NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
Introduction
In D. melanogaster, a well-characterized cascade of genes, called the ‘sex determination cascade,’ is known to control somatic sex determination and differentiation.1-4 The primary genetic signal for sex determination is the ratio of X-chromosomes to sets of autosomes. A ratio of 1.0 leads to female, and a ratio of 0.5, to male development. This is because the master switch gene, Sex-lethal (Sxl), can be activated only when the ratio of X chromosomes to sets of autosomes exceeds 1.0. The SXL protein acts as a splicing factor on the RNA produced by the tra gene, resulting in the production of the active TRA protein in females. TRA and TRA-2 determine the female-specific splicing of the dsx RNA to produce the female-specific DSX protein (DSXF). In males, all these regulatory decisions do not occur, and, by default, the male-specific product of the dsx (DSXM) is produced. The DSXF and DSXM proteins regulate the sex-specific transcription of target genes that encode the sexual phenotype of the body.
The regulation of yolk protein gene expression is by far the best-characterized DSX function at the molecular level. DSXM and DSXF bind directly in vitro5 and in vivo6 to several specific sites in an enhancer sequence designated as the fat body enhancer (FBE), which plays a role in directing the adult female fat body-specific transcription characteristic of the adjacent yolk protein genes, yp-1 and yp-2.7 A zinc finger-like domain in the common amino-terminal region of the DSX polypeptides is responsible for this sequence-specific DNA-binding activity. 8 Binding of DSXM to the FBE represses the expression of the yolk protein genes dramatically, whereas binding of DSXF to the same sequences cooperates with other factors to activate transcription through binding to minimal yolk protein enhancer constructs in transgenic flies.9,10 The DSX polypeptides can thus function in this case as either activators or repressors.
In the silkworm, Bombyx mori, the chromosomal sex determination mechanism is distinct from that of D. melanogaster, with the female (ZW) being the heterogametic sex and the male (ZZ), the homogametic sex. In D. melanogaster, the X: A ratio, a balance mechanism in which X chromosomal gene products are titrated against autosomal gene products, governs sex determination. 11,12 On the other hand, it has been shown genetically that female sex in B. mori is determined by the presence of a dominant feminizing factor on the W chromosome.13 Despite such a difference, we have recently identified a dsx homologue from B. mori. It has been found to be sex-specifically expressed in various tissues at the larval, pupal, and adult stages in the silkworm.14 The primary transcript of the Bmdsx gene is alternatively spliced in males and females to yield sex-specific mRNAs that encode male-specific (BmDSXM) and female-specific (BmDSXF) polypeptides.14,15 However, since no information is to date available about the biological function of BmDSX proteins, the question remains open as to whether the Bmdsx gene is used as a final regulatory gene in the sex-determination cascade, even if the primary sex-determination signal is different.
To confirm whether or not Bmdsx acts as a final regulatory gene in the sex-determination cascade of B. mori in the same way as Drosophila doublesex, the regulation of the vitellogenin and pheromone-binding protein (PBP) genes provides an ideal system because these genes are expressed sex-specifically in a common tissue to both sexes, as mentioned below. In B. mori, vitellogenin is synthesized by the fat body cells of females at about larval-pupal ecdysis and released into the hemolymph before being taken up by the developing oocytes.16,17 The biosynthesis of vitellogenin is regulated transcriptionally in a female-dependent manner in the fat body.18 In the male moth's antennae, PBP conveys a sex-attractant pheromone released by females to a membrane-bound receptor on a nerve cell.19 The PBP is predominantly expressed in male antennae and localized in pheromone-sensitive sensilla.19,20
As a first step to learn about the role of Bmdsx in sexual differentiation, we introduced chimeric genes expressing either female-specific or male-specific Bmdsx cDNAs under the control of the Drosophila hsp70 heat-shock promoter into the Bombyx genome according to the protocol developed by Tamura.21 We observed the phenotypic consequences of BmDSXM or BmDSXF expression on male and female differentiation.22 Moreover, we investigated the effects of the transgenic expression of BmDSXM or BmDSXF on the regulation of the vitellogenin and PBP genes that are expressed sex-specifically in a common tissue to both sexes.22,29 Moreover, the experiments described below supply compelling evidence that a product of the Bmdsx gene is a direct link between the sex-determining cascade of B. mori and the sex-specific enhancer of a particular target gene. Here, we present the first direct evidence that a doublesex homologue plays an essential role in sexual development in lepidopteran insects.
Generation of Hs:BmdsxM Transformants22
To study the role of Bmdsx in sexual differentiation, the piggyBac vector pBac[3xP3-EGFPaf ]23-25 containing a Bmdsx male cDNA under the control of a Drosophila hsp70 promoter was coinjected with a piggyBac transposase plasmid pHA3PIG21 into eggs at the preblastodermal stage. From 443 eggs injected with the pBac[hspBmdsxM] plasmid, 225 larvae survived to the first larval stage (Table 1). After sibling mating, eleven of the G0 mating yielded progeny with EGFP eye fluorescence (Table 1). The yield of mating in G0 adults with transformed gametes was thus 12.5 % (Table 1). In the GFP-positive G1 broods, on average, 6.0 % of the larvae displayed fluorescence (Table 1). Sexing the G1 GFP-positive larvae at the fifth larval stage showed that all of these were normal females and males in roughly equal proportion (Table 1). Sublines were generated by single matings of each transformant with the parental pnd-w1 strain. Each line carried only one insert, and each insert was shown to be independent by Southern blot analysis probed with the EGFP-encoding sequence (data not shown).
Table 1
Results of injection of the construct DNAs in pnd-w1 embryos (modified from ref. , with permission).
Effects of the Expression of Hs:BmdsxM in Females on Genital Morphology22
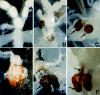
Four lines carrying the hs:BmdsxM (hsp70 promoter:Bmdsx male cDNA) construct (lines A, B, C, and D) were produced. All the transformant lines exhibited morphological abnormalities in the female external genitalia, as shown in Figure 1. While normal females have an almost degenerated 8th abdominal segment with no scaly hairs, transgenic females had well-developed 8th tergites with scaly hairs (fig.1). Variable expressivities of the transgenes were indicated by different degrees of development of the 8th tergite and the density of scaly hairs formed on the tergite. In addition to this abnormal phenotype, some chitinous structures were seen in all transformant lines. The shape of the chitinous structures was similar to that of a male uncus or clasper. Normal females have a pheromone gland that is represented as a pair of eversible, ventrolateral sacs (sacculi laterales) formed between the 8th and 9th abdominal segments. Different from those in the normal female, the pheromone glands seen in line D were atrophied. More severe effects on the phenotype of the pheromone gland were observed in lines A, B, and C. Transgenic females in these lines had no distinct pheromone glands.
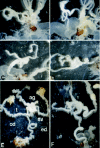
Next, we observed the internal genital organs of the transgenic females. Several abnormal structures were seen in all transformant lines. First, some chitinous structures existed at the inner side of the external genitalia (fig.2). There was a notable difference in the extent of development of the chitinous structures shown by these transformant lines. Transgenic females in line A had one or few spherical chitinous structures (fig.2B). Such abnormal structures observed in line B were more developed and displayed more complex shapes than those seen in line A (fig.2C). The chitinous structures observed in lines C and D showed the greatest extent of complexity and displayed a U-shaped structure (fig.2E,F). These abnormal structures seemed to correspond to the male-specific uncus and clasper complex when compared with male genitalia. Occasionally, needle-shaped structures resembling a male penis appeared together with the U-shaped chitinous structures (fig.2F).
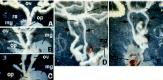
Second, abnormal organs that developed from the region surrounding the base of ovipositors were present in the transgenic females (fig.3). Transgenic females in line A had an abnormal organ showing a tubulous structure (fig.3A). Such abnormal organs as those observed in line B displayed a more complicated structure than those seen in line A (fig.3B). The abnormal organs observed in lines C and D showed the greatest extent of complexity (fig.3C,D). A comparison of the genitalia between normal males and the transgenic females verified that the abnormal organs seen in lines C and D were similar to a male internal genital complex comprising a spermiduct, an ejaculatory duct, a seminal vesicle, and an accessory gland (fig.3F).
Third, in the transgenic females, tubular structures branched out from the oviducts (fig.4). There was a strong correlation between the severity of the abnormal organ phenotype and the extent of development of the tubular structure. In lines A, B, and C, this tubular structure was occasionally connected to the abnormal organ developed from the base of the ovipositors. The frequency of such a connection was increased in proportion to the extent of complexity of the abnormal organ phenotype. In line D, all the tubular structures were fully connected to the abnormal organs, resulting in the formation of a spermiduct-like duct (fig.4E). In such individuals, several female-specific genital organs, such as a glandula receptaculi, a receptaculum seminis, a rectum, a bursa copulatrix, and an ovipositor, were absent.
These observations indicate that the BmDSXM protein acts as an activator regulating the development of male-specific morphological structures. It remains unclear whether transgenic BmDSXM activates the expression of genes required to form the male-specific genital structures or repress the expression of genes involved in the formation of the female-specific genital structures to develop male-specific morphological structures in the BmdsxM transgenic animals. However, from the findings that the pheromone glands seen in many of the transformant lines showed an abnormal phenotype and that several female-specific genital organs were absent in the transgenic females in line D, it is quite likely that BmDSXM has an activity to repress the differentiation of several female-specific structures.
These transgenic females did not have the gonads developed as sperm-producing testes and, instead, had ovarioles containing normal-shaped eggs. They attracted males and displayed ovipositional behavior, but many of them oviposited very few eggs. Contrary to normal females, the eggs in the ovarioles in all transformant lines were significantly ill-arranged and sometimes formed a cluster (fig.5), and there were no eggs near the base of the ovarioles. Such aggregation of eggs may interfere with the extrusion of eggs by the peristaltic motion of the ovarioles, causing defectiveness in oviposition. Since the hatched larvae were obtained from the eggs oviposited by the transgenic females (data not shown), eggs produced by the transgenic females appeared to have fertility. This result demonstrates that the expression of the male BmDSX protein in females does not affect the normal development of the female germline cells. Therefore, it is quite likely that the male Bmdsx is not involved in germ cells for spermatogenesis. This hypothesis is strongly supported by previous reports that doublesex is not required within the female or male germline for normal development in D. melanogaster26,27 and that the female germline cells undergo normal oogenesis when surrounded by a soma masculinized by the dominant male gain-of-function dsx allele, dsxDom.28 Expression of the male BmDSX protein in females may cause some defects in the development of ovarioles, resulting in the ill arrangement or aggregation of eggs.

Figure 5
Photographs of ovarioles in control females and BmdsxM transgenic females (modified from ref. 22, with permission). Note that eggs in the ovarioles in all transformant lines are significantly ill-arranged and sometimes formed a cluster.
It is important to note that the transformations we have described are incomplete. Because both BmDSXF and BmDSXM bind to the same binding site in the vitellogenin promoter, 29 they should compete with each other for a target site when both are present. This competition would interfere with the masculinizing activity of the BmDSXM protein and also inhibit the feminizing activity of the endogenous BmDSXF protein in the BmdsxM transgenic females. The intersexual genital structures observed in our BmdsxM transgenic animals may be viewed as a result of such a competition effect. Alternatively, the heat-shock promoter activity may be insufficient to achieve and maintain appropriate levels of BmDSXM to direct a complete sex reversal. Needless to say, complete removal of endogenous Bmdsx is essential to fully understand the biological functions of the BmDSX proteins. However, since techniques such as gene targeting or gene silencing by RNA interference are not fully developed in B. mori, we cannot eliminate the influence of endogenous Bmdsx at the present time. Efforts to perform a targeted mutagenesis of Bmdsx using a gene-targeting technique according to the protocol recently developed by Golic30 are being made to overcome such a problem.
Males in all transformant lines displayed normal male morphology and also showed normal courtship behavior. There was neither obvious developmental delay nor decreased survival to eclosion in any of the transgenic animals.
Effects of Expression of hs:BmdsxM in Females on Gene Expression22
To investigate the effects of the expression of BmDSXM in females on the expression of vitellogenin and PBP genes, the mRNA levels of these genes in the line D females, whose genital phenotype was most significant among the transformed lines, were determined.
The level of vitellogenin mRNA in the line D females was reduced to approximately 25% of that in normal females. To eliminate the possibility that the examined transgenic females were all males, we typed transgenic females by PCR using oligonucleotide primers derived from the W chromosome-specific retrotransposon. This PCR analysis confirmed that all the examined transgenic females were chromosomally females. Next, we examined whether the expression level of the PBP gene was altered in the antennae of the BmdsxM transgenic females. The amount of PBP mRNA in the transgenic females was approximately two times greater than that of normal females.
As just described, in addition to the abnormal differentiation of certain female genital structures, expression of male Bmdsx in females induced the repression of a gene predominantly expressed in females (vitellogenin) and the activation of a gene preferentially expressed in males (PBP). On the other hand, the expression of female Bmdsx in males resulted in the converse situation (see below). The levels of vitellogenin mRNA in BmdsxF transgenic males were higher than those of normal males, while the amount of PBP mRNA in BmdsxF transgenic males was approximately two times lower than that in wild-type males. Taken together with the results that both BmDSXF and BmDSXM bind to the same binding site,29 these results make it reasonable to suppose that BmDSXF and BmDSXM have opposite regulatory effects on a common set of target genes.
Generation and Characterization of hs:BmdsxF and ie1:BmdsxFTransformants
In addition to the hs:BmdsxM transformant lines described above, two viable lines bearing a hs:BmdsxF construct were generated in the same way as described for the creation of hs:BmdsxM transformants. Each line possesses a single insert of a construct DNA that has been shown by Southern analysis to be unique (data not shown).
In contrast to the hs:BmdsxM transformants, the hs:BmdsxF transformants displayed no unexpected phenotypes. There were no phenotypic differences in the morphologies of genital organs between wild-type individuals and individuals carrying the hs:BmdsxF construct. Therefore, we tried to create transgenic animals carrying a Bmdsx female cDNA under the control of the Bombyx mori nucleopolyhedrovirus ie1 promoter, which has very strong promoter activity. From 1,314 eggs injected with pBac[ie1BmdsxF] plasmid, 303 larvae survived to the first larval stage (Table 1).29 After sibling mating, 18 of the G0 mating yielded progeny with EGFP eye fluorescence (Table 1). The yield of mating in G0 adults with transformed gametes was thus 16.1% (Table 1). In the GFP-positive G1 broods, on average, 5.5 % of the larvae displayed fluorescence (Table 1). Two independent ie1:BmdsxF transgenic lines were recovered. Southern blot analysis demonstrated that all transformants carried a single insertion of the construct DNA (data not shown).
The level of vitellogenin mRNA in males carrying the ie1:BmdsxF transgene was ten times higher than that observed in the normal males.29 In conjunction to this result, the amount of PBP mRNA was reduced to approximately 50% of that in the normal males.29 However, the levels of vitellogenin mRNA in the BmdsxF transgenic males were approximately 20-fold less than those of normal females. Since both BmDSXF and BmDSXM bind to the same binding site in the vitellogenin promoter, they should compete with each other for a target site in the vitellogenin promoter when both are present. This competition would cause the inhibition of BmDSXF binding to the vitellogenin promoter in the BmdsxF transgenic males. However, since the expression level of the BmdsxF transgene was only two-fold less than that of endogenous BmdsxM, the antagonistic effects of endogenous BmDSXM do not seem to give a full explanation for the significantly low levels of vitellogenin in transgenic males. In D. melanogaster, the intersex gene product and DSXF are dependent on each other to promote female differentiation, and this dependent relationship also holds for the yp genes.31 Additionally, the expression of DSXF in ix mutant males is not sufficient to activate Yp expression.32 Like Drosophila DSXF, BmDSXF may require some cofactor(s) to activate the vitellogenin gene expression in females. If this is the case, it is conceivable that the lack of expression of such cofactor(s) in the BmdsxF transgenic males as a result of female-specific expression of the cofactor(s) causes extremely low levels of vitellogenin in transgenic males. Consistent with this hypothesis, the mRNA expression levels of vitellogenin in the female fat body increased according to BmdsxF gene dosage.29
In contrast to the BmdsxM transformants, the BmdsxF transformants displayed normal morphological characteristics. As is the case in the activation of the vitellogenin expression in the BmdsxF transgenic males, we expected that BmDSXF would require some female-specific cofactor(s) to promote the development of female-specific organs. This hypothesis is strongly supported by the fact that the female DSX protein needs to interact with the intersex (ix) gene product for the regulation of female-specific differentiation in D. melanogaster.31 Recently, we have successfully cloned an ix homologue of B. mori (unpublished data). Further studies are in progress to confirm whether female Bmdsx requires interaction with the ix homologue to direct female differentiation.
Conclusions
To learn about the biological activities of Bmdsx, we expressed the BmDSXM protein in females and the BmDSXF protein in males by germline transformation with the piggyBac transposon vector according to the protocol developed by Tamura.21 Our data demonstrate that the piggyBac gene-transfer system can provide a powerful tool to perform a functional genetic analysis. From the findings presented above, we can safely conclude that Bmdsx acts as a double-switch gene at the final step in the sex-determination cascade of B. mori in the same way as Drosophila dsx, even if the primary sex-determination signal is different between D. melanogaster and B. mori. The demonstrated similarity supports the “bottom-up” hypothesis of Wilkins for the evolution of sex-determining pathways,33 which suggests that the most ancient genes operate at the bottom of the cascade and that, during evolution, new regulatory elements are recruited upstream. Other downstream sexual regulators, for example, the fruitless, intersex, hermaphrodite, and dissatisfaction genes of Drosophila, may also be conserved in B. mori. Downstream regulators such as dsx and Bmdsx may be less likely to evolve because they control multiple genes: modifying such downstream regulators might require coordinate modification of all of their target genes. In fact, we have successfully cloned homologs of fruitless and intersex from B. mori. However, in addition to the fact that the primary genetic signal for sex determination is completely different between D. melanogaster and B. mori (see Introduction), our previous report demonstrates that the regulation of sex-specific alternative splicing of Bmdsx premRNA is very different from the case of dsx.15 In other words, genes forming the sex determination cascade show a difference at the upstream of dsx between D. melanogaster and B. mori. The identification of the genes forming the sex-determination cascade of B. mori will continue to be the focus of future research.
Acknowledgements
The authors' transgenic studies on Bmdsx were supported by the “Research for the Future Program,” MEXT-JSPS, and the “Program for Promotion of Basic Research Activities for Innovative Biosciences,” BRAIN, Japan. We thank Toshiki Tamura and Shunsuke Funaguma for discussions and permission to cite their unpublished results.
References
- 1.
- Baker BS. Sex in flies: The splice of life. Nature. 1989;340:521–524. [PubMed: 2505080]
- 2.
- Cline TW. The affairs of daughterless and the promiscuity of developmental regulators. Cell. 1989;59:231–234. [PubMed: 2680106]
- 3.
- Slee R, Bownes M. Sex determination in Drosophila melanogaster. Q Rev Biol. 1990;65:175–204. [PubMed: 2117298]
- 4.
- Steinmann-Zwicky M, Amrein H, Nothiger R. Genetic control of sex determination in Drosophila. Adv Genet. 1990;27:189–237. [PubMed: 2112300]
- 5.
- Burtis KC, Coschigano KT, Baker BS. et al. The doublesex proteins of Drosophila melanogaster bind directly to a sex-specific yolk protein gene enhancer. EMBO J. 1991;10:2577–2582. [PMC free article: PMC452955] [PubMed: 1907913]
- 6.
- Coschigano KT, Wensink PC. Sex-specific transcriptional regulation by the male and female doublesex proteins of Drosophila. Genes Dev. 1993;7:42–54. [PubMed: 8422987]
- 7.
- Garabedian MJ, Shepherd BM, Wensink PC. A tissue-specific transcription enhancer from the Drosophila yolk protein 1 gene. Cell. 1986;45:859–867. [PubMed: 2423251]
- 8.
- Erdman SE, Burtis KC. The Drosophila doublesex proteins share a novel zinc finger-related DNA-binding domain. EMBO J. 1993;12:527–535. [PMC free article: PMC413235] [PubMed: 8440242]
- 9.
- An W, Wensink PC. Integrating sex- and tissue-specific regulation within a single Drosophila enhancer. Genes Dev. 1995a;9:256–266. [PubMed: 7851798]
- 10.
- An W, Wensink PC. Three protein-binding sites form an enhancer that regulates sex- and fat body-specific transcription of Drosophila yolk protein genes. EMBO J. 1995b;14:1221–1230. [PMC free article: PMC398199] [PubMed: 7720712]
- 11.
- Bridges CB. Triploid intersexes in Drosophila melanogaster. Science. 1921;54:252–254. [PubMed: 17769897]
- 12.
- Cline TW. The Drosophila sex determination signal: How do flies count to two? Trends Genet. 1993;9:385–390. [PubMed: 8310535]
- 13.
- Hashimoto H. The role of the W chromosome for sex determination in the silkworm, Bombyx mori. Jap J Genet. 1933;8:245–258.
- 14.
- Ohbayashi F, Suzuki MG, Mita K. et al. A homologue of the Drosophila doublesex gene is transcribed into sex-specific mRNA isoforms in the silkworm, Bombyx mori. Comp Biochem and Physiol Part B. 2000;128:145–158. [PubMed: 11163313]
- 15.
- Suzuki MG, Ohbayashi F, Mita K. et al. The mechanism of sex-specific splicing at the doublesex gene is different between Drosophila melanogaster and Bombyx mori. Insect Biochem Mol Biol. 2001;31:1201–1211. [PubMed: 11583933]
- 16.
- Mine E, Izumi S, Katsuki M. et al. Developmental and sex-dependent regulation of storage protein synthesis in the silkworm, Bombyx mori. Dev Biol. 1983;97:329–337. [PubMed: 6852368]
- 17.
- Yano K, Sakurai MT, Izumi S. et al. The vitellogenin gene of the silkworm, Bombyx mori: Structure and sex-dependent expression. FEBS Lett. 1994a;356:207–211. [PubMed: 7805839]
- 18.
- Yano K, Sakurai MT, Watabe S. et al. Structure and expression of mRNA for vitellogenin in Bombyx mori. Biochim Biophys Acta. 1994b;1218:1–10. [PubMed: 8193154]
- 19.
- Pelosi P, Maida R. Odorant-binding proteins in insects. Comp Biochem Physiol B Biochem Mol Biol. 1995;111:503–514. [PubMed: 7613772]
- 20.
- Steinbrecht RA, Laue M, Ziegelberger G. Immunolocalization of pheromone-binding protein and general odorant-binding protein in olfactory sensilla of the silk moths Antheraea and Bombyx. Cell Tissue Res. 1995;282:203–217.
- 21.
- Tamura T, Thibert C, Royer C. et al. Germline transformation of the silkworm Bombyx mori L. using a piggyBac transposon-derived vector. Nat Biotechnol. 2000;181:81–84. [PubMed: 10625397]
- 22.
- Suzuki MG, Funaguma S, Kanda T. et al. Role of the male BmDSX protein in the sexual differentiation of Bombyx mori. Evol Dev. 2005;7(1):58–68. [PubMed: 15642090]
- 23.
- Berghammer AJ, Klingler M, Wimmer EA. A universal marker for transgenic insects. Nature. 1999;402:370–371. [PubMed: 10586872]
- 24.
- Horn C, Jaunich B, Wimmer EA. Highly sensitive, fluorescent transformation marker for Drosophila transgenesis. Dev Genes Evol. 2000;210:623–629. [PubMed: 11151299]
- 25.
- Horn C, Wimmer EA. A versatile vector set for animal transgenesis. Dev Genes Evol. 2000;210:630–637. [PubMed: 11151300]
- 26.
- Marsh JH, Wieschaus E. Is sex determination in germ line and soma controlled by separate genetic mechanisms? Nature. 1978;272:249–251. [PubMed: 628449]
- 27.
- Schupbach T. Autosomal mutations that interfere with sex determination in somatic cells of Drosophila have no direct effect on the germline. Dev Biol. 1982;89:117–127. [PubMed: 7054003]
- 28.
- Horabin JI, Bopp D, Waterbury J. et al. Selection and maintenance of sexual identity in the Drosophila germline. Genetics. 1995;141:1521–1535. [PMC free article: PMC1206884] [PubMed: 8601491]
- 29.
- Suzuki MG, Funaguma S, Kanda T. et al. Analysis of the biological functions of a doublesex homologue in Bombyx mori. Dev Genes Evol. 2003;213:345–354. [PubMed: 12733073]
- 30.
- Rong YS, Golic KG. Gene targeting by homologous recombination in Drosophila. Science. 2000;288:2013–2018. [PubMed: 10856208]
- 31.
- Garrett-Engele CM, Siegal ML, Manoli DS. et al. Intersex, a gene required for female sexual development in Drosophila, is expressed in both sexes and functions together with doublesex to regulate terminal differentiation. Development. 2002;129:4661–4675. [PubMed: 12361959]
- 32.
- Waterbury JA, Jackson LL, Schedl P. Analysis of the doublesex female protein in Drosophila melanogaster: Role on sexual differentiation and behavior and dependence on intersex. Genetics. 1999;152:1653–1667. [PMC free article: PMC1460680] [PubMed: 10430590]
- 33.
- Wilkins AS. Moving up the hierarchy: A hypothesis on the evolution of a genetic sex determination pathway. Bioessays. 1995;17:71–77. [PubMed: 7702596]
- Transgenic Analysis of the Biological Functions of a Doublesex Homologue in Bomb...Transgenic Analysis of the Biological Functions of a Doublesex Homologue in Bombyx mori - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...