stool culture, and occasionally by blood culture.
Control
Control depends on measures to prevent transmission from animal reservoirs to humans.
Helicobacter Pylori and other Gastric Helicobacter-like Organisms
Clinical Manifestations
Helicobacter pylori is associated with chronic superficial gastritis (stomach inflammation) and plays a role in the pathogenesis of peptic ulcer disease. Increasing evidence indicates that H pylori infection is important in causing gastric carcinoma and lymphoma. Acute infection may cause vomiting and upper gastrointestinal pain; hypochlorhydria and intense gastritis develop. Chronic infection usually is asymptomatic.
Structure
This Gram-negative curved or spiral rod is distinguished by multiple, sheathed flagellae and abundant urease.
Classification and Antigenic Types
The antigenic structures are not completely defined and no universal typing scheme has been developed; strains may be differentiated by genotypic methods including restriction endonuclease analysis, and polymerase chain reaction (PCR).
Pathogenesis
Helicobacter pylori is sheltered from gastric acidity in the mucus layer and a small proportion of cells adheres to the gastric epithelium. The microorganism does not appear to invade tissue. Production of urease, a vacuolating cytotoxin, and the cagA-encoded protein is associated with injury to the gastric epithelium.
Host Defenses
Local and systemic humoral immune responses are essentially universal, but are not able to clear infection.
Epidemiology
H pylori infection has a worldwide distribution; about 1/3 of the world's population is infected. The prevalence of infection increases with age. The major, if not exclusive, reservoir is humans but the exact modes of transmission are not known. H pylori has now been isolated from feces and dental plaque.
Diagnosis
Examination of gastric biopsy or stained smears allows presumptive diagnosis; definitive diagnosis is made by culture. Recently, non-invasive techniques such as the urea breath test and serologic tests have been developed to diagnose H pylori infection, with accuracy exceeding 95 percent.
Control
Several indications have emerged for the use of antimicrobial therapies that eradicate H pylori infection. No vaccine is yet available.
Other Pathogenic Camplyobacter and Helicobacter Species
Campylobacter fetus causes bacteremia in compromised hosts and self-limited diarrhea in previously healthy individuals. Helicobacter cinaedi and H fennelliae cause enteric and extraintestinal diseases and are more common in homosexual men and in travelers.
Introduction
Campylobacter and Helicobacter are Gram-negative microaerophilic bacteria that are widely distributed in the animal kingdom. They have been known as animal pathogens for nearly 100 years. However, because they are fastidious and slow-growing in culture, they have been recognized as human gastrointestinal pathogens only during the last 20 years. They can cause diarrheal illnesses, systemic infection, chronic superficial gastritis, peptic ulcer disease, and can lead to gastric carcinoma.
Table 23-1 lists the Campylobacter species known to be pathogenic for humans. Campylobacter jejuni, and, less often, C coli and C lari are the most common bacterial causes of acute diarrheal illnesses in developed countries. Helicobacter pylori (formerly known as Campylobacter pylori), which was first cultured from gastric biopsy tissues in 1982, causes chronic superficial gastritis and is associated with the pathogenesis of peptic ulcer disease and gastric cancer. Campylobacter fetus subspecies fetus occasionally causes systemic illnesses in compromised hosts.
Table 23-1
Campylobacter, Helicobacter and Related Species Associated With Clinical Manifestations of Human Infection.
Campylobacter Jejuni and other Enteric Campylobacters
Clinical Manifestations
The symptoms and signs of Campylobacter enteritis are not distinctive enough to differentiate it from illness caused by many other enteric pathogens. Symptoms range from mild gastrointestinal distress lasting 24 hours to a fulminating or relapsing colitis that mimics ulcerative colitis or Crohn's disease (Figure 23-1). The predominant symptoms experienced by individuals in developed countries are diarrhea, abdominal pain, fever, nausea, and vomiting. A history of grossly bloody stools is common, and many patients have at least one day with eight or more bowel movements; fecal leukocytes are usually present. A cholera-like illness with massive watery diarrhea may also occur. Campylobacter enteritis usually is self-limiting with gradual improvement in symptoms over several days. Most patients recover within a week, but 10%–20% experience relapse or a prolonged severe illness. Toxic megacolon, pseudomembranous colitis, and massive lower gastrointestinal hemorrhage also have been described. Mesenteric adenitis and appendicitis have been reported in children and young adults. Bacteremia is uncommon (<1%) in immunocompetent patients with C jejuni infection.
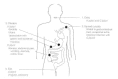
Figure 23-1
Pathogenesis of Campylobacter and Helicobacter infection in humans.
Among populations in developing countries, infection by C jejuni and closely related organisms is associated with much milder illness, without bloody diarrhea, fever or fecal leukocytes. Asymptomatic infection is much more common than in the developed countries, especially in older children and adults. However, when travelers from developed countries acquire C jejuni infections in developing countries, the symptoms are those associated with an inflammatory process. This indicates that organisms in the developing countries are fully pathogenic. Guillain-Barré syndrome is an uncommon consequence of C jejuni infection that is present 2–3 weeks after the diarrheal illness. However, because of the high incidence of Campylobacter infection, it has been estimated to be the trigger of 20 to 40 percent of all cases of Guillain-Barré syndrome.
Structure
Campylobacter jejuni, like all Campylobacter species, is a microaerophilic, non-fermentative Gram-negative organism. The name Campylobacter, meaning “;curved rod,” describes the appearance of the organisms (Figure 23-2). In young cultures, organisms are comma shaped, spiral, S shaped, or gull-winged shaped; as cultures age or are subjected to atmospheric or temperature stresses, round or coccoid forms appear.

Figure 23-2
Forty-eight-hour culture of C jejuni (originally King's “related vibrios”), showing typical thin, comma-, S-, or gull-winged shaped forms. In broth cultures, chained organisms may appear as elongated forms. All forms are Gram negative (more...)
C jejuni, which is structurally similar to other Gram-negative bacilli, is motile, with a single flagellum at one or both poles of the cell. The cell envelope has an inner bipolar lipid cell membrane, a thin peptidoglycan layer, an outer bipolar lipid layer with the lipid moiety of a lipopolysaccharide layer embedded in it, and the carbohydrate portion extending to the surface of the cell. Interspersed in the outer membrane layer are membrane proteins, some of which are exposed to the surface and are antigenic for infected hosts. Many Campylobacter species contain surface proteins that are external to the outer membrane. Campylobacter lipopolysaccharide has endotoxin activity similar to that of other Gram-negative bacteria.
All Campylobacter species except H pylori are similar in structure and appearance. The Campylobacter and Helicobacter species and subspecies may be differentiated by biochemical markers (Table 23-2).
Table 23-2
Differentiation of Campylobacter and Helicobacter Species Related to Human Disease.
Classification and Antigenic Types
Based on heat-labile antigens, at least 108 serogroups of both C jejuni and C coli have been described. In addition, 47 and 18 different heat-stable somatic (O) antigens have been described among isolates of C jejuni and C coli organisms, respectively. Although geographic differences in the prevalence of serogroups exist, 10 O-groups account for about 70% of human infections. Similarly, only a few serogroups account for most human isolates in any geographic region. Serotyping has been of value in numerous epidemiologic investigations.
Despite the antigenic diversity of these organisms, C jejuni possesses several common surface-exposed antigens which have been used for development of serological tests. Major antigens include the porin protein (Mr 45,000), flagellin (Mr 63,000), and a group of proteins around Mr 30,000 that appear important in adhesion. The flagellar proteins undergo antigenic phase variation.
Pathogenesis
As with other enteric pathogens, the attack rate of C jejuni varies with the ingested dose. In outbreaks of Campylobacter enteritis, the incubation period has ranged from 1–7 days, with most illness developing 2–4 days after infection. Infection leads to multiplication of organisms in the intestines. Patients shed 106 to 109 Campylobacter per gram of feces, concentrations similar to those shed in Salmonella and Shigella enteric infections. The sites of tissue injury include the small and large intestines, and the lesions show an acute exudative and hemorrhagic inflammation. Patients frequently have colonic involvement consisting of inflammation of the lamina propria with neutrophils, eosinophils, and mononuclear cells. Destruction of epithelial glands with crypt abscess formation occurs in severe cases (Figure 23-3). The pathologic lesions seen in Campylobacter colitis are difficult to distinguish from those in ulcerative colitis. Therefore, before ulcerative colitis can be diagnosed, infection by Campylobacter and related organisms should be ruled out.

Figure 23-3
Rectal biopsy from a patient with Campylobacter colitis. There is increased cellularity of the lamina propria with neutrophils, plasma cells, and eosinophils. Glandular epithelial cells are degenerated and thinned, with loss of goblet cells. A crypt abscess (more...)
The mechanisms by which C jejuni causes illness are uncertain. Cellular infiltration in colonic biopsy specimens of patients with Campylobacter infections and the occasional presence of bacteremia suggest that these organisms may be invasive. That most Campylobacter enteritis in developed countries is associated with fever and the presence of fecal leukocytes and blood in the stool also is consistent with the invasive characteristics of the organisms. C jejuni is invasive in vitro in chicken embryo cells and causes bacteremia in experimentally infected mice, rabbits, calves, chickens and monkeys.
Some C jejuni isolates elaborate very low levels of cytotoxins similar to Shiga toxin. Some isolates have been reported to elaborate an enterotoxin similar to cholera toxin. Enterotoxin production has been more frequently observed in isolates from developing countries, where infection by C jejuni has been associated with watery diarrhea. However, the clinical significance of the toxigenicity of these organisms is still unclear. Strains lacking detectable enterotoxin production and with low level in vitro cytotoxin production were fully virulent in volunteers. Campylobacter jejuni may adhere in vitro in several tissue culture lines. This may be important in intestinal colonization or may enhance tissue invasion. A superficial antigen (PEB1) that appears to be the major adhesin is conserved among C jejuni strains. However, the actual in vivo significance of adherence remains undefined.
Host Defenses
C jejuni and related organisms are capable of infecting healthy persons as well as immunocompromised patients. The minimal infection-causing dose of C jejuni is not known, although volunteers who have ingested as few as 800 organisms have become ill. Campylobacter jejuni can be killed by hydrochloric acid, suggesting that normal gastric acidity may be an important barrier against infection.
Neutrophils often are observed in the feces of patients infected with C jejuni, and colonic biopsy specimens from patients with Campylobacter colitis have shown marked infiltration with neutrophils, suggesting that these cells may be important in host defense. In mice, macrophages are important for clearance of bacteremia, and, in vitro, C jejuni antigens stimulate a T-cell response.
Acutely infected persons frequently develop elevated specific serum Immunoglobulin A (IgA), IgG, and IgM titers, which may persist for several weeks. Experimentally infected animals and humans manifest specific intestinal IgA production. Whether the antibody response eliminates the infection or protects against reinfection is not known. However, upon challenge with C jejuni, human volunteers with elevated specific serum IgA levels were likely to develop asymptomatic infection with only a brief duration of pathogen excretion. In contrast, hypogammaglobulinemic persons and those with acquired immune deficiency syndrome (AIDS) are at increased risk for severe, recurrent or bacteremic infections. C jejuni isolates are usually susceptible to complement-mediated killing by normal serum. Regardless of the exact host defense mechanisms involved, most C jejuni infections resolve spontaneously.
Epidemiology
In developed countries, C jejuni is an important cause of diarrhea, particularly in children and young adults (Figure 23-4). Between 3 and 14 percent of patients with diarrhea who seek medical attention are infected with C jejuni. Prolonged asymptomatic carriage is rare. The attack rate is highest in children less than 1 year old, and gradually decreases throughout childhood. A second peak occurs in young adults (18 to 29 years old). Although C jejuni enteritis occurs throughout the year, the highest isolation rates occur in summer, as with other enteric pathogens. In contrast, up to 40 percent of healthy children in developing countries may carry the organism at any time. This is an age-related phenomenon, with the highest excretion rates in very young children. Case-to-infection ratios decline with age, which probably is indicative of acquisition of immunity due to recurrent exposure.
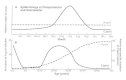
Figure 23-4
Comparison of the epidemiology of C jejuni(-----) and H pylori (--- ) by seasonal distribution by month (panel A) and by age (panel B) in the United States.
The ultimate reservoir for C jejuni is gastrointestinal tract of many wild animals, and a variety of domestic animals, including food animals (cattle, sheep, poultry, swine, and goats). More than 50 percent of poultry sold in the United States is contaminated with C jejuni. Transmission from food sources accounts for most human infections. Rodents and pets including dogs, cats, and birds also may transmit infection to humans, and excreta from wild animals may contaminate water supplies. Therefore, C jejuni infection may be transmitted via food, water, or direct contact with infected animals; in rare cases it may be transmitted from person-to-person.
Diagnosis
Campylobacter enteritis is hard to distinguish from enteritis caused by other pathogens. The presence of neutrophils or blood in the feces of patients with acute diarrheal illnesses is an important clue to Campylobacter infection. Darting motility in a fresh fecal specimen observed by dark-field or phase-contrast microscopy or characteristic vibrio forms visible after Gram staining permit a presumptive diagnosis. The diagnosis is confirmed by isolating the organism from a fecal culture or, rarely, from a blood culture (Figure 23-5). Because of its growth requirement for microaerobic atmosphere, special laboratory methods are needed to isolate C jejuni. Plating methods must be selective to inhibit the growth of competing microorganisms in the fecal flora. The traditional approach to isolating C jejuni has been to use media that contain antibiotics to which C jejuni is resistant but most members of the usual flora are susceptible. However, owing to their motility and small diameter, Campylobacter organisms have been isolated by filtration methods that do not use antibiotic-containing media. Use of filters (pore size 0.6μm) in conjunction with non-selective media improves stool culture yields of both C jejuni and the atypical enteric Campylobacters. Polymerase chain reaction (PCR)-based techniques have been developed for rapid detection, culture confirmation and for typing of C jejuni strains.
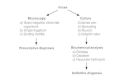
Figure 23-5
Detection of C jejuni and related enteric bacteria.
Because Campylobacter is microaerophilic, cultures must be incubated in an environment with reduced oxygen, optimally between 5 and 10 percent. The optimal temperature for growth is 42°C for C jejuni, and 37°C for many of the other enteric Campylobacters. When selective methods are used, suspicious colonies can be readily identified by their spreading character, mucoid appearance, and grayish color. The series of biochemical reactions outlined in Table 23-2 can differentiate the Campylobacter species. Serologic methods for diagnosis are only research tools at the present. A non-radioactive gene probe is available for rapid identification of C jejuni and C coli from isolated colonies.
Control
Control of Campylobacter enteritis depends largely on interrupting the transmission of the organism to humans from farm and domestic animals, food of animal origin, or contaminated water. Individuals can reduce the risk of Campylobacter infection by properly cooking and storing meat and dairy products, avoiding contaminated drinking water and unpasteurized milk, and washing their hands after contact with animals or animal products.
Fluid and electrolyte replacement are the cornerstones for treatment. Specific treatment with antimicrobial agents indicated for persons with severe or prolonged symptoms. However, for mild infections, the efficacy of treatment with antimicrobial agents has not yet been demonstrated. When treatment is required, erythromycin or ciprofloxacin appear to be the agents of choice. The presence of several surface-exposed, broadly specific proteins may permit vaccine development.
Other Pathogenic Campylobacter Species
Campylobacter fetus subsp fetus, well known as an animal pathogen, may cause bacteremia and other extraintestinal infections in compromised hosts, as well as an uncommon self-limited diarrheal illness in previously healthy persons. Recognized complications of C fetus infection include meningitis, endocarditis, pneumonia, thrombophlebitis, septicemia, arthritis, and peritonitis.
Virtually all C fetus isolates from humans possess lipopolysaccharide molecules with long polysaccharide side chains. Two major serogroups, A and B, have been identified. A microcapsule of high molecular-weight, antigenically related surface-array proteins has been associated with serum and phagocytosis resistance. These proteins apparently mediate serum resistance by inhibiting the binding of complement component C3b, thereby conferring to the organism a significant survival advantage. C fetus can undergo antigenic variation, switching the particular S-layer protein expressed.
Helicobacter Pylori and other Gastric Helicobacter-Like Organisms
Clinical Manifestations
Helicobacter pylori has repeatedly been shown to be associated with chronic superficial gastritis (CSG), which involves the antrum and the fundus of the stomach (Figure 23-1). Essentially all infected persons develop chronic superficial gastritis and it has clearly been shown worldwide that H pylori is the major cause of this lesion. Most of the patients with H pylori-induced chronic superficial gastritis are asymptomatic. The organisms are present on the luminal surface of mucus-secreting cells and within gastric pits, but do not invade tissue. Colonization of the affected areas of the gastric mucosa may be patchy (heavily colonized areas may be adjacent to those with no colonization). Organisms are generally not present over areas of intestinal metaplasia in the gastric mucosa. This CSG is nearly always present in patients with either gastric or duodenal ulcers. Essentially all patients with duodenal ulcers harbor H pylori in the duodenum. In duodenal ulcer disease, H pylori is associated with gastric metaplasia, but not with normal duodenal mucosa. The association of H pylori infection and gastric metaplasia is highly associated with active duodenitis.
H pylori causes the most common form of chronic gastritis (CSG), and chronic gastritis is a well known risk factor for the development of gastric carcinoma. The epidemiologic characteristics of H pylori infection are similar to those observed in the epidemiology of adenocarcinoma of the stomach. In addition, the development of intestinal metaplasia and atrophic gastritis, two risk factors for gastric cancer, are associated with H pylori infection. All these data and prospective epidemiologic studies indicate that infection of humans with H pylori is causally associated with the risk of developing gastric cancer. H pylori infection also is associated with risk of developing gastric lymphoma.
Structure
H pylori differs genetically from members of the genus Campylobacter, and has been reclassified from Campylobacter (where it was initially placed) to the separate genus Helicobacter. H pylori organisms are microaerophilic, nonsporulating, Gram-negative curved rods, 3.5 μm long and 0.5 to 1 μm wide, with a spiral periodicity in fresh cultures and spherical (coccoid) forms present in older cultures. H pylori further differs from Campylobacter species in having multiple polar sheathed flagellae (Figure 23-6), a unique composition of cell wall fatty acids, and a smooth surface. Most Campylobacter species contain either unipolar or bipolar single unsheathed flagellae and have a wrinkled surface. Unlike most campylobacters, H pylori produces urease and does not grow when incubated below 30°C. Growth is best on chocolate or blood agar plates after incubation for 2 to 5 days; for liquid media, either a blood or a hemin source appears essential.

Figure 23-6
Seventy-two hour culture of H pylori showing typical thin, comma- or S-shaped forms. All forms are Gram-negative and motile with multiple sheathed flagella. Old cultures may presented coccoid forms (X 1000) (Courtesy of Donna R. Murray, PhD.)
Classification and Antigenic Types
The antigenic nature of H pylori has not been completely defined. The whole-cell and outer-membrane profiles of all H pylori isolates have major similarities and are substantially different from those of C jejuni and C fetus. However, H pylori has strain-specific protein and lipopolysaccharide antigens, so it may be possible to type the organism. Simple systems for biotyping and serotyping H pylori are not yet available, but strains can be differentiated by genotypic-based techniques such as restriction endonuclease analysis, polymerase chain reaction, and restriction fragment length polymorphism (RFLP).
Pathogenesis
Helicobacter pylori are readily killed by brief exposure to hydrochloric acid solutions with pH below 4.0. This is paradoxical for an organism whose primary residence is the gastric lumen. However, several factors may explain this phenomenon. Firstly, H pylori lives in the mucus layer overlaying the gastric mucosa, a niche protected against gastric acid. This mucus is relatively thick and viscous and maintains a pH gradient from approximately pH 2 adjacent to the gastric lumen to pH 7.4 immediately above the epithelial cells. Secondly, H pylori is among the most efficient producers of urease. An important effect of this metabolic activity is the release of ammonia, which buffers acidity. Third, H pylori is highly motile even in very viscous mucus. This motility may allow organisms to migrate to the most favorable pH gradient. Finally, acute H pylori infection is associated with hypochlorhydria. H pylori only overlays gastric-type but not intestinal-type epithelial cells; a proportion of the bacterial cells are adherent.
Inflammatory infiltrates with polymorphonuclear leukocytes, eosinophils, and an increased number of lymphocytes are observed in the epithelium and lamina propria (Figure 23-7). The exact mechanism by which H pylori causes tissue injury is unknown. At present there is little evidence for direct tissue invasion by H pylori. For pathogenic organisms that do not invade tissue, the lesions are likely to reflect a response to extracellular products such as exotoxins. An 87kDa cytotoxin that induces vacuolation of eukaryotic cells is expressed in vitro by about 50% of strains. However, vacA, the gene encoding this toxin, is present in all strains but has substantial variability. Strains from patients with ulcers are more often toxin-producing than are strains from patients with gastritis only. H pylori also appears to affect the gastric mucus layer in which it resides. Isolates cultured in vitro produce an extracellular protease. This proteolytic activity affects the ability of mucus to retard diffusion of hydrogen ions. Mucus depletion over inflamed tissues is characteristic of H pylori-associated gastritis. Ammonia, produced by urease, is known to be toxic to eukaryotic cells and may potentiate mucosal injury. H pylori strains from patients with duodenal ulceration more frequently express a highly antigenic protein of 120–128kDa than H pylori strains from patients with gastritis. The gene encoding this protein, termed cagA, only is present in about 60% of H pylori strains.
Figure 23-7
Antral gastric biopsy from a patient with H pylori gastritis. There is increased cellularity of the epithelium and lamina propria with neutrophils, eosinophils, and lymphocytes (Hematoxylin and eosin stain, x 100.) (Courtesy of Donna R. Murray, PhD.) (more...)
Host Defenses
Although gastric acid plays an important role in protection against many enteric organisms, it is not a sufficient barrier to prevent colonization of the gastric mucosa by H pylori. Infected persons develop high titers of serum and local IgA and IgG antibodies to H pylori. Longitudinal serologic studies show that H pylori can persist for years or longer despite these high antibody levels. It is not known whether these specific antibodies play any protective role, such as inhibiting adherence or promoting opsonophagocytosis. The role of cell-mediated immunity to these persistent pathogens has only been explored in recent years. Activation of mononuclear cells by H pylori induces production of tumor necrosis factor α, Interleukin-1 and other cytokines. Differences among infected hosts in cell-mediated immunity are possible determinants of outcome variability.
Epidemiology
H pylori is found worldwide and affects persons from diverse socioeconomic strata. The prevalence of these infections, as documented by both histologic and serologic studies, rises with age, as does gastritis. Person-to-person transmission is the major, if not exclusive, source of infection. H pylori has been isolated from dental plaque, and DNA products may be detected in saliva by PCR. H pylori has been isolated from feces. These data indicate potential routes of transmission of H pylori. H pylori is frequently isolated from asymptomatic persons who have no dyspeptic or ulcer-related symptoms. H pylori infection is more common among populations from developing areas than in more industrialized countries. Moreover, high prevalence of infection has been observed among persons in settings where sanitary conditions are suboptimal suggesting that fecal-oral transmission occurs. Infection, defined by seropositivity, persists for years and possibly for life. The annual incidence of infection in adult populations in developed countries is approximately 1 percent. On occasion, transmission occurs from person to person via contaminated endoscopes.
Other gastric Helicobacter-like organisms have now been observed in a variety of animals, including rodents, primates, swine, and ferrets, but with the exception of primates and possibly cats, these isolates are clearly different from human isolates. Human exposure to non-human primates is not sufficiently frequent to explain the wide prevalence of H pylori infection in humans. Food-borne transmission would not be unusual for an enteric pathogen, but no other environmental reservoirs of H pylori have been identified.
Diagnosis
H pylori can be presumptively identified in freshly prepared gastric biopsy smears by phase-contrast microscopy, based on the characteristic motility of the microorganisms, and by staining histologic sections from gastric biopsies with Gram (carbol fuchsin counterstain), Warthin-Starry silver, Giemsa, or acridine orange stains. Organisms also can be seen directly in fixed tissue stained with hematoxylin and eosin. H pylori may be isolated from gastric tissue or from biopsies of esoageal or duodenal tissue containing gastric metaplasia (Figure 23-8) using nonselective media, such as chocolate agar, or antibiotic-containing selective media, such as those of Skirrow or Goodwin. Spiral organisms that are oxidase-, catalase-, and urease-positive can be identified as H pylori. Culture allows determination of antimicrobial susceptibilities. In gastric biopsies, H pylori also can be diagnosed presumptively, on the basis of the presence of preformed urease. DNA probe and PCR methodologies have been developed as well.

Figure 23-8
Detection methods for H pylori.
All of the above tests require endoscopy and biopsy. A non-invasive technique known as the urea breath test has been developed to diagnose H pylori infection. Infection can also be diagnosed accurately by detecting serum antibodies to H pylori antigens. These methods may be more sensitive than diagnostic methods involving biopsies. These non-invasive methods will greatly facilitate diagnosis in individual patients, aid studies of the epidemiology of this infection, and be useful for evaluation of the efficacy of antimicrobial therapy. A number of kits now are commercially available.
Control
Antimicrobial therapy for treatment of this infection has emerged as the most important means to resolve H pylori infection. Antimicrobial therapy is now one of the primary therapies for duodenal ulceration. Studies to identify the best combinations of antibiotics are being done. However, for most cases of H pylori-associated non-ulcer dyspepsia, data related to efficacy of antimicrobial therapy are not clear.
Other Pathogenic Helicobacter Species
Helicobacter cinaedi and Helicobacter fennelliae are two newly recognized Helicobacter species, formerly identified as Campylobacter species which have been associated with enteric and extraintestinal diseases; they are more common in homosexual men, and in travelers to developing countries.
References
- Blaser MJ, Parsonnet J. Parasitism by the “slow” bacterium Helicobacter pylori leads to altered gastric homeostasis and neoplasia. J Clin Invest. 1994;94:4–8. [PMC free article: PMC296275] [PubMed: 8040281]
- Dooley CP, Cohen H, Fitzgibbons PL. et al. Prevalence of Helicobacter pylori infection and histologic gastritis in asymptomatic persons. N Engl J Med. 1989;321:1562. [PubMed: 2586553]
- Goodwin CS, Armstrong JA, Chilvers T. et al. Transfer of Campylobacter pylori and Campylobacter mustelae to Helicobacter mustelae comb. nov. respectively. Int J Syst Bacteriol. 1989;39:397.
- Graham DY, Klein PD, Evans DJ Jr, et al.: Campylobacter pylori detected non-invasively by the 13C-urea breath test. Lancet i: 1174, 1988 . [PubMed: 2883491]
- Allos-Mishu B, Blaser MJ: Campylobacter jejuni and the expanding spectrum of related infections. Clin Infect Dis 20: in press, 1995 . [PubMed: 7619982]
- Penner JL. The genus Campylobacter: A decade of progress. Clin Microbiol Rev. 1988;1:157. [PMC free article: PMC358040] [PubMed: 3069194]
- Perez-Perez GI, Dworkin BM, Chodos JE. et al. Campylobacter pylori antibodies in humans. Ann Intern Med. 1988;109:11. [PubMed: 3288028]
- Nachamkin I, Blaser MJ, Tompkins LS, editors. Campylobacter jejuni - current strategy and future trends. Washington D.C.: American Society for Microbiology, 1992, p.3-296.
- Review The pathobiology of Campylobacter infections in humans.[Annu Rev Med. 1989]Review The pathobiology of Campylobacter infections in humans.Cover TL, Blaser MJ. Annu Rev Med. 1989; 40:269-85.
- The role of endotoxin in infection: Helicobacter pylori and Campylobacter jejuni.[Subcell Biochem. 2010]The role of endotoxin in infection: Helicobacter pylori and Campylobacter jejuni.Moran AP. Subcell Biochem. 2010; 53:209-40.
- [Campylobacter pylori in patients with gastroduodenal disease].[Gan To Kagaku Ryoho. 1990][Campylobacter pylori in patients with gastroduodenal disease].Fukuda Y, Yamamoto I, Inoue H, Mikami J, Tamura K, Satomi M, Shimoyama T. Gan To Kagaku Ryoho. 1990 Apr; 17(4 Pt 1):575-88.
- [Campylobacter pylori in patients with chronic gastritis and gastric and duodenal peptic ulcer].[Vutr Boles. 1989][Campylobacter pylori in patients with chronic gastritis and gastric and duodenal peptic ulcer].Kolarski V, Tsenova V, Shopova K, Vasileva E, Petrova D. Vutr Boles. 1989; 28(2):58-62.
- Review Campylobacter pylori: a newly recognized infectious agent in the gastrointestinal tract.[Am J Surg Pathol. 1988]Review Campylobacter pylori: a newly recognized infectious agent in the gastrointestinal tract.Yardley JH, Paull G. Am J Surg Pathol. 1988; 12 Suppl 1:89-99.
- Campylobacter and Helicobacter - Medical MicrobiologyCampylobacter and Helicobacter - Medical Microbiology
Your browsing activity is empty.
Activity recording is turned off.
See more...
