NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
Multidrug resistance, inherent or acquired, is a frequent characteristic of cancer cells and is difficult to predict and to manage. Multidrug resistance is caused by multiple mechanisms, including the dysfunctional metabolism of the lipid second messenger ceramide. The cytotoxic effect of various chemotherapeutics is decreased when the generation of ceramides is impaired, which results in the ineffectiveness of routine dosage and the need for higher, even more toxic drug levels. Needless to emphasize, this is a most undesirable situation; patients and oncologists would welcome its possible correction. Here we review ceramide metabolism with relationship to both blocking and potentiating the toxic response of cancer cells to chemotherapy. It is hoped that administering agents to target ceramide metabolism in combination with chemotherapy will improve response rates, especially in those patients with metastatic disease.
The link between ceramide metabolism and chemotherapy response was alluded to by earlier studies with anthracyclines. Shortly thereafter, the association between anthracycline resistance and accelerated ceramide metabolism through glucosylceramide synthase (GCS) was revealed. The signaling pathways activated by daunorubicin, including a SMase-initiated ceramide pathway, have been the subject of a recent review.1
The enzyme ceramide synthase, by way of the de novo pathway, was shown to mediate daunorubicin-induced apoptosis in leukemia cells.2 Exposing cells to drug-heightened ceramide synthase activity, promoting ceramide formation and increasing thepercentage of apoptotic cells. Introducing Fumonisin B1 to inhibit ceramide synthase negated the cytotoxic impact ofdaunorubicin, a result which clearly affirmed ceramide's role in anthracycline action. In a round of similar studies using human leukemia cell models, Jaffrezou et al3 found that daunorubicin likewise increased ceramide levels, albeit via SM hydrolysis and not ceramide synthase; nevertheless, apoptosis was the end result. Doxorubicin also promotes ceramide elevation, this work being conducted in MCF-7 breast cancer cells.4,5Moving into the realm of multidrug resistance (MDR) brought recognition to the enzyme catalyzing ceramide glycosylation, GCS, as an important facet of cellular response to chemotherapy.
Studies comparing chemotherapy-sensitive and chemotherapy-resistant cancer cells demonstrated a clear distinction in cerebroside content shown by an accumulation of a glycosylated form of ceramide, glucosylceramide, in the drug-resistant cells.6 This finding holds true for drug-resistant cancer cells derived from breast, ovary, melanoma,7 and prostate (author's unpublished observations), and recent studies have confirmed this in a multidrug-resistant cell line derived from HT29 human colon carcinoma.8
Investigations into the mechanism underlying the glucosylceramide surplus in chemotherapy-resistant cells revealed that exposure of wild-type cells to doxorubicin prompted an increase in ceramide and cell death attributable to apoptosis, whereas in chemotherapy-resistant cells treated in the same fashion, the ceramide generated was converted to glucosylceramide and the cytotoxic response was nil.4 This indicated that drug-resistant cells had an enhanced capacity for ceramide metabolism through the glycosylation pathway. An evaluation of ceramide toxicity showed wild-type cells susceptible to cell-permeable C6-ceramide, whereas drug-resistant cells were tolerant.9 Analysis of the metabolic fate of ceramide in these cells showed wild-type MCF-7 contained only free C6-ceramide whereas doxorubicin-resistant cells contained only high amounts of glucosyl-C6-ceramide. With this work, the importance of GCS in regulating cellular response to ceramide and perhaps to ceramide-generating drugs such as the anthracyclines, became apparent.
To more clearly establish the influence of ceramide metabolism on drug resistance, the GCS gene was introduced into MCF-7 cells using a retroviral tetracycline-on expression system.10 The cell line that was developed, “MCF-7/GCS,” expressed an 11-fold higher level of GCS activity in the expression-on mode, compared to the parent cell line, and demonstrated strong resistance to doxorubicin and to ceramide analogs.11 Ceramide resistance displayed by MCF-7/GCS cells paralleled the activity of the expressed GCS. In addition, resistance to TNF-α, which employs ceramide as a second messenger in the cell-killing response, was also a property of MCF-7/GCS cells.12 It was shown that TNF-α had little influence on the induction of apoptosis or on growth arrest in MCF-7/GCS cells, and lipid metabolism studies revealed that TNF-α promoted a ceramide increase in MCF-7 cells and a glucosylceramide increase in MCF-7/GCS cells. Further, TNF-α-induced caspase activity was halted as a result of GCS transfection. Doxorubicin and TNF-α resistance in MCF-7/GCS cells was related to hyperglycosylation of ceramide and not to shifts in the levels of P-gp, Bcl-2, or TNF receptor 1 expression.11,12
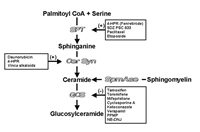
The role of ceramide metabolism in regulating cellular the response to chemotherapy has also been demonstrated employing a reverse tactic, by introducing GCS-antisense into doxorubicin-resistant cells. These studies showed that decreasing cellular glycosylation potential through GCS antisense transfection heightened chemotherapy sensitivity in doxorubicin-resistant cells, effectively reversing resistance.13 The antisense cell line displayed by RT-PCR, Western blot, and in vitro assays, decreased GCS mRNA, GCS protein, and GCS enzymatic activity. These cells were 28 times more sensitive to doxorubicin compared to the parent MCF-7-AdrR cell line. Under doxorubicin stress, GCS-antisense transfected cells displayed time- and dose-dependent increases in endogenous ceramide and dramatically higher levels of caspase activity, compared to control cells. GCS antisense transfection also heightened sensitivity to Vinca alkaloids and taxanes but had little impact on sensitivity to either 5-FU or cisplatin.14 Inasmuch as the data from gene transfection studies positions GCS as a force to overcome multidrug resistance in chemotherapy (Fig. 1), manipulation of ceramide metabolism by drug intervention can also provide a viable therapeutic avenue. Ceramide levels are readily enhanced by the introduction of ceramide generating agents alone or in conjunction with inhibitors of ceramide metabolism ( Fig. 2).

Figure 1
Shifting cellular chemotherapy tolerance through GCS gene transfection.
A number of classical P-glycoprotein substrates influence ceramide metabolism by hindering its conversion to glucosylceramide. Tamoxifen, used in the Dartmouth regimen for treatment of melanoma,15 evaluated for treatment of pancreatic carcinoma and malignant gliomas,16,17 and employed in a biochemotherapy regimen for patients with metastatic melanoma,18 inhibits glucosylceramide synthesis in vinblastine-resistant carcinoma.19 In doxorubicin-resistant cells, clinically relevant concentrations of tamoxifen, verapamil, and cyclosporin A, markedly decrease glucosylceramide levels with IC50 values of 1.0, 0.8, and 2.3 mM, respectively.20 Toremifene, an anti-estrogen with chemistry and pharmacology similar to tamoxifen, is equally effective.9 These studies suggest that the P-glycoprotein-targeted agents act not only by blocking cellular drug efflux but also through enhancing ceramide levels. RU486 (mifepristone) inhibits growth and induces apoptosis in MCF-7 cells,21 and retards ceramide glycosylation in MCF-7-doxorubicin-resistant cells.9 Ketoconazole overcomes resistance to doxorubicin and vinblastine in cancer cells,22 displays activity in advanced prostate cancer patients,23 and it inhibits glucosylceramide synthesis (author's unpublished observations). Studies with tamoxifen analogs corroborate the idea that MDR reversal and inhibition of cellular glucosylceramide synthesis are allied. For example, exposure of doxorubicin-resistant cells to triphenylethylene, the tamoxifen nucleus minus the dimethylethanolamine moiety, neither inhibits ceramide glycosylation nor sensitizes cells to doxorubicin, but inclusion of tamoxifen with doxorubicin decreases glucosylceramide production, enhances ceramide generation, and sensitizes cells.20,24 Similarly, cis-tamoxifen is devoid of chemosensitizing activity, indicating a stereochemical requirement.
Studies using chemical inhibitors of GCS further highlight a relationship between ceramide metabolism and chemotherapy efficacy. PPMP (1-phenyl-2-palmitoylamino-3-morpholino-1-propanol), a commercially available inhibitor of ceramide glycosylation,25 sensitizes breast cancer cells to doxorubicin,20 and neuroblastoma to Taxol and vincristine.26 These enzyme inhibitors are structural analogs of the natural GCS substrate. A chemical cousin, PPPP (1-phenyl-2 palmitoylamino-3-pyrrolidino-1-propanol) completely blocks glucosylceramide synthesis in vincristine-resistant leukemia and in combination, enhances vincristine-induced cytotoxicity,27 and pretreatment of melanoma cells with PPPP markedly reduces tumor formation and metastatic potential in mice.28 In metastatic human colon cancer, raising the levels of ceramide by direct administration of ceramide analogs and ceramidase inhibitors induces apoptotic cell death, preventing tumor growth.29 Preferential killing of drug-resistant epidermoid carcinoma cells over drug-sensitive counterparts upon exposure to PDMP and PPPP has also been shown.30 A reduction in glucosylceramide levels accompanied PDMP-induced apoptosis, prompting the authors to suggest that manipulation of glucosylceramide levels is an avenue for preferential destruction of drug-resistant cancer cells. Spinedi et al31 showed that PDMP suppresses glucosylceramide synthesis and potentiates the apoptotic effect of C6-ceramide in neuroepithelioma, suggesting that glucosylceramide synthesis is a mechanism to escape ceramide-governed apoptosis. Similarly, by targeting ceramide metabolism, Sietsma et al26 showed that PDMP increases neuroblastoma sensitivity to chemotherapy, and that the increase involves a sustained elevation of ceramide.
Work with SDZ PSC 833 ([3'-keto-bmt1]-[val-2]-cyclosporine), signaled an important step forward in demonstrating both the cytotoxic principles of ceramide and the impact of manipulating the ceramide pathway for therapeutic purposes. SDZ PSC 833 is a second generation MDR modulator that interferes with P-glycoprotein-mediated drug efflux.32 Our group first reported that SDZ PSC 833 alone strongly activates cellular ceramide formation, and that the increase in ceramide was mirrored by a progressive decline in cell survival.33 Cells having an enhanced capacity for ceramide glycosylation were more resistant to SDZ PSC 833, as opposed to drug-sensitive cells.6,24 Lipid metabolism studies revealed that SDZ PSC 833 resistance was allied with rapid conversion of ceramide to glucosylceramide.24 Studies with P-glycoprotein negative cells and with mdr-1-transfected cells showed that SDZ PSC 833 elicits ceramide generation independently of P-glycoprotein.34 In other studies it was revealed that drug sensitivity in leukemia could be enhanced by SDZ PSC 833 through perturbations in SM-ceramide pathways and not by modified drug efflux parameters.35
Tilly and colleagues, using an inhibitor of ceramide-induced cell death, showed that cell destruction by chemotherapy was preventable if ceramide metabolism was manipulated.36 Numerous studies now clearly demonstrate that co-administration of agents that target ceramide metabolism enhances the cytotoxic impact of chemotherapy. For example, vinblastine toxicity is intensified by SDZ PSC 833 in P-glycoprotein-poor cells, and both drugs at low dose promote ceramide formation.37 Doxorubicin and tamoxifen synergize to increase ceramide formation, complementing cytotoxicity.5,20 Another example of enhanced drug efficacy through ceramide targeting is shown by the influence of RU486 on doxorubicin.5,9 A three-component regimen consisting of doxorubicin and two agents that enhance ceramide formation, tamoxifen and SDZ PSC 833, can bring cell viability to zero.24 Suramin, a polysulfonated naphthylurea, disrupts glycolipid metabolism and elicits apoptosis through ceramide pathways in a number of cancer cell lines.38 In Bcl2-transfected prostate cancer cells that are resistant to doxorubicin, a doxorubicin/suramin combination was shown effective in circumventing resistance.39 Suramin is currently in clinical trials for treatment of breast and prostate cancer.40–42 Aberrant ceramide generation is also involved in prostate cancer cell resistance to chemotherapy,43 and it was recently shown that ceramidase is overexpressed in prostate cancer.44 These findings justify exploring a ceramide-targeted approach for the treatment of prostate cancer.
More evidence that the ceramide pathway can be manipulated for therapeutic purposes stems from work with the novel synthetic retinoid, N-(4-hydroxyphenyl) retinamide (4-HPR). 4-HPR has been shown to significantly increase the levels of ceramide and induce mixed apoptosis/necrosis in highly drug-resistant human neuroblastoma cell lines.45 The cytotoxicity of 4-HPR can be enhanced by adding modulators of ceramide metabolism,46 such as L-threo-dihydrosphingosine (safingol), a sphingosine analog and protein kinase-C inhibitor,47 and PPMP and tamoxifen. Safingol has been shown to be synergistic with 4-HPR and produces a 100- to 10,000-fold increase in cytotoxicity, compared to 4-HPR alone, in neuroblastoma, lung, melanoma, prostate, colon, and pancreatic cancer cell lines.46The safingol/4-HPR combination is minimally toxic to fibroblasts and bone marrow myeloid progenitor cells.
The involvement of ceramide metabolism in cancer cell response to chemotherapy is far-reaching. Exogenous addition of ceramide enhances Taxol-induced apoptosis in leukemic T cells.48 Ceramide also enhances Taxol-induced apoptosis in head and neck squamous cell carcinoma, suggesting that a Taxol/ceramide combination therapy may be a promising alternative to conventional treatment of head and neck cancers.49 Research in breast cancer shows that Taxol elicits de novo ceramide generation and apoptosis; however, addition of ceramide synthesis inhibitors, such as L-cycloserine, checks Taxol-induced apoptosis.50 These data imply that ceramide is obligate for Taxol-elicited cytotoxicity. The theme on the interplay of ceramide with chemotherapy response continues to expand. Recent reviews by Norman Radin caption this tract.51,52
Many studies now indicate that, in addition to SMase, de novo enzymes of ceramide synthesis are also viable targets for chemotherapeutic agents. Etoposide regulates serine palmitoyltransferase, the rate-limiting enzyme in de novo ceramide synthesis.53 In human neuroblastoma, 4-HPR elevates ceramide via coordinate activation of serine palmitoyltransferase and ceramide synthase.54
Controlling ceramide metabolism is an attractive approach to cancer treatment. The majority of drugs that would be used are already available in the clinic, expediting the application of principle to practice. Ceramide metabolism can also be impacted by gene therapy. This has been illustrated through the application of antisense GCS, which is effective in the complete reversal of doxorubicin resistance in a breast cancer cell model.14
References
- 1.
- Laurent G, Jaffrézou J -P. Signaling pathways activated by daunorubicin. Blood. 2001;98:913–924. [PubMed: 11493433]
- 2.
- Bose R, Verheij M, Haimovitz-Friedman A. et al. Ceramide synthase mediates daunorubicin-induced apoptosis: an alternative mechanism for generating death signals. Cell. 1995;82:405–414. [PubMed: 7634330]
- 3.
- Jaffrezou JP, Levade T, Bettaieb A. et al. Daunorubicin-induced apoptosis: triggering of ceramide generation through sphingomyelin hydrolysis. EMBO J. 1996;15:2417–2424. [PMC free article: PMC450173] [PubMed: 8665849]
- 4.
- Cabot MC, Giuliano AE. Apoptosis-a cell mechanism important for cytotoxic response to adriamycin and a lipid metabolic pathway that facilitates escape. Breast Cancer Res Treat. 1997;47:71.
- 5.
- Lucci A, Han TY, Liu YY. et al. Modification of ceramide metabolism increases cancer cell sensitivity to cytotoxics. Int J Oncol. 1999;15:541–546. [PubMed: 10427137]
- 6.
- Lavie Y, Cao H, Bursten SL. et al. Accumulation of glucosylceramides in multidrug-resistant cancer cells. J Biol Chem. 1996;271:19530–19536. [PubMed: 8702646]
- 7.
- Lucci A, Cho WI, Han TY. et al. Glucosylceramide: a marker for multiple-drug resistant cancers. Anticancer Res. 1998;18:475–480. [PubMed: 9568165]
- 8.
- Kok JW, Veldman RJ, Klappe K. et al. Differential expression of sphingolipids in MRP1 overexpressing HT29 cells. Int J Cancer. 2000;87:172–178. [PubMed: 10861470]
- 9.
- Lucci A, Giuliano AE, Han TY. et al. Ceramide toxicity and metabolism differ in wild-type and multidrug-resistant cancer cells. Int J Oncol. 1999;15:535–540. [PubMed: 10427136]
- 10.
- Liu YY, Cabot MC. Development of a mammalian tet-on expression cell line: glucosylceramide synthase regulates TNF-α-induced apoptosis In: DeLey M, ed. Human Cytokine Protocols in Methods in Molecular Biology Totowa: Humana Press, Inc., 2002. :in press . [PubMed: 14573900]
- 11.
- Liu YY, Han TY, Giuliano AE. et al. Expression of glucosylceramide synthase, converting ceramide to glucosylceramide, confers adriamycin resistance in human breast cancer cells. J Biol Chem. 1999;274:1140–1146. [PubMed: 9873062]
- 12.
- Liu YY, Han TY, Giuliano AE. et al. Glycosylation of ceramide potentiates cellular resistance to tumor necrosis factor-alpha-induced apoptosis. Exp Cell Res. 1999;252:464–470. [PubMed: 10527636]
- 13.
- Liu YY, Han TY, Giuliano AE. et al. Uncoupling ceramide glycosylation by transfection of glucosylceramide synthase antisense reverses adriamycin resistance. J Biol Chem. 2000;275:7138–7143. [PubMed: 10702281]
- 14.
- Liu Y -Y, Han T -Y, Giuliano AE. et al. Ceramide glycosylation potentiates cellular multidrug resistance. FASEB J. 2001;15:719–730. [PubMed: 11259390]
- 15.
- Del Prete SA, Maurer LH, O'Donnell J. et al. Combination chemotherapy with cisplatin, carmustine, dacarbazine, and tamoxifen in metastatic melanoma. Cancer Treat Rep. 1984;68:1403–1405. [PubMed: 6541973]
- 16.
- Gelmann EP. Tamoxifen for the treatment of malignancies other than breast and endometrial carcinoma. Semin Oncol. 1997;24(1 Suppl 1):S1-65–S1-70. [PubMed: 9045318]
- 17.
- Couldwell WT, Weiss MH, DeGiorgio CM. et al. Clinical and radiographic response in a minority of patients with recurrent malignant gliomas treated with high-dose tamoxifen Neurosurgery 199332485–489. discussion 489-490. [PubMed: 8384328]
- 18.
- O'Day SJ, Boasberg PD, Kristejda TS. et al. High-dose tamoxifen added to concurrent biochemotherapy with decrescendo interleukin-2 in patients with metastatic melanoma. Cancer. 2001;92:609–619. [PubMed: 11505406]
- 19.
- Cabot MC, Giuliano AE, Volner A. et al. Tamoxifen retards glycosphingolipid metabolism in human cancer cells. FEBS Lett. 1996;394:129–131. [PubMed: 8843149]
- 20.
- Lavie Y, Cao HT, Volner A. et al. Agents that reverse multidrug resistance, tamoxifen, verapamil, and cyclosporin A, block glycosphingolipid metabolism by inhibiting ceramide glycosylation in human cancer cells. J Biol Chem. 1997;272:1682–1687. [PubMed: 8999846]
- 21.
- El Etreby MF, Liang Y, Wrenn RW. et al. Additive effect of mifepristone and tamoxifen on apoptotic pathways in MCF-7 human breast cancer cells. Breast Cancer Res Treat. 1998;51:149–168. [PubMed: 9879777]
- 22.
- Siegsmund MJ, Cardarelli C, Aksentijevich I. et al. Ketoconazole effectively reverses multidrug resistance in highly resistant KB cells. J Urol. 1994;151:485–491. [PubMed: 7904313]
- 23.
- Small EJ, Baron AD, Fippin L. et al. Ketoconazole retains activity in advanced prostate cancer patients with progression despite flutamide withdrawal. J Urol. 1997;157:1204–1207. [PubMed: 9120902]
- 24.
- Lucci A, Han TY, Liu YY. et al. Multidrug resistance modulators and doxorubicin synergize to elevate ceramide levels and elicit apoptosis in drug-resistant cancer cells. Cancer. 1999;86:300–311. [PubMed: 10421266]
- 25.
- Abe A, Inokuchi J, Jimbo M. et al. Improved inhibitors of glucosylceramide synthase. J Biochem (Tokyo). 1992;111:191–196. [PubMed: 1533217]
- 26.
- Sietsma H, Veldman RJ, Kolk D. et al. 1-phenyl-2-decanoylamino-3-morpholino-1-propanol chemosensitizes neuroblastoma cells for taxol and vincristine. Clin Cancer Res. 2000;6:942–948. [PubMed: 10741719]
- 27.
- Olshefski RS, Ladisch S. Glucosylceramide synthase inhibition enhances vincristine-induced cytotoxicity. Int J Cancer. 2001;93:131–138. [PubMed: 11391632]
- 28.
- Deng W, Li R, Ladisch S. Influence of cellular ganglioside depletion on tumor formation. J Natl Cancer Inst. 2000;92:912–917. [PubMed: 10841826]
- 29.
- Selzner M, Bielawska A, Morse MA. et al. Induction of apoptotic cell death and prevention of tumor growth by ceramide analogues in metastatic human colon cancer. Cancer Res. 2001;61:1233–1240. [PubMed: 11221856]
- 30.
- Nicholson KM, Quinn DM, Kellett GL. et al. Preferential killing of multidrug-resistant KB cells by inhibitors of glucosylceramide synthase. Br J Cancer. 1999;81:423–430. [PMC free article: PMC2362922] [PubMed: 10507766]
- 31.
- Spinedi A, Bartolomeo SD, Piacentini M. Apoptosis induced by N-hexanoylsphingosine in CHP-100 cells associates with accumulation of endogenous ceramide and is potentiated by inhibition of glucocerebroside synthesis. Cell Death Differ. 1998;5:785–791. [PubMed: 10200538]
- 32.
- Archinal-Mattheis A, Rzepka RW, Watanabe T. et al. Analysis of the interactions of SDZ PSC 833 ([3'-keto-Bmtl-Val2]-cyclosporine), a multidrug resistance modulator, with P-glycoprotein. Oncol Res. 1995;7:603–610. [PubMed: 8704277]
- 33.
- Cabot MC, Giuliano AE, Volner A. et al. Tamoxifen retards glycosphingolipid metabolism in human cancer cells. FEBS Lett. 1996;394:129–131. [PubMed: 8843149]
- 34.
- Goulding CW, Giuliano AE, Cabot MC. SDZ PSC 833 the drug resistance modulator activates cellular ceramide formation by a pathway independent of P-glycoprotein. Cancer Lett. 2000;149:143–151. [PubMed: 10737718]
- 35.
- Pallis M, Russell N. P-glycoprotein plays a drug-efflux-independent role in augmenting cell survival in acute myeloblastic leukemia and is associated with modulation of a sphingomyelin-ceramide apoptotic pathway. Blood. 2000;95:2897–2904. [PubMed: 10779437]
- 36.
- Perez GI, Knudson CM, Leykin L. et al. Apoptosis-associatedsignaling pathways are required for chemotherapy-mediated femalegerm cell destruction. Nat Med. 1997;3:1228–1232. [PubMed: 9359697]
- 37.
- Cabot MC, Giuliano AE, Han T -Y. et al. SDZ PSC 833, the cyclosporine A analogue and multidrug resistance modulator, activates ceramide synthesis and increases vinblastine sensitivity in drug-sensitive and drug-resistant cancer cells. Cancer Res. 1999;59:880–885. [PubMed: 10029079]
- 38.
- Gill JS, Windebank AJ. Role of ceramide in suramin-induced cancer cell death. Cancer Lett. 1997;119:169–176. [PubMed: 9570368]
- 39.
- Tu SM, McConnell K, Marin MC. et al. Combination adriamycin and suramin induces apoptosis in bcl-2 expressing prostate carcinoma cells Cancer Lett 199593147–155. Erratum in Cancer Lett 1996; 99:247. [PubMed: 7621422]
- 40.
- Lawrence JB, Conover CA, Haddad TC. et al. Evaluation of continuous infusion suramin in metastatic breast cancer: impact on plasma levels of insulin-line growth factors (IGFs) and IGF-binding proteins. Clin Cancer Res. 1997;3:1713–1720. [PubMed: 9815555]
- 41.
- Beedassy A, Cardi G. Chemotherapy in advanced prostate cancer. Semin Oncol. 1999;26:428–438. [PubMed: 10482185]
- 42.
- Smith DC. Chemotherapy for hormone refractory prostate cancer. Urol Clin North Am. 1999;26:323–331. [PubMed: 10361555]
- 43.
- Wang XZ, Beebe JR, Pwiti L. et al. Aberrant sphingolipid signaling is involved in the resistance of prostate cancer cell lines to chemotherapy. Cancer Res. 1999;59:5842–5848. [PubMed: 10582708]
- 44.
- Seelan RS, Qian C, Yokomizo A. et al. Human acid ceramidase is overexpressed but not mutated in prostate cancer. Genes Chromosomes Cancer. 2000;29:137–146. [PubMed: 10959093]
- 45.
- Maurer BJ, Metelitsa LS, Seeger RC. et al. Increase of ceramide and induction of mixed apoptosis/necrosis by N-(4-hydroxyphenyl)retinamide in neuroblastoma cell lines. J Natl Cancer Inst. 1999;91:1138–1146. [PubMed: 10393722]
- 46.
- Maurer BJ, Melton L, Billups C. et al. Synergistic cytotoxicity in solid tumor cell lines between N-(4-hydroxyphenyl)retinamide and modulators of ceramide metabolism. J Natl Cancer Inst. 2000;92:1897–1909. [PubMed: 11106681]
- 47.
- Schwartz GK, Ward D, Saltz L. et al. A pilot clinical/pharmacological study of the protein kinase C-specific inhibitor safingol alone and in combination with doxorubicin. Clin Cancer Res. 1997;3:537–543. [PubMed: 9815717]
- 48.
- Myrick D, Blackinton D, Klostergaard J. et al. Paclitaxel-induced apoptosis in Jurkat, a leukemic T cell line, is enhanced by ceramide. Leuk Res. 1999;23:569–578. [PubMed: 10374850]
- 49.
- Mehta S, Blackinton D, Omar I. et al. Combined cytotoxic action of paclitaxel and ceramide against the human Tu138 head and neck squamous carcinoma cell line. Cancer Chemother Pharmacol. 2000;46:85–92. [PubMed: 10972477]
- 50.
- Charles AG, Han T -Y, Liu YY. et al. Taxol-induced ceramide generation and apoptosis in human breast cancer cells. Cancer Chemother Pharmacol. 2001;47:444–450. [PubMed: 11391861]
- 51.
- Radin NS. Killing cancer cells by poly-drug elevation of ceramide levelsa hypothesis whose time has come? Eur J Biochem. 2001;268:193–204. [PubMed: 11168352]
- 52.
- Radin NS. Apoptotic death by ceramide: will the real killer please stand up? Med Hypotheses. 2001;57:96–100. [PubMed: 11421634]
- 53.
- Perry DK, Carton J, Shah AK. et al. Serine palmitoyltransferase regulates de novo ceramide generation during etoposide-induced apoptosis. J Biol Chem. 2000;275:9078–9084. [PubMed: 10722759]
- 54.
- Wang H, Maurer BJ, Reynolds CP. et al. N-(4-Hydroxyphenyl)retinamide elevates ceramide in neuroblastoma cell lines by coordinate activation of serine palmitoyltransferase and ceramide synthase. Cancer Res. 2001;61:5102–5105. [PubMed: 11431347]
- Ceramide Glycosylation and Chemotherapy Resistance - Madame Curie Bioscience Dat...Ceramide Glycosylation and Chemotherapy Resistance - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...