NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Félétou M. The Endothelium: Part 1: Multiple Functions of the Endothelial Cells—Focus on Endothelium-Derived Vasoactive Mediators. San Rafael (CA): Morgan & Claypool Life Sciences; 2011.

The Endothelium: Part 1: Multiple Functions of the Endothelial Cells—Focus on Endothelium-Derived Vasoactive Mediators.
Show detailsThe term “endothelium-dependent responses” was coined after the seminal work of Robert Furchgott in 1980 [496], although endothelium-derived vasoactive substances have been identified previously (e.g., prostacyclin, adenosine). However, it was only after this princeps publication that the fundamental role of the endothelial cells in controlling the tone of the underlying vascular smooth muscle was fully perceived. Indeed, endothelial cells control the tone of the underlying vascular smooth muscle cells by releasing various relaxing and contracting factors and by interacting directly with the vascular smooth muscle cells [425].
4.1. NITRIC OXIDE SYNTHASES
4.1.1. Historical Notes
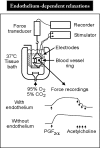
Furchgott and Zawadzki [496] first showed that the presence of the endothelial lining is required to observe the relaxation of isolated strips of rabbit aorta in response to acetylcholine (Figure 10). These authors attributed the relaxation to the calcium-dependent release of an endothelial factor of unknown origin and termed it endothelium-derived relaxing factor abbreviated as EDRF. The main target of EDRF in the smooth muscle cells is the soluble guanylyl cyclase [698,1281]. The mechanism of EDRF-induced relaxation is similar to that of nitrovasodilators [1089] and its half-life in physiological solution is short, in the order of a few seconds [584,1314]. EDRF is inactivated by superoxide anion [1316] and scavenged by oxyhemoglobin [989]. These observations prompted Dr Furchgott and Dr Ignarro to propose independently, during the meeting “Mechanism of Vasodilatation” held in Rochester (MN) in July 1986, 7 years after Dr Furchgott's seminal observation, that EDRF and NO are one and the same, or are very similar molecules [494,699]. Subsequently, Moncada and colleagues demonstrated that the amount of NO released by the endothelial cells could account for the biological activity of EDRF [1181]. Finally, the same group identified the enzymatic origin of NO production in the endothelial cells: the L-arginine–NO-synthase pathway [1180]. Diverse analogues of L-arginine have been identified as fairly specific inhibitors of NO-synthase [1287]. Some of these guanido-substituted L-arginine analogues, NG monomethyl-L-arginine, asymmetric dimethylarginine and symmetric dimethylarginine, are naturally occurring and have the potential to affect L-arginine handling and/or NO synthesis in various biological systems [896]. NO is not only a powerful vasodilator, but is also a potent anti-thrombotic agent and it plays important biological roles in the gastrointestinal, respiratory, nervous and immune systems [1070,1173] (Figure 11).
4.1.2. The Endothelial NO-Synthase (eNOS)
4.1.2.1. Characterization of the Three NO-Synthases.
NO derived from L-arginine has been involved in the responses of macrophages to inflammatory stimuli, such as bacterial lipopolysaccharides and interferon-γ, and in their ability to kill tumor cells or bacteria [643,644,986,1462,1463]. A NO-forming enzyme is present in the brain and the L-arginine-NO-cyclic-GMP pathway is activated by neurotransmitters in various neurons [516,820]. These different observations prompted the conclusion that distinct types of NO production were associated with three different subtypes of NO biosynthetic enzymes. These were cloned, characterized and termed according to the chronology of their characterization: NO-synthase I (or neuronal, nNOS, NOS-1) [144,146], NO-synthase II (or inducible, iNOS, NOS-2) [964,1781] and NO-synthase III (or endothelial, eNOS, NOS-3) [1233,1394]. In fact, the expression of the various NOS isoforms is much more diverse than their name would suggest. For instance, in the vascular wall, the three NOS can be present. nNOS is expressed not only in perivascular nerves but can also be detected in endothelial and smooth muscle cells [131,566,1186]. The expression of iNOS has been documented in all nucleated cells in the cardiovascular system. In the blood vessel wall this includes endothelial and smooth muscle cells as well as fibroblasts, leukocytes and mast cells [1187]. Actually, eNOS is expressed not only in endothelial cells but also in cardiac myocytes and platelets [57,1350]. Nevertheless, the epigenetic regulation of vascular endothelial gene expression means that the preponderant NOS isoform in healthy endothelial cells is the calcium-dependent eNOS and that eNOS is predominantly expressed in endothelial cells [1000].
The three NOS enzymes contain a reductase and an oxygenase domain. In the presence of molecular oxygen, they catalyze a five-electron oxidation of one of the guanido nitrogens of L-arginine. They all require the cofactors NADPH, FAD, FMN, heme, tetrahydrobiopterin (BH4) and zinc in order to catalyze this reaction [145]. The conversion of L-arginine to NO by eNOS involves two independent steps. The first one is the NADPH-dependent hydroxylation of L-arginine to NG-hydroxy-arginine, a reaction requiring calcium-calmodulin, which is accelerated by the presence of BH4. This hydroxylation step resembles cytochrome P450 monoxygenation. The second step leading to the generation of NO with the stoichiometric formation of L-citrulline is also calcium-calmodulin-dependent, requires O2 and NADPH and is also accelerated by BH4 [143]. An essential feature of the three NOS, despite the ability of the reductase or the oxygenase domain to function independently, is that the NO-synthase activity requires dimerization. The heme and a tetracoordinated zinc ion held by four thiols, two from each monomer, play an essential role in the formation of the homodimer and BH4 stabilizes the dimer once formed [38,906]. The zinc thiolate center and BH4 are subject to attack by peroxynitrite, which disrupts and releases the zinc ion, and oxidizes the thiols. Upon thiol reduction, eNOS dimers dissociate into monomers and become uncoupled [234,1817,1819] (Figure 11).
4.1.2.2. Transcriptional and Post-Translational Regulation of eNOS in Endothelial Cells.
Since NO is a gaseous free radical with a high biological reactivity, its synthesis has to be tightly controlled in order to be delivered at the right time, the right place and in the right amount [841].
The transcription of eNOS is under epigenetic control and can be regulated by various physical, chemical and pharmacological stimuli. The endothelial cell-restricted expression of eNOS is under the control of an epigenetic mechanism of gene regulation involving DNA methylation and a specific endothelial histone code. The transcription of eNOS is enhanced, for instance, by laminar and oscillatory shear stress, hydrogen peroxide, inhibition of histone deacetylase, micro-RNA, statins and, possibly, estrogens [56,170,1000,1383]. In response to laminar flow, the transcription of eNOS is coordinated with the regulation of the expression of many other genes that not only control vascular tone but also promote an atheroprotective, anti-thrombotic and anti-inflammatory phenotype. This phenomenon involves the up-regulation of the endothelial transcription factor KLF-2 (Kruppel like factor-2) [56,548,585].
The half-life of eNOS mRNA has been reported to be as long as 10 to 35 hours, indicating that post-transcriptional regulation of the transcripts half-life could be a more rapid and efficient strategy than to regulate eNOS transcription [213]. Indeed, post-transcriptional regulation (mRNA processing and polyadenylation, mRNA export from the nucleus and mRNA degradation) is heavily controlled by various different stimuli, some of those also regulate eNOS transcription [1383].
Increasing the amount of eNOS protein can only partially predict overall enzymatic activity since eNOS is subjected to several overlapping post-translational regulatory modifications, including lipidation, phosphorylations, S-nitrosylations, acetylation and protein–protein interactions [56,347,550]. The activation of eNOS also requires the calcium-dependent binding of calmodulin [38]. Furthermore, the sub-cellular location of eNOS is critical for its state of activation and multiple levels of regulation are responsible for the trafficking of the enzyme [1144].
In endothelial cells, eNOS is predominantly located in the plasma membrane and in the Golgi. A two-step process involving the enzymatic acylation of eNOS residues is responsible for this targeting. The myristoylation of the N-terminal glycine targets eNOS to membrane structures. The subsequent reversible palmitoylation of cysteine residues Cys15 and Cys26 (according to the human eNOS sequence) then directs the enzyme specifically to caveolin-1, a protein that serves as a scaffold for the assembly of multi-protein signaling complexes. Caveolin-1 is preferentially located in caveolae, invaginations of the cell membrane and is also present in the Golgi. The association of eNOS with caveolin-1 inhibits its activity [347,439,1144].
Additionally, eNOS activity is regulated by serine, threonine and tyrosine phosphorylations. The phosphorylation of Ser1177, unphosphorylated at rest, and the coordinated dephosphorylation of Thr495, constitutively phosphorylated at rest, enhance the sensitization of eNOS to calcium–calmodulin binding. The prevention of Ser1177 phosphorylation by non-specific O-linked glycosylation of eNOS can contribute to a decreased NO formation in diabetes [56,347,455]. The phosphorylation of Tyr657 is associated with a decreased production of NO. This proline-rich tyrosine kinase 2 (PYK2)-dependent phenomenon is elicited not only in response to oxidative stress but also in response to shear stress and insulin stimulation. The former may contribute to the endothelial dysfunction occurring, for instance, in hypertension. The concomitant phosphorylation of Ser117 and Tyr657 by shear stress or insulin can allow low but regular and prolonged NO generation without being confronted with co-factor depletion (BH4) and/or excessive peroxynitrite formation [450,948]. Additionally, dephosphorylation of Ser114, a constitutive inhibitor of eNOS activity, and phosphorylation of Ser615 and Ser633 and, possibly, Tyr81 can also enhance eNOS activity [56,76,347,455,1084].
The AMP-activated protein kinase (AMPK) is the only kinase identified that can phosphorylate eNOS on different amino acid residues, Ser1177, Ser633 and Thr495. AMPK-dependent activation of eNOS has been reported following various physical, chemical, neurohumoral and pharmacological stimuli, including shear stress, hypoxia, vascular endothelial growth factor (VEGF), adiponectin, peroxisome proliferator-activated (PPAR) receptor agonists and statins [449,455].
S-nitrosylation, the reversible covalent modification of a cysteine thiol by NO, is an additional level of post-translational control of eNOS activity. When anchored to caveolae, resting eNOS activity can be self-inhibited by S-nitrosylation of Cys94 and Cys99. Conversely, during activation, the enzyme is denitrosylated in the cytosol. Whether or not the mechanism of the inhibition by S-nitrosylation is associated with eNOS monomerization is still a matter of debate [347,922,1285].
Acetylation (or ethanoylation)/deacetylation, i.e., the introduction/removal of an acetyl functional group on lysine residues, is emerging as a significant post-translational regulatory mechanism. Histone acetyltransferase and histone deacetylase can modify the acetylation status of histone and non-histone proteins as well [550]. Sirtuin-1 (SIRT-1), a class III histone deacetylase, enhances acetylcholine- and shear stress-induced NO production by deacetylating eNOS, possibly at Lys494 and Lys504, and enhancing its binding to calmodulin [239,1011]. This mechanism is susceptible to pharmacological manipulations since aspirin enhances eNOS activation by acetylating Lys609, via a mechanism that is totally independent of cyclooxygenase inhibition [758].
Furthermore, eNOS is part of a large signaling complex that, besides calmodulin and caveolin-1, involves many other different proteins which are associated with eNOS at different stages of its trafficking. When associated with caveolin-1, this includes, for instance, the cation L-arginine transporter CAT-1 or some G-protein-coupled receptors such as the ETB endothelin and the bradykinin B2 receptor subtype [455,841]. The heat shock protein, Hsp90, is a chaperone protein involved in the folding of eNOS and in the heme insertion. Hsp90 increases eNOS activity by cooperatively enhancing the affinity of eNOS for calmodulin and facilitating the interaction with Akt. Endoglin, a cell membrane glycoprotein, promotes the association of eNOS with Hsp90. Furthermore, the trafficking of eNOS involves various types of proteins such as actin cytoskeleton, NOSIP (eNOS interacting protein), NOSTRIN (eNOS trafficking inducer protein), Gab1 (growth factor receptor-bound protein-associated binding protein-1), PECAM-1 (platelet–endothelial cell adhesion molecule-1) or dynamin. These various eNOS-associated proteins can be also associated with various kinases and phosphatases, and their own post-translational phosphorylations and S-nitrosylations play an important regulatory role [347,455,841,1037].
Finally, the degradation of eNOS determines its availability and must be regulated. Unfortunately, very little is known at present on these processes [841].
4.1.2.3. Bradykinin and Shear Stress as Examples of Two Different Mechanisms of eNOS Activation.
In the resting state, the myristoylated and palmitoylated eNOS is anchored to the plasma membrane, associated with caveolin-1, which inhibits its activity, and clustered with other proteins located in the caveolae. Inhibitory Ser114 and Thr495 are phosphorylated, Cyst94 and Cyst99 are S-nitrosylated and some lysine residues are acetylated.
By binding to the endothelial B2 kinin receptor subtype, a G-protein-coupled receptor, bradykinin, promotes calcium entry and calcium release from internal stores, increasing [Ca2+]i. This stimulates calmodulin binding to eNOS and the concomitant dissociation from caveolin-1. In addition, bradykinin stimulates the binding of Hsp90 and reduces that of other associated proteins such as the B2 receptor. The production of NO is further enhanced by the phosphorylation of Ser1177 and the coordinated dephosphorylation of Thr495, mediated by the calcium/calmodulin-dependent protein kinase II (CaMKII) pathway and facilitated by the Hsp90-dependent recruitment of Akt. Phosphorylation of Ser633 and possibly Ser615, via the protein kinase A pathway, dephosphorylation of Ser114 and the denitrosylation of the two cysteins could contribute to the overall activation of eNOS [347,455,841,1084].
Shear stress stimulates NO production in two phases. A first brief transient calcium-calmodulin-dependent NO burst followed by a second sustained phase of low NO production, lasting as long as shear is imposed, which occurs at resting [Ca2+]i levels. In the first phase, the rapid dissociation to caveolin and the following association with Hsp90 are again observed. Then, the protein kinase A (PKA)-dependent phosphorylation of Ser1177 is rapidly induced. In the second phase, the PKA-dependent phosphorylation of Ser633 and the PYK2-dependent phosphorylation of Tyr657, which respectively increase and reduce eNOS activity, contribute to the sustained and moderate NO generation. In endothelial cells exposed to shear stress, eNOs is predominantly localized at the level of homocellular cell junctions and is associated with PECAM-1 and the adapter Gab-1, serving as a scaffold for kinases such as PKA and phosphatases [56,126,455].
After activation, eNOS can be recycled to the Golgi or the plasma membrane caveolae and can re-associate with caveolin-1. However, eNOS can also be inactivated following depalmitoylation and redistribution to sub-cellular membrane structures, a phenomenon associated by interactions with NOSTRIN and NOSIP. Complex and not completely understood, dephosphorylations and phosphorylations as well as S-nitrosylations and denitrosylations of eNOS take place during the trafficking of the enzyme [347,841].
4.1.3. NO Storage and NO-Synthase-Independent Production of NO
NO is a reactive radical with a half-life of a few seconds in tissues and physiological fluids since it reacts with superoxide anion to form peroxynitrite or is rapidly inactivated by oxyhemoglobin to form nitrate and methemoglobin [590,1316,1317,1666]. However, NO can also produce remote and long-lasting effects in the cardiovascular system, either by being transported by protein carriers or by being stored locally. S-nitrosylation/denitrosylation of protein cystein residues is a dynamic process that involves hundreds of proteins in intact cells and two major circulating ones, albumin and hemoglobin. Not only can S-nitrosylation of proteins affect the activity of these proteins, but also the generation of S-nitrosoglutathione allows the transfer of NO activity at a distance from the site where it has been generated [922]. Besides S-nitrosylation of local proteins, NO can also be stored by forming protein-bound dinitrosyl iron complexes and by being displaced, again, by low molecular thiols [1085].
Additionally, NO can also be oxidized into nitrite, either spontaneously in the presence of oxygen or enzymatically by the copper-storage plasma enzyme, ceruloplasmin [885,1420]. Additionally, nitrite can originate directly from ingested food or indirectly from the reduction of nitrates either by reductase enzymes from commensal bacteria colonizing the mouth and the digestive tract or by xanthine oxidase [1592]. However, deoxyhemoglobin and heme-globins in general, as well as xanthine oxidase, cytochrome P450 monooxygenase, cytochrome C and eNOS itself can act as nitrite reductases and produce NO [72,549,1592]. These mechanisms can produce vasodilatation, including in the human circulation, and are likely to play an important role in hypoxic vasodilatation. Indeed, heme-globins are oxygen sensors, which under hypoxic conditions generate NO from surrounding nitrites and initiate vasodilatation in these poorly oxygenated vascular beds. Furthermore, NO derived from nitrite can modulate mitochondrial respiration and cellular activity and be involved in the regulation of cardiac energetics and in the myocyte protection following ischemic insults [277,634,1283,1419,1592] (Figure 11).
4.1.4. Multiple Targets of NO
Most of the physiological effects of NO are associated with the activation of soluble guanylyl cyclase [663,1281]. The high affinity of this enzyme to NO confers to the cells expressing it in an exquisite sensitivity to subsecond, picomolar NO transients [73]. Additionally, NO forms S-nitrosylated proteins (see above) [922] and can interact with the heme iron moiety of various proteins. For instance, the reduction in the intracellular concentration of calcium that contributes to the inhibition of platelet activation and initiates vascular relaxation involves the uptake of calcium by sarcoplasmic reticulum ATPase (SERCA) [264,1565]. This guanylyl cyclase-independent effect is attributed to the redox regulation of SERCA via the NO-dependent S-glutathiolation of a reactive cysteine [5,1557]. NO binds reversibly to cytochrome C oxidase and is in competition with oxygen. The NO-mediated inhibition of cytochrome C oxidase activity plays a fundamental role in the regulation of mitochondrial signaling and cellular response to hypoxia [397,1524].
The soluble guanylyl cyclase is an heterodimer, consisting of an α- and a β-subunit, the latter containing the prosthetic ferrous heme group (Fe2+) that catalyzes the conversion of guanosine-5′ triphosphate (GTP) to the second messenger cyclic 3′,5′-guanosine monophosphate (cGMP) [697]. cGMP predominantly activates cGMP-dependent protein kinases, cGKI and cGKII, the former being preferentially expressed in vascular smooth muscle cells, platelets and to a lesser extent in the endothelium [663,1569]. Furthermore, cGMP interacts with phosphodiesterases and regulates cAMP concentration [1326] and activates cyclic nucleotide gated cation channels [105].
In vascular smooth muscle cells, activation of cGKI produces relaxation by multiple mechanisms, which either reduces the intracellular calcium concentration or affects the calcium sensitivity of the contractile proteins [663]. The phosphorylation of RGS-2 (regulator of G-protein signaling-2) [1516] and IRAG (InsP3R-associated cGMP kinase substrate) [534] prevents the formation of IP3 and the activation of IP3 receptors, respectively, decreasing calcium release from internal stores. The inhibition of non-selective cationic channels (TRP channels such as TRPC3) [709] and the inactivation of CaV (voltage-gated calcium channels), following the stimulation of various populations of potassium channels (BKCa, KV, KATP or KIR) [430], reduce calcium entry. By interacting with MYPT1 (myosin phosphatase target subunit), cGKI activates the myosin light chain phosphatase, which dephosphorylates the myosin light chain kinase and produces calcium desensitization [888,1477] (Figure 11).
4.1.5. NO-Synthases and Cardiovascular Functions
Although NO has long been known to be synthesized by bacteria, it was completely unexpected that this potentially noxious and reactive free radical would play such a vital role in mammals.
The evaluation of the role of each of the NOS isoforms in pathophysiology has to await the availability of genetically engineered nitric oxide synthase knock-out mice [(NOS(-/-)] since conventional pharmacological inhibitors are poorly isoform-specific [992]. eNOS is clearly the preponderant isoform in the control of vascular tone since only eNOS(-/-) mice have elevated systemic and pulmonary arterial pressures and since large arteries (but not the more peripheral blood vessels) of these animals no longer show endothelium-dependent relaxations in response to acetylcholine [142,211,685]. By contrast, mice overexpressing eNOS are hypotensive [1146]. Nevertheless, NO derived from iNOS also plays a role in vasodilatation, especially in pathological states. For instance, iNOS is responsible for the severe systemic hypotension observed in toxic shock as iNOS(-/-) mice are resistant to sepsis-induced hypotension [589]. Blood vessels are under the control of excitatory and inhibitory nerves, and NO derived from nNOS, expressed in non-adrenergic non-cholinergic nerves, is an inhibitory neurotransmitter [684,1031,1556]. NO from both eNOS and nNOS provide parallel pathways for vasodilatation, and the respective knockout mice show that one pathway can compensate for the dysfunction of the other [1031].
In fact, this compensatory system occurs with all three NOS isoforms. The recent engineering of double and triple NOS knockout mice shows that, in resistance arteries, the endothelium-dependent relaxations to acetylcholine of mesenteric arteries were progressively reduced as the number of disrupted NOS genes increased [1505]. The increase in blood pressure is similar in triple NOS knockout mice and single eNOS knockout mice but the survival and fertility of the former is markedly reduced [1103]. Only the triple deletion of the NOS genes leads to the spontaneous development of arteriosclerotic vascular lesions (neointimal formation, medial thickening, perivascular fibrosis, plaque formation) under normal conditions and standard chow diet. Additionally, these mice show spontaneous myocardial infarction, sudden cardiac death and have a phenotype closely resembling that of metabolic syndrome in humans and exhibit nephrogenic diabetes insipidus [1078,1103,1571,1762].
The endothelium maintains the balance between vasodilatation and vasoconstriction, inhibition and promotion of the proliferation and migration of smooth muscle cells, prevention and stimulation of the adhesion and aggregation of platelets as well as thrombogenesis and fibrinolysis. NO is involved directly or indirectly in all these functions and upsetting this tightly regulated balance leads to endothelial dysfunction. In cardiovascular diseases, including hypertension, oxidative stress appears to be a common denominator underlying endothelial dysfunction and can be involved in the reduced NO production and/or a decrease in its bioavailability and/or an impairment of its effects on the cellular targets [431].
4.1.6. L-arginine–eNOS Pathway and Potential Therapeutic Targets in Cardiovascular Diseases
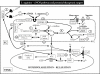
Besides NO itself, various potential therapeutic targets have been identified along the L-arginine-NOS pathway, for instance, at the level of the substrate, L-arginine, at the level of the NO-generating enzyme, eNOS, at the level of the soluble guanylyl cyclase, its main target, and at the level of the main effector of NO action, cGMP (Figure 12).
4.1.6.1. Availability of the Substrate L-Arginine.
When L-arginine is deficient, eNOS can generate both superoxide anions and nitric oxide leading to the detrimental production of peroxynitrite [1724]. L-arginine supplementation shows beneficial effects in pathophysiological conditions such as hypercholesterolemia and hypertension. However, since the L-arginine plasma concentration is approximately 100 μM, much above the KM of eNOS for L-arginine, it is unlikely that the circulating concentrations of this semi-essential amino acid become critical. In addition, human endothelial cells can effectively recycle L-citrulline to L-arginine and can obtain L-arginine from protein breakdown [468]. However, L-arginine could be locally submitted to competition with endogenously produced analogues such as symmetric dimethyl-L-arginine, asymmetric dimethyl-L-arginine (ADMA) and N(G)-monomethyl-L-arginine (L-NMMA). L-arginine is transported through the plasma membrane via the system Y+, a member of the family of cationic amino acid transporters, and is in competition with other amino acids such as L-lysine as well as with these analogues. Furthermore, ADMA and L-NMMA directly compete at the NOS site and are inhibitors of the enzyme [247]. The levels of ADMA represent a novel independent predictor for all causes of cardiovascular mortality. Free dimethylarginines are the products of proteolytic degradation of arginine-methylated proteins following the preferential action of protein arginine N-methyltransferase type I (PRMT-I). ADMA is mainly metabolized by dimethylarginine dimethylaminohydrolase, the DDAH-2 isoform being preponderant in the endothelial cells. Following oxidative stress or angiotensin II administration, the observed elevation in ADMA levels is associated with an increase in the enzymatic activity of PRMT and a decrease in the activity of DDAH. Silencing the DDAH-2 gene with siRNA impairs endothelium-dependent relaxation and NO production. Therefore, the inhibition of PRMT-1 and the activation and/or the enhanced expression of DDAH-2 could be beneficial in cardiovascular diseases [1179].
Additionally, endothelial cells express arginases, arginase-II being the predominant isoform, which metabolize L-arginine to L-ornithine and urea. Arginase-II competes with eNOS for the substrate and its expression and/or activity are enhanced in cardiovascular diseases, possibly following oxidative stress, a redox-sensitive element having been identified on its promoter. In vascular smooth muscle cells, the arginase-dependent formation of polyamines and L-proline may promote aberrant vessel wall remodeling and neointima formation. In animal models of cardiovascular diseases, arginase-II inhibition or gene deletion improves endothelium-dependent relaxations and vascular NO production, prevents the development of hypertension and decreases the generation of endothelial reactive oxygen species and the formation of atherosclerotic plaque [357,1327]. Arginase-II may therefore represent a promising novel therapeutic target in hypertension that could reverse vascular dysfunction.
4.1.6.2. Proper Expression and Function of eNOS.
A reduced expression of eNOS could be responsible for a decrease in NO production. However, in most situations, where endothelial dysfunction is encountered, the expression of eNOS is paradoxically increased. The increased endothelial levels of reactive oxygen species, especially hydrogen peroxide, which increase the expression of eNOS at the transcriptional and translational level, is likely to explain this paradox. An endothelial dysfunction associated with an increased expression of eNOS shows that the ability to generate NO is reduced and/or that its bioavailability is decreased. The reduction in NO generation can be attributed to the eNOS uncoupling phenomenon where the NOS itself is a source of superoxide anion and a cause of endothelial dysfunction. The decrease in NO bioavailability is associated with an oxidizing environment, i.e., superoxide anion generation from different sources, and the formation of peroxynitrite [1375].
The two independent enzymatic steps required for the eNOS-dependent conversion of L-arginine to NO necessitate the presence of BH4. Decreased endothelial levels of this essential pteridine cofactor are not only responsible for a reduction in NO production but also for the uncoupling of eNOS. In such an uncoupled state, electrons, which normally flow from the reductase domain of one subunit to the oxygenase domain of the other subunit, are diverted to molecular oxygen rather than to L-arginine, resulting in production of superoxide anion rather than nitric oxide [1602].
BH4 is synthesized de novo from guanosine triphosphate (GTP) by the enzymes GTP-cyclohydrolase I (GTPCH-I), 6-pyruvoyl-tetrahydrobiopterin synthase and sepiapterin reductase (SR). An alternative pathway for BH4 synthesis, the so-called salvage pathway, involves the formation of sepiapterin and its subsequent reduction in dihydrobiopterin (BH2), which is further reduced by dihydrofolate reductase (DHFR) to form BH4. BH4 is very susceptible to oxidation by peroxynitrite, forming BH2 and ultimately biopterin. Following oxidation, BH4 can be regenerated either by DHFR or by dihydropteridine reductase. Increased vascular homocysteine is a potent risk factor for atherosclerosis and endothelial dysfunction, and some of this effect may be mediated by the inhibition of BH4 de novo synthesis [1064].
Direct supplementation with BH4, or with its precursor sepiapterin, improves not only endothelial function in numerous animal models of cardiovascular diseases but also in patients with hypertension, diabetes, hypercholesterolemia or coronary diseases and in smokers. Enhancing the expression or the activity of GTPCH-I, the first and rate-limiting step of de novo BH4 synthesis, or that of DHFR, involved in BH4 regeneration, could prevent the occurrence of endothelial dysfunction. Alternative strategies include folic acid, which enhances the binding-affinity of BH4 to NOS, chemically stabilizes BH4 and, by stimulating DHFR, enhances its regeneration. Preventing peroxynitrite formation and therefore BH4 oxidation or facilitating the recycling of BH4 regeneration with vitamin C may also be considered [1365,1375].
Inhibition of eNOS causes accelerated atherosclerosis in rabbits [202] and mice [794]. When apoE-/- mice are subjected to the additional deletion of eNOS, they also display accelerated atherosclerosis and develop abdominal aortic aneurysm formation and ischemic heart disease [230,853]. These data indicate that endogenous eNOS has an anti-atherosclerotic role. However, apoE-/- mice overexpressing eNOS develop paradoxically larger atherosclerotic lesions than control apoE-/- mice [1172]. This paradox can easily be explained. In a model of vascular oxidative stress, reactive oxygen species from various sources (kindling radicals) and the formation of peroxynitrite would lead to eNOS uncoupling and the subsequent eNOS contribution to superoxide production (bonfire radicals). Therefore, the up-regulation of eNOS itself is a double-edged sword strategy. However, if the up-regulation of eNOS is associated with an increase in the availability of essential cofactors, such as BH4, the eNOS uncoupling can be prevented. Some compounds, such as AVE9488 and AVE3085, which enhance eNOS promoter activity, have demonstrated this type of coordinated activity and validated the concept that enhanced transcriptional expression of eNOS can be beneficial in cardiovascular diseases [1691].
Furthermore, the so-called pleiotropic effects of statins (3-hydroxy-3methylglutaryl-CoA reductase inhibitors), which are independent of their intended lipid-lowering effect, and the activation of SIRT-1 by resveratrol involve an increase in the expression of the transcription factor KLF-2 [573,1392], which is also activated by laminar shear stress [56]. KLF-2 is described as a master regulator of the transcription of anti-inflammatory, anti-thrombotic and vasoactive genes, including eNOS, leading to a vasoprotective phenotype [548]. Additionally, statins and polyphenols as well as other drugs such as fibrates, metformin and peroxisome proliferator-activated receptor-gamma (PPARγ) agonists activate AMP-kinase. This could lead to an increased expression of eNOS and to posttranslational modifications, such as phosphorylations, that enhance protein expression and enzymatic activity [449,856,1695].
4.1.6.3. Increasing the Levels of NO And/Or Its Bioavailability.
Besides preventing eNOS uncoupling (see above), different therapeutic strategies can be designed to restore proper NO levels. These include drugs that stimulate the release of NO from endothelial eNOS, NO donor drugs, antioxidant compounds, drugs that boost the antioxidant defense mechanisms and inhibitors of the enzyme(s) involved in the generation of reactive oxygen species.
4.1.6.3.1. Release of NO.
Some anti-hypertensive drugs can stimulate the release of endothelial NO, for instance, nebivolol, a third-generation β-blocker [506,1088] or various dihydropyridines, by a mechanism that is unrelated to the calcium channel-blocking properties of these compounds [325,1614,1799]. They produce endothelium-dependent relaxations in isolated arteries and/or an increase in forearm blood flow in hypertensive patients. However, whether or not this additional property offers a measurable therapeutic advantage when compared to other anti-hypertensive drugs of the same class must be tested comparatively in large clinical trials. Inhibitors of the angiotensin-converting enzyme (ACE-I) indirectly produce endothelium-dependent relaxations in vitro or vasodilatations, in vivo, by preventing bradykinin degradation [395,426,1066]. Independently of bradykinin metabolism, these ACE-I can also enhance endothelium-dependent relaxations by regulating the sequestration and the sensitivity of the bradykinin B2 receptors [99,1065]. Similarly, in various arteries including human coronary arteries, antagonists of the angiotensin AT1 receptor (ARB) unmask endothelium-dependent and NO-dependent vasodilatation via the preferential activation of AT2 receptor subtype during AT1 receptor blockade. The activation of AT2 receptor can also lead to kinin-mediated effects [74,781,939]. Additionally, chronic treatments with ACE-I or ARB increase eNOS expression in both animal models of hypertension and in humans [907,1539]. While in animal models of hypertension, chronic treatment with ACE-I or ARB consistently improves endothelial function, in hypertensive patients, variable effects have been reported, possibly because of the multi-factorial etiology of essential hypertension and/or the differences in the experimental protocols performed in these clinical studies [907,1450,1488]. In animal models of cardiovascular diseases, the fragmental information available with the recently developed renin inhibitor, aliskiren, shows that this third means of inhibiting the renin–agiotensin system is also likely to improve endothelium-dependent relaxations and to increase eNOS expression [334,702].
4.1.6.3.2. Reduction of Oxidative Stress.
Besides uncoupled eNOS, potential sources of superoxide anion in the vascular wall include NAD(P)H oxidases, xanthine oxidase, cyclooxygenases, lipooxygenases, cytochrome P450 monooxygenases and the mitochondrial respiratory chain. An increased production of reactive oxygen species has been demonstrated in numerous cardiovascular diseases including in patients with essential hypertension, renovascular hypertension, malignant hypertension and preeclampsia. In essential hypertension, an increase in the levels and/or activity of vascular NAD(P)H oxidase has been identified as a predominant source of excess reactive oxygen species [1730] (Figures 11, 12 and 13). Under pathological conditions, dysregulation of reactive oxygen species generation, due to enhanced production and/or reduced antioxidant potential, results in oxidative stress, which leads to vascular dysfunction and remodeling through oxidative damage. Superoxide anion not only decreases NO bioavailability but also the peroxynitrite generated exacerbates vascular injury by producing the oxidation of sulfhydryl groups as well as the nitration and hydroxylation of aromatic groups, including tyrosine, tryptophan and guanine [431,1387].
Thus, the scavenging of reactive oxygen species with antioxidant or its inactivation with superoxide dismutase mimetics would seem an obvious means of increasing NO bioavailability. However, if such a strategy has been successful in various animal models of hypertension, large clinical trials in hypertensive patients showed that chronic antioxidant therapy did not produce any major reduction in arterial blood pressure and did not improve the associated morbidity and mortality, with the possible exception of the long-term intake of polyphenols that are present in red wine, fruit and vegetables, i.e., the so-called beneficial effect of a Mediterranean diet [1205,1433]. The reasons underlying these failures are still not completely understood.
Direct inhibition of the generation of reactive oxygen species might be a better alternative. Over the recent years, major efforts in the synthesis of NADPH oxidase inhibitors have been made in both academia and pharmaceuticals companies. However, it is not yet clear which isoform(s) of NADPH should be inhibited, and the inhibitors available are still not selective, are not very potent and have poor pharmacokinetics properties [83].
The activation of the renin–angiotensin system and the stimulation of AT1 receptor subtype are major stimulators of NAD(P)H oxidase activation and ROS production, including human hypertension [883,1730]. Preventing the generation of reactive oxygen species, for instance, by deleting a subunit of the NADPH oxidase, induces resistance to angiotensin II-induced hypertension and markedly reduces the associated endothelial generation of superoxide anions [871]. In rat models of hypertension, ACE-I and ARB decrease the generation of superoxide anions [151,1511,1677]. They diminish the amplitude of endothelium-dependent contractions [1295] and restore the amplitude of both NO- and EDHF-mediated endothelium-dependent relaxations [571,1295]. Statins not only increase eNOS expression but also decrease the expression of NADPH subunits, thus improving the balance between NO and reactive oxygen species. These endothelial effects may contribute to the therapeutic effect exerted by these compounds, although no clinical data exist demonstrating that the reversal of endothelial dysfunction is associated with a reduction in cardiovascular events [638,899,907,1450,1488].
4.1.6.3.3. NO-Donors.
Substituting NO with nitric oxide donor drugs started empirically in the late 19th century with the nitrate compound nitroglycerin to alleviate the symptoms of angina. However, the development of nitrate tolerance, a complex multi-factorial phenomenon, limits their continuous clinical use and is associated with the generation of oxidative stress and endothelial dysfunction [1014]. Different chemical classes of NO donors, some of which are able to spontaneously release NO, have been synthesized and may have a therapeutic interest beyond coronary disease and heart failure. S-nitrosothiols, which are not prone to tolerance and are not cross-tolerant with nitrates, have vasodilator and anti-aggregating properties and could boost the antioxidant capacity of the plasma and the vascular wall [1027].
Alternatively, hybrid compounds possessing a dual activity could demonstrate therapeutic interest in hypertension and associated end organ damage. Earlier compounds, such as nicorandil, an organic nitrate that also opens potassium channels, or nipradilol, a non-selective adrenoceptor blocker with weak β-adrenoceptor-antagonistic and NO-releasing properties, are still predominantly prescribed for angina and glaucoma, respectively [638,1027]. However, among the new hybrid compounds that have been designed, i.e., NO-releasing anti-adrenergic drugs, NO-releasing-dihydropyridines, NO-releasing statins, NO-releasing ARB or NO-releasing ACE-I, some may be of therapeutic value in hypertension and other cardiovascular diseases [988]. For instance, a NO-releasing derivative of the ACE-I enalapril, NCX-899, has demonstrated superior activity when compared to enalapril alone in animal models of heart failure and hypertension [721,1152].
4.1.6.4. Soluble Guanylyl Cyclase and cGMP Production.
When NO binds to the heme moiety of soluble guanylyl cyclase, its main physiological target, the activity of the enzyme, which produces the signaling molecule cGMP, is enhanced 400-fold. Thus, regulation of nitric oxide bioactivity is not only achieved by modulation of NOS but also by affecting soluble guanylyl cyclase [398] or the half-life of cGMP, which is readily hydrolyzed by phosphodiesterases [1013].
4.1.6.4.1. Soluble Guanylyl Cyclase.
NO cannot activate the soluble guanylyl cyclase when it is heme-free [951] or when the heme moiety is oxidized (Fe3+), following oxidative stress [517,1457]. Pharmacological agents that interact with the soluble guanylyl cyclase and generate cGMP independently of NO have been synthesized. Based on their mechanisms of action, these compounds can be separated into two different classes, the stimulators and the activators of soluble guanylyl cyclase [1361,1456]. The former class of compounds stimulates the enzyme in an NO-independent but heme-dependent manner and, as allosteric modulators, markedly enhance NO-dependent cGMP production [398,1455]. In contrast, activators of soluble guanylyl cyclase target the soluble guanylyl cyclase when oxidized, i.e., when the heme iron is in the oxidized ferric form, or more likely when the enzyme is under the heme-free form. These compounds do not synergize with NO [1312,1363,1454,1457]. Both activators and stimulators of soluble guanylyl cyclase are potent vasodilators and inhibitors of platelet aggregation, where they synergize with the prostacyclin pathway [655,1297,1298,1454,1455,1457]. The former are currently undergoing clinical trials in acute decompensated heart failure and peripheral artery occlusive diseases, while the latter are being evaluated in pulmonary hypertension. However, the potential therapeutic indications of these new compounds include hypertension, myocardial ischemia, erectile dysfunction, atherosclerosis or thrombosis [1361,1456] (Figure 14).
4.1.6.4.2. Phosphodiesterases.
Phosphodiesterases are a diverse family of enzymes that hydrolyze cyclic nucleotides. In the cardiovascular system, phosphodiesterase 5 (PDE5) was the first identified selective cGMP esterase and is the major isoform involved in the hydrolysis of the cGMP pools generated by the activation of soluble guanylyl cyclase [779,1783]. Specific PDE5 inhibitors are currently approved for erectile dysfunction but, in the future, their therapeutic indications may also include pulmonary hypertension, heart failure and essential hypertension [129,540,780].
4.1.7. Hydrogen Peroxide, The Other NOS-Derived Vasoactive Factor
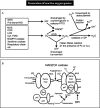
Metabolism of oxygen by cells generates potentially deleterious reactive oxygen species (ROS). Under normal conditions, the rate and magnitude of oxidant formation is balanced by the rate of oxidant elimination. ROS play an important role as intracellular signaling molecules and as paracrine messengers. However, an imbalance between prooxidants and antioxidants results in oxidative stress, which has been associated with multiple pathological states including cardiovascular diseases. Superoxide anion, the one electron reduction of molecular oxygen (O2.-), can be generated by different enzymes (e.g., NADPH oxidase, xanthine oxidase, cyclooxygenases, NO-synthases, cytochrome P450 monooxygenases, enzymes of the mitochondrial respiratory chain) in virtually all cell types including vascular smooth muscle and endothelial cells. Superoxide, either spontaneously or enzymatically through dismutation by superoxide dismutase (SOD), is reduced to the uncharged H2O2. H2O2 in the presence of the enzyme catalase or glutathione peroxidase and is then dismutated into water and oxygen. In the presence of transition metals (copper, iron) or superoxide anion, H2O2 generates, through the Fenton or the Haber–Weiss reaction, the highly reactive hydroxyl radicals (.OH) [389,1559] (Figure 13).
Both endothelial [49,140,631,998,1476] and smooth muscle cells [909,1564,1784] generate significant amounts of reactive oxygen species, either spontaneously or in response to receptor-mediated and non-receptor-mediated stimuli. H2O2 can act locally close to its site of production or, since it is an uncharged molecule, diffuse through the cell membrane and act on neighboring cells. H2O2 modulates different aspects of endothelial cell function including endothelial cell growth and proliferation, endothelial apoptosis, endothelial cytoskeletal reorganization and barrier dysfunction, endothelial inflammatory responses, endothelium-regulated vascular remodeling and endothelium-dependent regulation of vascular tone. Indeed, H2O2 can, depending on the tissue, the experimental conditions or the concentrations studied, possess dilator or constrictor properties [182].
4.1.7.1. H2O2 as a Vasorelaxing Agent.
H2O2 can induce relaxations in a number of blood vessels including human coronary and mesenteric arteries [65,98,479,624,701,997–999,1351,1545,1663,1756]. Additionally, endogenously produced H2O2 contributes to flow-induced relaxation and to the endothelium-dependent relaxation evoked, for instance, by acetylcholine or the endothelium-independent relaxation evoked by cromakalim [621,716,811,935,1055].
In most blood vessels, which relax in response to exogenously added H2O2, the relaxation is endothelium-independent. However, in some arteries, the relaxing effect of H2O2 can be partially endothelium-dependent, such as in the canine and porcine coronary artery. In the latter coronary artery, the endothelium-dependent relaxation elicited by H2O2 involves the COX-1-dependent release of PGE2 [1317,1545]. The vasodilator effects of H2O2 are totally endothelium- and NO-dependent in the canine basilar artery [1756], in the rabbit and in the rat aorta [103,1757].
The endothelium-independent relaxations of arterial myocytes to H2O2 again involve multiple pathways and include activation of soluble guanylyl cyclase [479,481,624,693,1351] and the metabolism of arachidonic acid either through cyclooxygenases and/or lipoxygenases [64,479,622,701]. H2O2 also produces hyperpolarization of the vascular smooth muscle cells by activating BKCa [624,999,1545], KATP [1663], KV [1299] and/or KIR channels [701]. The activation of smooth muscle KV by H2O2 involves redox-sensitive elements, thiol groups, on the channel itself or on associated regulatory proteins [1299]. The activation of BKCa by H2O2 is evoked by a direct action on the channel as well as by a soluble guanylyl cyclase-dependent effect [624]. In the isolated murine mesenteric artery, H2O2 produces an endothelium-independent relaxation providing that KCa channels are operational but, at the same concentrations, elicits a potent contractile response if the activity of these channels is compromised [952].
H2O2 also modulates the ionic channel activity of the endothelial cells which, in turn, can influence the membrane potential and thus the contractile activity of the underlying smooth muscle cells. In cultured endothelial cells of the human umbilical vein, H2O2 elicits both depolarization and hyperpolarization. In the micromolar range, it inhibits KIR, while at 1 mM, it activates BKCa [179]. The activation of BKCa is due to an increase in [Ca2+]i since, in concentrations higher than 100 μM, H2O2 activates a calcium-permeable, non-selective cation current in these cells [743]. In human aortic endothelial cells, H2O2 causes the release of Ca2+ from the endoplasmic reticulum [678].
4.1.7.2. H2O2 as an Endothelium-Derived Relaxing/Hyperpolarizing Agent.
Most of the evidence in favor of H2O2 being an endothelium-derived relaxing/hyperpolarizing factor comes from the observation that, in certain blood vessels, including human coronary arteries, the agonist- and flow-induced dilatations, studied in the presence of inhibitors of NO-synthases and cyclooxygenases, are partially or totally sensitive to catalase, the enzyme that dismutates H2O2 into water and oxygen. In these arteries, exogenously added H2O2 appears to mimic these responses, which rely on KCa activation [935,997–999,1055,1076,1733]. Furthermore, in murine mesenteric [999,1076] and porcine coronary arteries [998], the agonist-induced relaxations/hyperpolarizations are associated with a catalase-sensitive endothelial production of H2O2. Similarly, in human coronary arterioles [935,1055], flow-induced dilatation increases the production of H2O2 from the endothelial cell layer. In addition, without actually being released by the endothelial cells, H2O2 per se or substances that promote its formation can enhance endothelial responses by potentiating calcium release from endothelial stores [371,514]. In various experimental models or in arteries from patients with cardiovascular disease, H2O2 generation can partially compensate the decreased NO production, at least in terms of endothelium-dependent relaxations [278,279,1222].
In eNOS-knockout murine arteries, the relaxations resistant to inhibitors of NO-synthases and cyclooxygenases are sensitive to catalase and have been attributed to the NOS-3/Cu,Zn-SOD-dependent formation of H2O2 [999,1076]. The maintenance of these endothelium-dependent relaxations/hyperpolarizations in NOS-3 knockout mice was explained by the compensatory endothelial expression of other NOS genes. Indeed, these responses are progressively reduced as the number of the disrupted NOS genes increases (single eNOS, double nNOS/eNOS and triple nNOS/iNOS/eNOS knockouts). In parallel, the production of H2O2 is preserved up to the total disruption of the three NOS genes [192,1504]. Nevertheless, whether or not the decrease in the EDHF-mediated responses is directly associated with the disruption of the NOS genes or is independently associated with the severe phenotype of these mice, especially with the one observed in the triple knockout mice (hypertension, dyslipidemia, myocardial infarction and nephrogenic diabetes insipidus) remains to be fully assessed.
In these murine arteries, the NOS appear to be the sole source of H2O2 without the involvement of other oxidases [1504]. However, in human coronary arterioles, flow-induced endothelium-dependent dilatation is associated with H2O2 generated by the mitochondrial respiratory chain, while bradykinin-induced endothelium-dependent relaxation requires NADPH oxidase-derived H2O2 [879,938] (Figure 15).
Not all available data support the hypothesis that H2O2 is involved in endothelium-dependent responses and in particular in EDHF-mediated responses. Indeed, catalase does not inhibit such responses in the porcine, canine and rat coronary [98,491,1235,1510], human radial and mesenteric artery [205,607], murine mesenteric [388], guinea-pig carotid [552] and in rabbit ileo-femoral and mesenteric [218,716] arteries or in the mouse aorta [388]. Furthermore, H2O2 does not always hyperpolarize vascular smooth muscle cells [552], and its relaxing effect is not necessarily associated with hyperpolarization of the vascular smooth muscle cells and/or the opening of K+ channels [218].
4.1.7.3. H2O2 as a Vasoconstricting Agent.
In various arteries from different species, H2O2 also directly induces contractions [45,621,788,1296,1400,1449,1758]. In some other blood vessels, such as the rabbit aorta or the rat mesenteric artery, low concentrations of H2O2 (<100 μM) evoke contractions, while higher concentrations (>0.3mM) produce transient relaxations followed by sustained contractions [103,503].
Additionally, the vasoconstricting (and proliferative) actions induced by receptor stimulation, for instance, α1-adrenergic, TP and AT1 receptors, or mechanical stimulation, for instance, stretch, involves the generation of H2O2 [597,1130,1142,1558,1784]. NAD(P)H is a frequent but not the exclusive source of the ROS involved in these contractile effects. In vivo in mice, the administration of either norepinephrine or angiotensin II produces a vasopressor response, which is also associated with the production of H2O2. In genetically modified mice overexpressing catalase, the basal arterial pressure and the basal level of H2O2 are similar to those of wild-type animals. However, the pressor response induced by the administration of either norepinephrine or angiotensin II is decreased in the transgenic animals and this is associated with a suppression of H2O2 production, indicating that endogenously produced H2O2 contributes to the vasopressor responses evoked by these cardiovascular hormones [1750]. Furthermore, mice with a specific endothelial overexpression of catalase show a strongly reduced steady-state concentration of vascular H2O2 and are hypotensive. This phenomenon is not dependent on the bioavailability of endothelial NO but is completely reversible by treatment with a catalase inhibitor, suggesting that endothelial-derived H2O2 exerts a tonic contractile effect on vascular smooth muscle cells [1478]. In agreement with these observations, H2O2 has been identified as a putative EDCF in rat arteries [505].
Numerous mechanisms can explain the contractile responses produced by endogenous and exogenous H2O2 and include activation of P2 purinergic receptors [1400], production of COX-derivatives, possibly thromboxane A2 and the activation of TP receptors [504,509,1744], stimulation of ERK-MAP kinase [502,1540] and PKC [597], inhibition of potassium channels, for instance, KV [263,1038] and BKCa by altering cysteine-mediated calcium sensing [1518], activation of chloride channels [578] and interplay with calcium signaling at multiple levels [1564].
In addition, H2O2 can impair endothelium-dependent vasodilatations. Thus, H2O2 promotes proline-rich tyrosine kinase-2 (Pyk2)-dependent phosphorylation of eNOS Tyr657, preventing NO generation [948], and directly inhibits cytochrome P450 epoxygenase and the production of vasodilatory EETs [881]. Furthermore, in endothelial cells of the porcine renal artery, patched in situ, H2O2 inhibits the NO-dependent activation of BKCa and the associated endothelial hyperpolarization [138]. Finally, H2O2 reduces the activity of IKCa [183] and may therefore prevent EDHF-mediated responses.
These apparently controversial results are still difficult to explain. Nevertheless, in different arteries, endothelium-derived H2O2 can evoke the hyperpolarization and/or the relaxation of smooth muscle cells and can compensate a decrease in NO bioavailability [1412]. Additionally, this peroxide appears to play an important role in flow-induced vasodilatation [935]. In vivo, this reactive oxygen species could play an important role in coronary autoregulation [1733] and a cardioprotective role during ischemia–reperfusion injury [1732].
4.1.8. Citrulline
It has been suggested that L-citrulline, the by-product of NO biosynthesis by the NO-synthase, could relax the rabbit aorta by a cyclic-GMP-dependent mechanism that may involve potassium channel activation [1320]. However, this observation has not been confirmed by subsequent work involving the same and different arteries [991].
4.2. OTHER GASEOUS MEDIATORS
The discovery and characterization of NO was much more than simply the identification of another intercellular messenger. Unlike other neurohumoral mediators, NO is not involved in classic ligand-membrane-bound receptor interactions. It is a gas, which readily crosses lipid membranes and therefore diffuses homogeneously in a non-polarized manner from its production site. NO can be considered as the first member of a new family of neurohumoral substances, the gaseous mediators. Three other low molecular weight compounds can be included in this family, carbon monoxide (CO) hydrogen sulfide (H2S) and possibly sulfur dioxide (SO2) [1644,1652].
4.2.1. Carbon Monoxide
4.2.1.1. The HO–CO Pathway.
The predominant biological source of CO is from the degradation of heme (hemoglobin, myoglobin, cytochrome P450, etc.) by heme-oxygenase (HO), which generates equimolar quantities of biliverdin, free ferrous iron and carbon monoxide. Biliverdin is rapidly converted to bilirubin, while free iron is promptly sequestered by ferritin [355]. These bile pigments are not simple by-products of heme catabolism, they have antioxidant properties which protect the vascular cells from oxidative stress. Three isoforms of heme oxygenase have been described: HO-1 (or heat shock protein 32, inducible isoform), HO-2 (constitutive isoform) and HO-3, which is closely related to HO-2 but is nearly devoid of catalytic activity [238,1643]. HO-1 is ubiquitously expressed in response to a variety of stimuli including its own substrate, heme, and several oxidants. HO-2 is also ubiquitously expressed with notably high levels in brain and testes. Both HO-1 and HO-2 are expressed in vascular smooth muscle and endothelial cells [355,1644].
In many pathophysiological situations, the HO-CO pathway compensates for the decrease in NO bioavailability [1709]. CO is a potent vasodilator in most, but not all, vascular beds. The biological activity of CO is generally associated with the modulation of heme-dependent proteins and although it has been postulated that CO could also bind to targets that lack a heme moiety, direct evidence for these effects is still missing [1083,1709]. The mechanisms of CO-induced vasodilatation can involve the stimulation of soluble guanylate cyclase [1647], the inhibition of cytochrome P450 [1300] and/or the activation of various populations of K+ channels [1709].
4.2.1.2. CO as a Vasodilator.
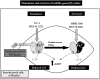
The involvement of guanylyl cyclase activation in CO-induced vascular relaxation was recognized early. However, this gaseous mediator is many times less potent than NO and is able to activate the purified guanylyl cyclase only in the high micromolar range [495,765]. Nevertheless, in canine cerebral arteries, cyclic-GMP-dependent activation of BKCa has been reported [840]. Additionally, CO can activate BKCa in a cGMP-independent manner. The inhibition of cytochrome P450 by CO suppresses the synthesis of 20-HETE and lifts the inhibition of BKCa produced by this cytochrome P450 derivative [262,1300]. Furthermore, CO can directly increase the open probability of BKCa [1648,1649] (Figure 16).
The direct action of CO on BKCa differs from that of NO on this population of potassium channels [1708]. CO increases the apparent calcium sensitivity of the α subunit, enhancing the probability of eliciting STOCs in response to calcium sparks [730,1721]. The molecular mechanism could involve the heme group that binds with high affinity to the α subunit of BKCa and inhibits its activity [730,1721]. Indeed, BKCa has recently been recognized as a hemoprotein since its activity is regulated by free heme [672,731,1519,1682]. CO can activate BKCa channels by binding to the channel-bound heme and altering the interaction between the heme and the heme-binding domain. Furthermore, HO and BKCa channels are colocalized in the membrane [1682]. HO activation of BKCa may not only be associated with the production of CO but also with the metabolism of the membrane-associated heme, the very substrate required for the generation of CO. Thus, it is conceivable that compartmentalization of the CO generator (HO), CO receptor (heme) and its downstream target (BKCa channel) may regulate cellular excitability through more than one local signaling pathway [731]. Additionally, CO and CO-donors can activate smooth muscle KATP [467] and/or KV [63].
4.2.1.3. Cardiovascular Effects of Endogenous CO Production.
Endogenously produced CO attenuates the sensitivity to vasconstrictors and to pressure-induced vasoconstriction in various vascular beds [764,894,1647,1793].
Angiogenic factors, such as VEGF, mediate some of their effects through the induction of HO-1 [350]. Nevertheless, HO-1 knockout mice are viable and normotensive but have an abnormal postnatal lung development. When subjected to stress or injury, these genetically deficient animals show exacerbated cardiovascular responses [238,1812]. For instance, HO-1 gene disruption accelerates the formation of arterial thrombosis and enhances oxidative damage to the endothelial cell [1568]. Induction of HO-1 protects against atherosclerotic disease in part by promoting reendothelialization. In HO-1-deficient mice, the generation of endothelial progenitor cells from bone marrow is significantly impaired [1704]. In contrast, overexpression of HO-1 plays a protective role in hypoperfusion and ischemia–reperfusion injury [238,1812]. The pharmacological induction of HO-1 expression by hemin or cobalt protoporphyrin administration produces long-lasting normalization of arterial blood pressure in SHR and reduces the elevation of blood pressure in angiotensinII hypertensive rats, respectively [1458,1646]. However, in the latter model, the lowering of arterial blood pressure was not associated with an improvement in endothelial function [1458].
HO-2 knockout mice are also normotensive but their endothelial cells show an activated phenotype characterized by increases in inflammatory, oxidative and angiogenic factors. In these animals, stroke damage in response to injuries is accentuated, indicating that HO-2 plays an endogenous neuroprotective role in the brain [91,341]. In vivo, during myocardial ischemia, HO-2 activation can evoke a cGMP-mediated vasodilation, but only as a back-up mechanism, which is activated when the other pathways of vasodilation are exhausted [1128].
An endothelial production of CO, contributing to endothelium-dependent relaxations in response to neurohumoral substances, has been demonstrated only in a limited number of arteries [62,1785]. As HO-1 is induced in response to a variety of stresses and especially oxidant stress, the endothelial and even more so the smooth muscle cell release of CO, since the latter is a more important source of CO production in the vascular wall [1644], could contribute to the regulation of vascular tone under pathological conditions such as atherosclerosis [713].
4.2.1.4. CO Contribution to Endothelial Dysfunction.
However, CO also contributes to endothelial dysfunction. Indeed, this gas, by binding to its prosthetic heme, acts as a tonic inhibitor of eNOS and, in resistance arteries, can produce vasoconstriction and impair flow-induced dilatation [356,750,751]. Furthermore, CO induces contractions by stimulating the production of ROS, generated by various enzymes (including NO-synthase, NADPH oxidase, xanthine oxidase and complex IV of the mitochondrial chain), and the subsequent activation of the TP receptors [870]. In transgenic mice overexpressing HO-1, specifically in vascular smooth muscle cells, the systemic arterial blood pressure is elevated when compared to the wild-type controls [1467].
Nevertheless, carbon monoxide releasing molecules have vasodilator, anti-ischemic and anti-inflammatory effects and may present some therapeutic value in cardiovascular diseases [1082,1580].
4.2.2. Hydrogen Sulfide
4.2.2.1. The L-Cysteine–H2S Pathways.
Hydrogen sulfide (H2S), like NO and CO, is a toxic gas. It is colorless with a strong smell of rotten eggs and is produced not only by bacteria but also by mammalian cells, including vascular cells [674,1644,1803]. Two main enzymes are predominantly responsible for the production of H2S, cystathionine β-synthase and cystathionine γ-lyase, and both use L-cysteine as substrate. A third pathway, recently identified, involves the production of H2S in the mitochondria, still from L-cysteine, via the combined action of 3-mercaptopyruvate sulfurtransferase and cysteine aminotransferase. In the cardiovascular system, the production of H2S is generally associated with the activation of cystathionine γ-lyase, while cystathionine β-synthase is the predominant enzyme involved in the nervous system. The major sources of H2S in the blood are likely to be the vascular smooth muscle and the red blood cells [1482,1644,1652]. However, in the endothelial cells, both the cystathionine γ-lyase and the 3-mercaptopyruvate sulfurtransferase–cysteine aminotransferase pathways are expressed and produce H2S [1407,1749] (Figure 17).
4.2.2.2. H2S as a Vasoactive Mediator.
The predominant effect of H2S is to produce vascular smooth muscle hyperpolarization and relaxation. H2S activates KATP channels by causing the S-sulfhydration of specific cystein residues of the SUR subunit protein of the KATP channel complex [1645,1803]. Additionally, H2S could stimulate the metabolism of arachidonic acid by activating phospholipase A2 [306] and acts as a non-specific inhibitor of phosphodiesterases, including PDE5, which enhance the smooth muscle cell levels of cAMP and cGMP. This latter effect could again be attributed to cystein sulfhydration or to the binding of this compound to the coordinated zinc atom, which is essential for the enzymatic activity of phosphodiesterase. Furthermore, H2S, in a cGMP-dependent or -independent manner, could activate other populations of potassium channels such as BKCa and KV [27,222,1436,1823]. An interesting property of H2S is its ability to act synergically with NO. Indeed, low concentrations of H2S greatly enhance the relaxation induced by NO [674]. Finally, in some blood vessels, H2S is an endothelium-dependent relaxing agent, possibly in part because it increases endothelial [Ca2+]i, by modulating calcium entry and calcium handling [75,306,1436].
Conversely, H2S can also be a vasoconstricting substance. This gaseous mediator can reduce NO bioavailability by forming with NO a nitrosothiol compound [25,1675], inhibit eNOS by preventing its interaction with BH4 [848,849], and produce a general down-regulation of the L-arginine-NO pathway not only by decreasing eNOS activity but also by reducing eNOS transcript abundance and L-arginine transport [536]. Additionally, H2S generates vasoconstricting prostaglandins [306] and impairs the cAMP-dependent relaxing pathway in vascular smooth muscle cells [921].
4.2.2.3. Cardiovascular Effects of H2S.
H2S is not only a vasodilator but also has anti-inflammatory and anti-oxidant properties, produces inhibition of vascular smooth muscle cell proliferation, has pro-angiogenic effects, depresses cardiac function and decreases arterial blood pressure [1482,1483,1644,1652]. Furthermore, microvascular endothelial cells and hepatocytes can release cystathionine γ-lyase and cystathionine β-synthase in the circulation. These transsulfuration enzymes generate H2S from homocysteine and protect the endothelium from oxidative stress [81].
Mice with deletion of the cystathionine γ-lyase are hypertensive and, in the mesenteric artery, the endothelium-dependent relaxations are virtually abolished [1749]. In this artery, H2S is produced and released by the endothelial cells in a calcium-dependent manner following neurohumoral stimulation, and evokes relaxation and hyperpolarization of vascular smooth muscle cells by activating KATP channels [1749]. Thus, these results suggest that H2S is an endothelium-derived relaxing and hyperpolarizing factor. However, the precise role of this mediator needs to be further substantiated. In most studies, the endothelium-dependent relaxations of the murine mesenteric artery involve NO release and EDHF-mediated responses, which are not necessarily associated with the activation of KATP channel [430]. The disappearance of both the NO- and EDHF-mediated component of the endothelium-dependent relaxation in cystathionine γ-lyase knockout mice is unexplained at present but could be attributed to the increase in homocysteine levels [313,1244], and/or to the disappearance of the facilitating role of H2S on NO-mediated relaxations [674].
H2S donors are currently being synthesized and have therapeutic potential in cardiovascular diseases associated with inflammatory processes such as reperfusion injury, circulatory shock, atherosclerosis, diabetes, pulmonary hypertension, ischemia–reperfusion injury and possibly hypertension [1652].
4.2.3. Sulfur Dioxide
Sulfur dioxide (SO2) another toxic sulfur-containing gas is also endogenously produced in mammalian tissues from sulfur-containing amino acids such as L-cysteine. Various metabolic pathways are involved and include aspartate aminotransferase or glutamic oxaloacetic transaminase, which is abundantly expressed in endothelial cells. Besides, H2S itself can be the source of SO2 either via NADPH oxidase or thiosulfate reductase. This compound is a vasodilator and a hypotensive agent that may have some relevance in the regulation of cardiovascular system under physiopathological conditions [1652] (Figure 17).
4.3. METABOLISM OF ARACHIDONIC ACID
The fatty acid arachidonic acid, the most common precursor of prostaglandins, is released from the cell membrane phospholipids. Two major phospholipases are implicated in prostanoid formation, phospholipase A2 (PLA2) acting on phosphatidyl-ethanolamine, phosphatidyl-choline or plasmalogens, as well as phospholipase C, which together with the diacylglycerol lipase, acts sequentially on phosphatidyl-inositols derivatives [1441].
4.3.1. Phospholipases A2
In mammals, the phospholipase A2 super family includes at least 25 enzymes identified with PLA2 activity and is subdivided into five main groups: secreted PLA2 (sPLA2), cytosolic PLA2 (cPLA2), calcium-independent PLA2 (iPLA2), the platelet-activating factor acetylhydrolases and the lysosomal PLA2 [160]. Although, sPLA2, cPLA2 and iPLA2 can generate arachidonic acid, cPLA2α (or Group IV-A) is the only PLA2 enzyme that shows significant selectivity toward phospholipids containing arachidonic acid. In the cardiovascular system, sPLA2 are involved in atherogenesis and coronary artery diseases as they modify circulating LDL and HDL and, in the arterial wall, matrix-bound lipoproteins [686].
cPLA2α is expressed ubiquitously and constitutively in most cells and tissues and is an essential component in the initiation of the metabolism of arachidonic acid. The translocation and activation of this intracellular enzyme is activated by submicromolar calcium concentrations and by phosphorylations, both of which are critical events in the post-receptor signaling transduction [850]. Cells isolated from cPLA2α knockout mice produce smaller amounts of prostaglandins and leukotrienes. The phenotype of these genetically modified mice includes urinary concentration defects, intestinal ulcerative lesions and some reproductive disorders, but they are protected in various inflammatory disorders such as anaphylaxis, acute lung injury or organ dysfunction following ischemia–reperfusion [1577]. In myocardial cytosol, the dominant PLA2 activity is calcium-independent, while cPLA2α contributes relatively little to the total measurable myocardial PLA2 activity, although cPLA2α-deficient mice show cardiac hypertrophy [203]. iPLA2s, as their name indicates, do not require calcium for membrane association or enzymatic activity and have been implicated in a number of physiologic and pathophysiologic processes. In both endothelial and smooth muscle cells, iPLA2β is an important effector of calcium signaling and contributes with STIM and ORAI to capacitative Ca2+ entry [118,203].
Following its release, arachidonic acid can be metabolized by several enzymatic systems including cyclooxygenases, lipooxygenases and cytochrome P450 monooxygenases.
4.3.2. Cyclooxygenases
Prostaglandin H (PGH) synthases are the first and rate-limiting enzymes involved in the biosynthetic pathways of prostaglandins. They possess both a cyclooxygenase catalytic activity leading to the formation of prostaglandin G2 (PGG2) and a peroxidase activity catalyzing the reduction of PGG2 to prostaglandin H2 (endoperoxide, PGH2). Although a single protein is associated with both cyclooxygenase and peroxidase activities, PGH-synthases are usually termed cyclooxygenases [1441,1590].
Two cyclooxygenases (COX-1 and COX-2) encoded by two different genes have been cloned and characterized. COX-1, an integral membrane protein found in microsomal membranes, was first purified in 1976 and cloned by three separate groups in 1988 [323,1032,1068,1770], and a few years later, in 1991, COX-2 was identified [653,1139]. Although COX-1 and COX-2 share a high level of homology (65%), the activity and expression of these enzymes are regulated differentially, and they can function independently within the same cell type [295]. An enzymatically active splice variant of COX-1, termed COX-3, is expressed in the heart and cerebral cortex, but the regulation of its transcription appears similar to that of COX-1 [1190]. Fatty acids, such as arachidonic acid, are the preferential substrate of COX-1, while COX-2 uses both fatty acids and 2-arachidonyl glycerol as substrates. Therefore, COX-2 can generate groups of products that COX-1 cannot synthesize. Both COXs depend on the presence of lipid peroxides for their activation but the activation of COX-2 requires 10-fold lower concentrations of hydroperoxide than COX-1, suggesting that COX-2 can function in the presence of COX-1, without the latter being activated [1442].
In healthy blood vessels, both endothelial and to a lesser extent vascular smooth muscle cells express the two COXs, COX-1 being the predominant isoform under static conditions or under chronic shear stress [322,1242,1515]. In most tissues, COX-1 is expressed constitutively, but can also be overexpressed, for instance, in endothelial cells, by shear stress [343]. COX-2 is often induced at sites of inflammation and, in endothelial cells, its expression is enhanced by various cytokines and increases in cyclic-AMP [295,1590]. However, COX-2 is also expressed constitutively in several organs and cell types, including endothelial cells where its expression is also up-regulated by shear stress [493,1515]. In humans, although COX-2 appears to be the major contributor of the overall systemic generation of prostacyclin, in endothelial cells of both healthy and diseased blood vessels, COX-1 is also an important source of prostaglandins [453,493,1016,1311].
Various biologically active eicosanoids are formed from the ephemeral, but also biologically active PGH2, through the action of a set of synthases, namely, PGD, PGE, PGF, PGI and thromboxane synthases. Furthermore, activation of COXs is a source of superoxide anions because of their ability to co-oxidize substances such as NADPH [1514,1694]. Prostaglandins interact with prostanoid (P) receptors, which belong to the G-protein-coupled seven transmembrane domain family. Nine different genes encode these receptors, which are classified into five subtypes, DP1 and 2 (or CRTH2), EP1, 2, 3 and 4, FP, IP and TP receptors, according to their preferential affinity toward the five primary prostaglandins (Figure 18). Splices variants of these receptors have been described in various species including humans. Due to their chemical and metabolic instability, prostanoids have a short half-life (seconds to minutes in biological fluids at physiological pH) and are believed to work as autocrine or paracrine mediators [382,1570]. Additionally, other prostanoids, such as isoprostanes, can activate prostaglandin receptors and produce, for instance, either vasoconstriction or relaxation [737,1748]. Isoprostanes are prostaglandin isomers that are generally produced from the oxidative modification of polyunsaturated fatty acids via a non-enzymatic, free radical-catalyzed mechanism [1081]. However, a cyclooxygenase-dependent production of 8-isoprostanes can take place in endothelial cells [1661]. For instance, the COX-2-dependent production of 8-iso-PGF2α, by activating TP receptors, may contribute to the hypoxia-induced hyper-responsiveness of pulmonary arteries [302]. Additionally, isoprostanes are often used as biological markers of oxidative stress [1247].
In mice, disruption of COX-1 is associated with a decrease in constitutive prostaglandin synthesis but the inducible synthesis remains unaffected. The knockout mice show normal survival but with an impaired platelet aggregation and inflammatory responses and with an increased tolerance to pain and some degree of airway hyper-responsiveness [943,1080]. They show a reduced hypertensive response to angiotensin II and, in isolated aortic rings, a complete inhibition of endothelium-dependent contractions [1444,1513]. COX-1 deletion prevents atherosclerotic lesion formation in ApoE null mice [1017].
The phenotype associated with COX-2 deletion is more severe. Thirty to 40% of the pups from COX-2 knockout mice die within 48 hours from a patent ductus arteriosus. Furthermore, the surviving animals have a shorter life span than the wild-type mice. The disruption of COX-2 is associated with normal constitutive prostaglandin synthesis but the inducible synthesis is impaired. Females have a deficient reproductive function and in both sexes, the inflammatory responses are altered. COX-2 knockout mice have a compromised postnatal kidney development associated with reduced renal blood flow, decreased urine production and the development of nephropathy. COX-2 disruption decreases plasma renin in mice subjected to a low-salt diet. The knockout mice are hypertensive and although platelet aggregation is normal, since mature platelets only express COX-1, they are more susceptible to thrombosis [943,1080,1778]. They show an enhanced pressor effect in response to angiotensin II and, in isolated arteries, endothelium-dependent contractions are virtually unaffected. In mice with a specific deletion of COX-2 in the cardiomyocytes, heart failure and fibrosis are observed. However, in mouse models of abdominal aortic aneurysms, the disruption of COX-2 decrease their formation, and in various experimental models of cancer, it reduces tumor formation [1444,1513].
The double deletion of COX-1 and COX-2 is lethal within hours after birth because of patent ductus arteriosus [295].
4.3.2.1. Prostaglandin D2 and DP Receptors.
PGD2 is a major prostaglandin in the central nervous system and in immune cells. It can also be synthesized in the vascular wall as both types of PGD-synthases are expressed in endothelial cells, where they are up-regulated in response to an increase in fluid shear stress [1485]. However, PGD2 is mainly involved in the regulation of sleep and in allergic responses [1444].
Two distinct types of PGD-synthases have been identified, the lipophilic ligand-carrier protein (lipocalin)-type enzyme and the hematopoietic enzyme. PGD2 is dehydrated to produce the J series of prostaglandin (PGJ2 analogues). PGJ2 acts as a ligand for the peroxisome proliferator-activated receptor-gamma (PPARγ), a transcription factor of the nuclear hormone receptor superfamily, which plays a predominant role in the differentiation of adipocytes [1578]. PPARγ is also expressed in endothelial and smooth muscle cells [722,886] and is involved in the regulation of vascular function and blood pressure, in addition to its well-recognized role in metabolism. Thiazolidinediones, PPARγ agonists, prevent proliferation and migration of smooth muscle and inhibit the interaction between leukocytes and endothelial cells [1629]. They lower blood pressure and have protective vascular effects. In contrast, loss-of-function dominant-negative mutations in human PPARγ cause insulin resistance and severe early onset hypertension [803].
Two PGD2 receptors, DP1 and DP2, have been identified, the latter being also known as CRTH2, a member of the chemokine receptor family [649]. Activation of DP1 increases blood flow and vascular permeability [1444]. For instance, flushing, an unwanted secondary effect of some drugs, such as nicotinic acid, involves in part the COX-1-dependent PGD2 production by Langherans cells and the subsequent activation of skin microvascular DP1 receptors [354]. PGD2 can produce not only endothelium- and NO-dependent relaxations [3] but also direct smooth muscle contractions, the latter effect involving activation of TP receptors [553]. PGD2 is also produced by COX-1 in platelets, where it can elevate cAMP and inhibit their aggregation [382].
4.3.2.2. Prostaglandin E2 and EP Receptors.
Prostaglandin E2 (PGE2), the most abundant prostaglandin in the human body, is involved in multiple physiological effects including gastric secretion and motility, bone formation, pain, inflammation, female reproductive function, body temperature, sleep–wake activity, and the regulation of kidney functions and blood pressure (glomerular filtration rate, tubular salt and water transport, renin release, renal blood flow and vascular tone). The diverse effects of PGE2 (for instance, this prostaglandin can produce both relaxation and contraction of vascular smooth muscle) can be attributed to the existence of four receptor subtypes, namely, EP1, EP2, EP3 (of which three splice variants exist in the mouse and at least eight in the human) and EP4, which are coupled to different signaling pathways [620,895,1190,1470]. In microvascular endothelial cells, PGE2 is the predominant COX product [539].
Three distinct PGE-synthases, responsible for the synthesis of PGE2, have been identified and characterized, one cytosolic, cPGES, and two membrane-bound, mPGES-1 and mPGES-2 [851]. cPGES is a constitutive enzyme expressed ubiquitously and in abundance in many tissues and cell types. This cytosolic form is preferentially associated with COX-1, suggesting that this isoform contributes physiologically to the production of PGE2 [1091,1190], and its function is thought to overlap that of COX-1. Knockout mice studies demonstrate that the deletion of cPGES is lethal [609].
mPGES-1 is localized in perinuclear membranes and shows significant homology with the superfamily of membrane-associated proteins involved in eicosanoid and glutathione metabolism (MAPEG), which includes 5-lipoxygenase-activating protein (FLAP) and leukotriene C4 synthase [734]. In the vascular wall, both the endothelium and the vascular smooth muscle can express this isoform. mPGES-1 is up-regulated in response to stimuli that induce COX-2 expression and is associated preferentially with COX-2 [1091]. Cells from mice deficient in mPGES-1 display impaired LPS-stimulated PGE2 generation but the basal formation, which is cPGES-dependent, is preserved [609]. The deletion of the mPGES-1 gene does not affect arterial blood pressure or thrombogenesis but delays atherogenesis and prevents angiotensin-II-induced oxidative stress and aortic aneurysm formation [243,1639,1640]. Conversely, mPGES-1-deficient mice show adverse left ventricular remodeling after myocardial infarction [299] and can, depending on the model, develop severe hypertension when subjected to a high-salt diet [472,744].
mPGES-2 is a constitutive, Golgi membrane-associated protein that can be coupled to both COX-1 and COX-2 [851]. The real significance of its contribution in PGE2 production under physiological or pathophysiological conditions is questionable since knockout of this enzyme does not result in a specific phenotype or a reduction in PGE2 generation [609].
Although PGE2 is the common ligand of the four receptor subtypes, the amino acid homology among these receptors is limited. The stimulation of the EP1 receptor is predominantly coupled with an increase in intracellular calcium concentration (calcium channel gating and phospholipase C-dependent IP3 formation), that of EP2 and EP4 with an increase in cAMP (activation of adenylyl cyclase) and that of EP3 with a decrease in cAMP (inhibition of adenylyl cyclase) [895,1470]. In the rat aorta, the mRNAs of the four receptor subtypes are expressed and in various rat arteries EP1 activation is associated with contraction [117,1512]. In addition, PGE2 can also produce contraction by activating TP receptors [553]. In contrast, the activation of EP2 and EP4 receptors produces vasodilatation, a phenomenon which also contributes to skin flushing, an unwanted side-effect of the drugs briefly described above [608]. PGE2 activates platelets via EP3 and possibly EP1 receptor stimulation, but at elevated concentrations can inhibit their aggregation by stimulating the IP receptor [399].
The deletion of the various EP receptor genes shows that in the kidney, PGE2 stimulates renin release via the stimulation of EP2 and EP4 receptors but that the four subtypes are involved in the control of renal vascular tone, with EP1 and EP3 increasing and EP2 and EP4 decreasing it [822,1378]. EP1-deficient mice have a urine concentration defect due to a decrease in vasopressin release resulting in hypotension. EP1 receptor deletion reduces the elevated blood pressure and cardiac hypertrophy induced by angiotensin-II [594,1444]. Paradoxically in EP1 knockout males, but not in females, the depressor response to exogenous administration of PGE2 is reduced. However, in both genders, EP2 receptors contribute to the decrease in blood pressure produced by PGE2 [52] and EP2 knockout mice develop salt-sensitive hypertension [965]. EP3 receptor-deficient mice have a bleeding tendency and are resistant to thromboembolism [1780]. In male mice, EP3 receptor activation opposes the vasodepressor response to PGE2, while in female mice, EP4 receptors also contribute to the depressor action of PGE2 [52]. Endothelium- and NO-dependent vasodilatation to PGE2 involves EP4 activation [677]. The germline disruption of the EP4 gene delays the closure of the ductus arteriosus [1119] and enhances infarct size in the heart following ischemia–reperfusion [1726] and specific deletion of EP4 in cardiomyocytes exacerbates the decline in cardiac function observed after myocardial infarction [1444]. Additionally, EP4 receptor activation has been associated with atherosclerotic plaque progression and destabilization [254,1507].
4.3.2.3. Prostaglandin F2α and FP Receptors.
Prostaglandin F2 isoforms are synthesized not only from PGH2, by the membrane-associated 9,11-endoperoxide reductase but also from PGD2 and PGE2 by cytosolic PGD2 11-ketoreductase and PGE2 9-ketoreductase (or in the endometrium by the 20α-hydroxysteroid dehydrogenase), respectively [632,1660].
Prostaglandin F2α interacts with its preferential receptor, the FP receptor, which generally generates an increase in the intracellular concentration of calcium. Prostaglandin F2α is required in the female reproductive system for normal parturition. In the kidney, this prostaglandin is involved in water absorption, causing natriuresis and diuresis and, in the eye, is involved in the regulation of intraocular pressure. Prostaglandin F2α is produced in the vascular wall, including by the endothelial cells. It is also a potent vasoconstrictor as it stimulates not only its preferential FP receptor but also TP receptors and could be involved in the development of cardiac hypertrophy [433,553,620,755,1444,1698]. Additionally, FP receptor can be expressed in endothelial cells and induce endothelium and NO-dependent relaxations when stimulated [229]. Deletion of FP receptors reduces arterial blood pressure and delays atherogenesis in hyperlipidemic mice [1779].
4.3.2.4. Prostaglandin I2 and IP Receptors.
Prostacyclin is an unstable substance formed by prostacyclin synthase, a member of the cytochrome P450 superfamily (in the human CYP8A1) of enzymes. In endothelial cells, prostacyclin synthase is highly expressed [1515] and the enzyme has been reported to be closely associated with COX-1 [796] or COX-2 [1136]. Prostacyclin synthase is also expressed in vascular smooth muscle cells, heart, lung, kidneys, gastric mucosa and intestinal epithelial cells, brain, macrophages, oviducts and embryonic cells [382,1707].
Prostacyclin is a potent platelet inhibitor, preventing their aggregation and their adhesion to the endothelial cell surface and an endothelium-derived vasodilator [1068,1069,1071,1269,1270]. However, this prostaglandin has numerous other physiological functions. Prostacyclin is involved in overall renal function by regulating renal blood flow, glomerular filtration rate and renin release. In addition, prostacyclin is involved in the development, transport and implantation of the embryo, in pain tolerance, in gastric acid secretion and in intracellular signaling by interacting with nuclear receptors and regulating gene transcription [1443,1444,1688,1707] (Figures 19 and 20).
![FIGURE 19. Multiple functions of the prostacyclin IP receptor. The IP receptor belongs to the G-protein-coupled seven transmembrane domain family [1461].](/books/NBK57147/bin/Fig19.gif)
FIGURE 19
Multiple functions of the prostacyclin IP receptor. The IP receptor belongs to the G-protein-coupled seven transmembrane domain family [1461].
The deletion of prostacyclin synthase generates hypertensive mice with thickening and sclerosis of the arterial wall and with kidney infarction associated with interstitial fibrosis and nephrosclerosis [1443,1707]. Conversely, overexpression of prostacyclin synthase, alone or in association with COX-1, prevents injury-induced intimal hyperplasia, pulmonary hypertension and vascular remodeling and protects brain tissue from ischemia–reperfusion injury [1707]. Prostacyclin synthase is among the most sensitive targets of peroxynitrite and is inactivated by concentrations as low as 50 nM [1364,1818,1819]. When PGI2 synthase is inactivated, the excess PGH2 is shunted toward other metabolic pathways leading to a variety of products that are, in general, deleterious to vascular function [433,553].
Prostacyclin is the preferential ligand of IP receptors (Figures 19 and 20). Although several types of G-proteins are likely to be coupled to the IP receptor, in platelets and to a lesser extent in vascular smooth muscle cells, the Gs-adenylyl-cyclase-cyclic adenosine monophosphate (cAMP)-PKA pathway is a preponderant signaling system [255,1443,1688,1689]. The genetic deletion of IP receptors is associated with increased injury-induced restenosis [242], thrombotic events [1093], atherosclerosis [383,823] and reperfusion injury [1725]. Some of the negative effects observed with IP receptor deletion, including restenosis and enhanced platelet activation, can be abrogated by coincidental TP receptor deletion, indicating that prostacyclin regulates the cardiovascular effects of thromboxane A2, and vice-versa [440] (Figure 20). A cross-talk between the two prostanoid-binding sites may involve the heterodimerization of these two receptors. TP receptor agonists stimulating these heterodimers would produce accumulation of cAMP. This and regulation of receptor endocytosis and trafficking would explain some of the braking effects of IP receptors on the cellular effects of TP receptor activation [1685,1686]. In humans, a defect in prostacyclin receptor signaling, as observed in patients with a dysfunctional IP receptor mutation, leads to accelerated atherothrombosis [46]. The accelerated cardiovascular disease associated with this IPR212C mutation is observed in individuals carrying only one copy of the variant allele and can thus be attributed to a dominant negative action not only when dimerized with the wild-type IP receptor but also through dimerization with the TP receptor since the activation of such dimers can no longer lead to cAMP production [692].
4.3.2.4.1. Prostacyclin and Vascular Function.
Since inhibitors of cyclooxygenases abolish the basal and stimulated generation of PGI2, and potent and specific antagonists of the IP receptor block the vasodilator responses that it elicits [553,562], the contribution of PGI2 in endothelium-dependent responses can be assessed. This prostaglandin plays a role in flow-mediated vasodilatation [348,834], but its contribution to acute endothelium-dependent relaxations in response to neurohumoral mediators is often considered as minimal because cyclooxygenase inhibitors, in particular indomethacin, do not affect these responses. However, the role of prostacyclin as an endogenous mediator contributing to endothelium-derived relaxation has generally been overlooked since in some vascular beds, a major vasodilator effect of COX derivatives can only be observed when the other pathways leading to endothelium-dependent responses have been inhibited [272,1830]. The contribution of PGI2 to endothelium-dependent responses is increased in eNOS knockout mice [211,1473]. In coronary arteries from insulin-resistant obese Zucker rats or in the mesenteric vascular bed of mice with streptozotocin-induced diabetes, endothelial dysfunction is prevented by a complementary up-regulation of COX-2 expression and activity [1094,1336]. Similarly, in humans with diabetes and cardiovascular diseases, COX-2-derived prostacyclin can play a compensatory role for the decreased NO bioavailability [157,1025,1484]. This increased contribution of COX-2-derived prostacyclin when NO bioavailability is reduced may explain some of the detrimental cardiovascular effects associated with COX-2 inhibitors [36].
The relaxation to PGI2, or its synthetic analogues, is often associated with the concomitant hyperpolarization of the smooth muscle cells and can involve the opening of KATP [274,1194], KV [914], KIR [1165], BKCa [256,1370] and/or two-pore domain (K2P) K+ channel family [1156]. The activation of K+ channels following IP receptor stimulation relies on cAMP-dependent and -independent pathways. The latter pathway could be associated with the direct activation of the K+ channel by Gs protein [1735]. The contribution of the hyperpolarizing mechanism in the relaxation process can be very significant in some tissues [724,1371] (Figure 21).
Therefore, in numerous vascular beds, PGI2 can act not only as an endothelium-derived relaxing factor but also as an endothelium-derived hyperpolarizing substance [430,1194]. Additionally, prostacyclin is also a potent anti-proliferative agent in vascular smooth muscle cells, and it reduces oxidant stress and prevents cellular adhesion to the vascular wall [382].
However, prostacyclin can also evoke depolarization, e.g., in the guinea-pig carotid artery [274], and contractile responses in various vascular beds including human coronary and umbilical arteries [127,297,904,963,1234,1683]. These depolarizations and/or contractions could be attributed in some instances to the release of endothelium-derived contracting factors [9] but are generally associated with the direct activation of thromboxane receptors by elevated concentrations of prostacyclin, showing that prostacyclin can contribute to endothelium-dependent contractions [274,419,433,1683,1804] (Figure 21). This latter phenomenon will be developed in a subsequent chapter.
Specific and potent agonists and antagonists of the IP receptors have been synthesized. IP receptor antagonists could be of interest for the treatment of pain, inflammation and overactive bladder, providing that cardiovascular side effects can be avoided [755]. IP receptor agonists are prescribed for pulmonary hypertension but are currently administered intravenously or by inhalation and are associated with serious side effects. The recent synthesis of orally active and long-acting IP receptor agonists may prove beneficial not only in pulmonary hypertension but possibly also in atherosclerosis obliterans [440,863].
4.3.2.5. Thromboxane A2 and TP Receptors.
The existence of an intermediate compound in the conversion of prostaglandin G2 into thromboxane B2 was first demonstrated in platelets [606]. This unstable compound, termed thromboxane A2, has a short half-life (approximately 30 seconds at 37°C and pH 7.4). In the cardiovascular system, thromboxane A2 is not only predominantly derived from platelet COX-1 [493] but can also be produced by COX-2, for instance, in macrophages. In the vascular wall, both the endothelial cells and the smooth muscle cells synthesize thromboxane A2 [434]. Thromboxane A2 is enzymatically produced from PGH2 as a substrate by a specific synthase, the thromboxane synthase, which also belongs to the cytochrome P450 superfamily (in the human, CYP5) [1771]. This enzyme catalyzes two distinct reactions, the formation of thromboxane A2 and that of 12-hydroxyheptadecatrienoic acid plus malondialdehyde. The human gene encoding for thromboxane synthase is a single copy gene, and the protein is widely expressed in mammalian tissues including platelets, vascular smooth muscle and to a lesser extent endothelial cells [1401,1417,1515,1638]. Thromboxane A2 elicits diverse physiological/pathophysiological reactions, including direct platelet aggregation and amplification of the responses to other aggregating agents, as well as contraction and proliferation of vascular smooth muscle. Additionally, thromboxane A2 is involved in allergies, modulation of acquired immunity, atherogenesis, neovascularization and metastasis of cancer cells [1098] (Figure 22).
Thromboxane A2 is the preferential ligand of the TP receptor, which was the first receptor of the prostaglandin/endoperoxide pathway to be cloned [651]. The transduction signal associated with the activation of TP receptors mainly involves two types of G-proteins, the Gq (Gq, G11 G15, G16) and the G13 (G12, G13) families, resulting in the activation of phospholipase C and RhoGEF, respectively (Figure 7). Additionally, TP receptors can also be coupled to Gh (phospholipase C) as well as Gi and Gs (adenylyl cyclase)-coupling proteins [1098]. In humans, but not in rodents, two alternatively spliced isoforms of TP receptors, TPα and TPβ, have been described, which differ only in their C-terminal portions (Figure 22). Both isoforms are coupled to phospholipase C activation but TPα stimulates adenylyl cyclase whereas TPβ inhibits it, at least in transfected cells [652]. Furthermore, TP receptors can be engaged in cross-talks with receptor tyrosine kinases, such as the EGF receptor, to induce cell proliferation and differentiation [1098] and with the IP receptor (see above). PGH2 is another potent agonist of TP receptor and higher concentrations of other prostaglandins, isoprostanes and hydroxyeicosatetraenoic acids (HETEs, generated by lipoxygenases and cytochrome P450 monoxygenases or formed by non-enzymatic lipid peroxidation in endothelial cells and leukocytes) can also activate it with various ranges of potency [419,433]. By contrast, epoxyeicosatrienoic acids (EETs), which act as endothelium-derived hyperpolarizing factors in some vascular beds [430], and their dihydro-derivatives (DiHETs), are endogenous antagonists of TP receptors [84].
Reactive oxygen species enhance the stability and increase the density of functional TP receptors at the cell membrane [1583,1684] and, in endothelial cells, the activation of TP receptors inhibits NO production [930]. The generation of deleterious eicosanoids, the post-transcriptional stabilization of TP receptors and the decreased production of NO are reactive oxygen species-dependent feed-forward loops further altering the unbalance between relaxing/anti-thrombosis and contracting/pro-thrombosis pathways. Taken in conjunction, this experimental evidence indicates that TP receptors are likely to play a pivotal role in cardiovascular diseases [419,433].
Mice genetically deficient in TP receptors are normotensive but have abnormal vascular responses to thromboxane A2 and show a tendency to bleeding [1550]. The deletion of TP receptors decreases vascular proliferation and platelet activation in response to intimal lesions [242], delays atherogenesis in apoE-/- mice [823], prevents angiotensin-II- and L-NAME-induced hypertension and the associated cardiac hypertrophy but reduces the extent of kidney injury only in the former hypertensive model [471,473]. TP receptor knockout mice are also protected against various lipopolysaccharide-induced responses such as the increase in iNOS expression [1734], acute renal failure [116] and inflammatory tachycardia [1508]. The phenotype of thromboxane A2 synthase knockout mice is much less pronounced [1776], most likely because thromboxane A2 is only one of the endogenous agonists of TP receptor and also because the deletion of this enzyme may redirect the arachidonic cascade toward less pathogenic synthases.
Metabolically stable agonists of TP receptors with high-affinity and potency, such as U46619, have been synthesized and numerous synthetic TP receptor antagonists have been designed, including SQ-29,548, ifetroban (BMS180291) or S 18886 (terutroban). One of them, seratrodast (AA-2414), is used for the treatment of asthma [755,1098,1430].
4.3.2.5.1. Thromboxane A2 and Vascular Function.
Activation of TP receptors not only causes vascular contraction and platelet aggregation but could also be directly implicated in chronic inflammatory responses [201,1822], contributing to the advancement of atherosclerotic vascular disease. Agonists of TP receptors are potent stimulators of the expression of VCAM-1, a principal mediator of leukocyte adhesion to the endothelium [201,1822]. In human endothelial cells, exposure to a hyperglycemic medium, which simulates diabetes, enhances the surface expression of VCAM-1, while TNF stimulation increases that of ICAM-1. These two phenomena are blocked by TP receptor antagonist [714,1822]. Stimulating TP receptors also increases VCAM-1 expression in smooth muscle cells [78]. Although leukocyte adhesion to endothelium is the primary event in inflammation, vascular smooth muscle inflammation is likely to play a role in the process by enhancing leukocyte emigration and sequestration in the vascular wall. This pro-inflammatory effect of TP receptor activation most certainly contributes to atherogenesis. Indeed, treating hyperlipidemic apolipoprotein E-deficient mice with a TP receptor antagonist decreases atherosclerotic lesion development without affecting hypercholesterolemia [201]. Furthermore, apolipoprotein E mice that are genetically deficient in TP receptors also develop less atherosclerosis [823]. Studies in which bone marrow harvested either from TP receptor-intact or -deficient mice was transplanted into wild-type or TP receptor knockout mice showed that the anti-atherosclerotic protection was conferred by the lack of vascular TP receptors, but not by the absence of TP receptors on bone marrow-derived platelets or leukocytes [1813]. The atherogenic properties of vascular TP receptors were confirmed in a rabbit model that lacked TP receptors only in the vasculature. When compared to control rabbits, a cholesterol-enriched diet produces a less severe impairment of endothelium-dependent relaxations and the incidence of aortic lesions caused by the diet is also diminished [1215]. Additionally, in atherosclerotic plaques taken either from a murine model of atherosclerosis or from atherosclerotic patients, thromboxane synthase is expressed and is associated with thromboxane A2 generation [498]. These results confirm that a TP antagonist can inhibit atherosclerosis development independent of its anti-platelet effects. Since anti-platelet therapy is a standard procedure in patients with arteriosclerotic cardiovascular disease, treatment with an antagonist of TP receptors, which possesses anti-platelet agents with additional direct effects on endothelial and vascular smooth muscle cells, may result in additional therapeutic benefit [414].
4.3.2.6. Endothelium-Dependent Contractions.
Very soon after the seminal discovery of Fuchgott and Zawadzki in 1980 [496] concerning the obligatory role of the endothelium in the relaxation of isolated arteries to acetylcholine, it was observed that in veins, the endothelial cells could cause contractions rather than relaxations of the surrounding vascular smooth muscle cells [305]. These responses were attributed to the production of a diffusible factor(s), termed “endothelium-derived contracting factor” (EDCF) and were shown to depend upon cyclooxygenase activation [1045]. Soon thereafter, cyclooxygenase- and endothelium-dependent contractions were also reported in arteries of different species in response to various agonists, substances that increase endothelial [Ca2+]i in a receptor-independent manner (calcium ionophores, thapsigargin, cyclopiazonic acid) and physical stimuli such as stretch [175,700,789,790,1129,1150,1418,1513,1746,1809]. These endothelium-dependent contractions were observed in healthy blood vessels, suggesting that they play a physiological role in the endothelium-dependent regulation of vascular tone.
However, endothelium-dependent contractions are also frequently associated with cardiovascular disease in both animals and humans [1599]. EDCF-mediated increases in tension associated with endothelial dysfunction were first observed in SHR [963] and then in other models of hypertension, in diabetic animals, in estrogen-deprived female rats, as well as in humans with essential hypertension and diabetes [419,431,433,1599].
4.3.2.6.1. SHR Aorta: The Archetypal Model.
Endothelium-dependent contractions have been extensively characterized in the isolated aorta of the SHR. In this blood vessel, the endothelium-dependent relaxations are impaired [940,963] and are associated with the generation of a diffusible EDCF that opposes the relaxing effect of nitric oxide with no or little alteration in its production [661,1745] (Figure 23). These endothelium-dependent contractions are positively correlated with the severity of hypertension and the aging process [720,824,825], are delayed in female SHR [574,795] and also occur in aging normotensive Wistar–Kyoto rats (WKY) [824,825]. The generation of EDCF is observed in response to the activation of endothelial G-protein-coupled receptors such as muscarinic M3 and purinergic receptors [133,825,1067,1746] and of tyrosine kinase receptors [931], as well as in response to receptor-independent stimuli, for instance, the calcium ionophore, A 23187 [1514,1746]. EDCF contributes to the contractile responses of endothelin [1489,1490] and in the presence of an inhibitor of NOS, a tonic generation of EDCF is observed in SHR aorta and in that of aging WKY [1].
In SHR, inhibitors of COX abolish the endothelium-dependent contractions and fully restore the impaired endothelium-dependent relaxations, indicating that there is no or little alteration in NO production [963], a conclusion strengthened by perfusion–superfusion bioassay studies [661]. Further bioassay studies using layered “sandwich” preparations demonstrated that endothelium-dependent contractions to ACh involve the endothelial release of diffusible contractile COX derivatives which oppose the relaxing effect of NO [1745] (Figure 23).
In SHR aorta, endothelium-dependent contractions are associated with multiple dysfunctions in both the endothelial and the smooth muscle cells. In the endothelial cells, they include: 1) abnormal calcium handling, 2) an increased expression of COX-1, 3) the associated enhanced production of reactive oxygen species, 4) a major increase in prostacyclin synthase expression, 5) the enhanced release of prostacyclin, thromboxane A2, and possibly PGH2. In vascular smooth muscle cells, they include: 1) an exacerbated response of the TP receptor to prostacyclin and PGH2, 2) a deficient IP receptor function and 3) an early dysfunction in the adenylyl cyclase pathway [419,433,434].
4.3.2.6.1.1. Abnormal Calcium Handling.
When compared to WKY aorta and in response to receptor mediated stimuli (acetylcholine), the increase in intracellular calcium ([Ca2+]i) in SHR endothelial cells and the amplitude of the endothelium-dependent contractions are exacerbated, while in response to receptor-independent stimuli (calcium ionophore, A 23187), the maximal amplitude of the endothelium-dependent contractions and the changes in [Ca2+]i in both strains are similar [553,554,1514]. Any event leading to an increase in endothelial [Ca2+]i activates the calcium-dependent phospholipase A2 (cPLA2) and provokes the mobilization of arachidonic acid. However, in response to receptor-dependent stimuli, such as acetylcholine, the activation of the calcium-independent phospholipase A2 (iPLA2) allows the store-operated calcium channel (SOC)-dependent influx of extracellular calcium and the subsequent activation of cPLA2. Substances, such as calcium ionophores, which bypass the cell membrane receptors, cause an increase in [Ca2+]i, and a direct activation of cPLA2 [1696]. Therefore, the iPLA2 pathway associated with calcium mobilization is defective in SHR endothelial cells.
4.3.2.6.1.2. Cyclooxygenases.
The subsequent steps involve the activation of cyclooxygenase and the production of reactive oxygen species along with that of prostanoids. Aortic endothelial cells express preferentially COX-1 versus COX-2 [796,1515]. In SHR endothelial cells, the mRNA and protein expression of COX-1 are enhanced when compared to that of WKY and, in the two strains, both are augmented by aging [528,1515]. In response to acetylcholine, endothelium-dependent contractions and the associated generation of prostaglandins are blocked consistently by specific inhibitors of COX-1 and partially inhibited, to a varying degree, depending on the experimental conditions, by specific inhibitors of COX-2 [528,553,1745] (Figure 23). However, if the endothelium-dependent contractions and the release of prostaglandins by A 23187 are also fully blocked by COX-1 inhibitors, these responses are less sensitive to COX-2 inhibition. This could possibly be explained by the fact that low concentrations of arachidonic acid are preferentially oxygenated by COX-2, while higher ones are preferentially metabolized by COX-1 [1080]. Alternatively, the effects observed with the COX-2 inhibitors could nevertheless be attributed to COX-1 inhibition. Indeed, the ability of COX-2 inhibitors to inhibit COX-1 depends obviously not only on the degree of selectivity of any given inhibitor but also on other factors such as substrate availability, endogenous lipid peroxide levels and plasma protein concentration, explaining why COX-2 inhibitors are systematically more potent in preventing the endothelial production of PGI2 than the platelet production of tromboxane A2 [1052,1658]. In agreement with a preponderant role for COX-1 in endothelium-dependent contractions, these responses are abolished in aortae taken from COX-1 knockout mice while they are maintained in aortic rings of COX-2 knockout animals [1513].
However, in addition to COX-1, COX-2 also can generate vasoconstrictor prostanoids in the SHR endothelial cells. In both WKY and SHR endothelial cells, not only the induction of COX-2 in the aorta but also in resistance arteries is accelerated by ageing and can be associated with the generation of endothelium-derived contractile prostanoids [33,107,642,1406,1619]. For instance, in WKY rats, the impairment of the aortic endothelium-dependent relaxations, observed after chronic treatment with fenofibrate, has been attributed to the endothelial release of COX-2-derived PGE2 acting on smooth muscle TP receptors [108]. In L-NAME-hypertensive rats, the up-regulation of COX-2 enhances the release of EDCFs [1258]. Additionally, the release of COX-2-derived PGF2α and 8-isoprostane augments α-adrenoceptor-induced contractions in SHR arteries [33] and COX-2 also mediates vasoconstrictions in response to tert-butyl hydroperoxide, a product of lipid peroxidation [508].
Additionally, COXs are also involved in the endothelial generation of reactive oxygen species, a key factor in the generation of endothelium-dependent contractions [1514,1744]. Reactive oxygen species decrease NO bioavailability [590,1316] and, as a positive feedback loop, the formation of hydroperoxides further activates COXs [1080]. In addition, since reactive oxygen species diffuse toward the vascular smooth muscle cells, they can stimulate COXs in these cells and produce more contractile prostanoids. Furthermore, oxidative stress enhances the response to TP receptor stimulation [1747]. Finally, the oxidative stress can produce isoprostanes [1081], which could theoretically act as EDCFs and contribute to the endothelial dysfunction [1619].
4.3.2.6.1.3. TP Receptor Activation.
Endothelium-dependent contractions are blocked by TP receptor antagonists, indicating that the generated prostaglandins diffuse toward the vascular smooth muscle cells and directly activate these receptors [51,963,1745] (Figure 23). In the rat aorta, the five major prostaglandins and 8-isoprostane produce contractions that predominantly involve TP receptor activation. However, the involvement of PGD2 and 8-isoprostane in endothelium-dependent contractions can be ruled out since their generation is not affected by acetylcholine [553]. In SHR aortic endothelial cells, the expression of thromboxane synthase is enhanced when compared to that in WKY endothelium [1515]. In response to ATP or the calcium ionophore A 23187, endothelium-dependent contractions are associated with an increased generation of thromboxane A2 and are partially inhibited by dazoxiben, a specific inhibitor of thromboxane synthase that abrogates the production of thromboxane A2 [554,555]. By contrast, acetylcholine produces only a minor dazoxiben-sensitive increase in thromboxane A2 production, and the endothelium-dependent contractions that it evokes are not affected by the presence of the thromboxane synthase inhibitor, indicating that thromboxane A2 is only one of the EDCFs that can be released from SHR aortic endothelial cells [528,553–555,787,825] (Figure 24).
4.3.2.6.1.3.1. PROSTACYCLIN INVOLVEMENT.
Paradoxically, prostacyclin is likely to be a major EDCF in SHR aorta. This conclusion is based on the following observations [553–555]:
- In the SHR aorta, prostacyclin is a contracting but not a relaxing factor [1282] (Figure 21). The absence of relaxation in response to prostacyclin is attributed to an early (as young as 12 weeks old) dysfunction of the IP-receptors of vascular smooth muscle. This dysfunction is tissue-specific since the platelet response to prostacyclin (or its analogues) is unaffected or even enhanced [35,562]. In order to explain this specific smooth muscle cell dysfunction, a decrease in the aortic expression of IP receptors [1138] and an early impairment of adenylyl cyclase signaling have been evoked [35,993]. However, these two hypotheses can only, at best, partially explain the total disappearance of IP-receptor-mediated relaxations in SHR aorta. Indeed, the decreased expression of the IP receptor was not confirmed in later experiments [1515] and when compared to WKY, the relaxations to prostacyclin in SHR aorta are much more severely affected than those produced by other agents that stimulate adenylyl cyclase, such as isoproterenol and forskolin [562]. A potential additional/alternative hypothesis, which requires proper validation, could be the oxidative damage of the IP receptor itself, which contains redox-sensitive cysteines that play an essential role in determining its structure, addressing and function [1461].
- Prostacyclin is a more potent contracting agent in SHR than in WKY.
- The contractions evoked by prostacyclin mimic the endothelium-dependent contractions produced by acetylcholine both in terms of duration and amplitude.
- Endothelium-dependent contractions and prostacyclin-induced contractions both involve activation of TP receptors (Figures 21 and 23).
- Prostacyclin is by far the most abundant prostaglandin released from the endothelial cells in response not only to receptor-dependent stimuli but also to calcium ionophores. This may come as a surprise since prostacyclin synthase is rapidly nitrosylated and inactivated by peroxynitrite [1364,1818,1819]. However, in the SHR aorta, the massive increase in the expression of prostacyclin synthase [1515] may compensate the loss of activity due to peroxynitrite-dependent tyrosine nitration (Figure 25).
- The release of prostacyclin is nearly two times greater in SHR than in WKY (Figure 25).
- The time course of the release of prostacyclin is compatible with the time course of the observed endothelium-dependent contractions.
- The release of prostacyclin correlates with the amplitude of the endothelium-dependent contractions over the full concentration range of ACh in both WKY and SHR (Figure 25).
- The endothelium-dependent contractions and the release of prostacyclin are affected similarly by COX inhibitors.
- The expression of PGIS is by far the most abundant of the prostaglandin synthases expressed in the rat aortic endothelial cells and PGIS and COX-1 co-segregate [796].
- The inhibition of prostacyclin synthesis enhances the ACh-induced endothelium-dependent contractions. Paradoxically, this latter observation also supports the hypothesis that prostacyclin contributes to endothelium-dependent contractions since the inhibition of PGIS may enhance PGH2 spillover, a more potent TP receptor agonist than prostacyclin itself [553,1282].
Prostacyclin has also been identified as a major contributing factor accounting for the endothelial dysfunction observed in the aorta and mesenteric artery of WKY and SHR treated with aldosterone [107,1719]. Thus, although, as a rule, prostacyclin is a vasodilator and an anti-aggregating agent, depending on the circumstances, it can also act as an EDCF.
4.3.2.6.1.3.2. OTHER PROSTAGLANDINS.
Any level of prostacyclin synthase inactivation would theoretically lead to an excess of free PGH2. Since PGH2 is the second most potent agonist at TP receptors and is more effective in activating TP receptors in vascular smooth muscle from SHR than that of WKY, the endoperoxide is also a suitable candidate as EDCF [528,553,787]. Finally, the shunting of PGH2 metabolism toward other metabolic pathways can lead to a variety of products, including PGE2 and/or PGF2α. Therefore, thromboxane A2, PGH2, PGI2, PGE2 and PGF2α can all theoretically act as EDCF and contribute to endothelial dysfunction [419,433,553] (Figure 26).
The EDCF- and TP-mediated responses, first observed in the aorta of the SHR, are not ubiquitous in SHR arteries, but have been reported in other vascular territories such as the mesenteric, skeletal muscle and renal vascular beds [434]. In these peripheral arteries the endothelial dysfunction additionally includes a marked attenuation of the EDHF-mediated component of the endothelium-dependent relaxations which in turn can favor the development of endothelium-dependent contractions.
4.3.2.6.2. Other Models of Hypertension.
In the more severe model of stroke prone SHR (SHRSP), the endothelial dysfunction is exacerbated. EDCF-mediated responses are more generalized than in the SHR of the same age, the NO bioavailability is reduced and the EDHF-mediated responses are diminished [419,1409]. The impaired endothelium-dependent relaxations are associated and co-segregate with the occurrence of stroke, suggesting a potential causal role of endothelial dysfunction in the pathogenesis of this cerebral disease [1622].
In other animal models of hypertension, such as chronic angiotensin II infusion, transgenic animals overexpressing the renin and the angiotensinogen genes, renovascular hypertension (one-kidney, one-clip, Goldblatt hypertension, renal mass reduction) and also in the so-called low renin hypertension models (Dahl salt-sensitive hypertension, deoxycorticosterone acetate-salt (DOCA-salt) hypertension, endothelin-1-induced hypertension), the high arterial blood pressure is generally also associated with an increased generation of reactive oxygen species and an impairment of endothelium-dependent relaxations [883]. Likewise, in different vascular territories of various rat models of hypertension, the production of endothelium-derived contractile prostanoids, derived from either COX-1 or COX-2 and activating TP receptors, contributes to the general endothelial dysfunction. This has been reported, for instance, in the salt-sensitive Dahl rat [70,1806,1807], the L-NAME-hypertensive rat [1072,1200], rats with aortic coarctation-induced hypertension [924], the angiotensin-II-hypertensive rat [775], the murine-Ren-2 transgenic rats [1135] or the transgenic preeclamptic rat model, which also involves an activated renin–angiotensin system [1610]. Additionally, in the DOCA-salt hypertensive rat, both endothelium-dependent and -independent COX-derived contracting factors activating TP receptors contribute to the vascular dysfunction [271,542] and the elevated COX-2 expression observed in this model contributes to the hypertension [10].
Similar observations have been made in various models of hypertensive mice such as angiotensin II-infused mice [1618], transgenic mice expressing both human renin and human angiotensinogen genes [327] or hypertensive eNOS knockout mice [1809]. Furthermore, in these eNOS-deficient mice, the vascular smooth muscle cells are also able to generate thromboxane A2, in a COX-2-dependent manner. Thus, the endothelin-1-induced contraction, which is mediated by ET-A receptors, relies on the production of thromboxane A2 and the subsequent activation of TP receptors [1808].
In line with the phenotype observed in TP receptor knockout mice, TP receptor antagonists given in vivo evoke no or only minor changes in arterial blood pressure, but they limit the endothelial dysfunction associated not only with hypertension [535,1304,1538] but also with diabetes and atherosclerosis.
4.3.2.6.3. Diabetes, Hypercholesterolemia, Atherosclerosis.
Microvascular and macrovascular complications are the major causes of morbi-mortality in patients with insulin-dependent (type I) and non-insulin-dependent (type II) diabetes mellitus, even in the presence of intensive glycemic control. The macrovascular complication is diabetes-accelerated atherosclerosis, while diabetic microvascular diseases include retinopathy, nephropathy and neuropathy. These are leading causes of blindness, end-stage renal disease, myocardial infarction, stroke and peripheral lesions leading to limb amputation. Animal and clinical studies show a strong relationship between plasma glucose levels and microvascular diseases, while both hyperglycemia and insulin resistance, with the associated dyslipidemia, play an important role in the pathogenesis of macrovascular diseases [430]. Hyperglycemia-induced reactive species generation and the ensuing endothelial dysfunction are important factors in the pathogenesis of these diabetic vascular complications [408,1353]. This involves not only a decrease in NO bioavailability and a reduction in EDHF-mediated responses but also an increase in the contribution of EDCFs.
In the aorta and various other arteries of alloxan-treated rabbits and streptozotocin-treated rats [models of insulinopenia and hyperglycemia reproducing some of the features of type I diabetes], endothelium-dependent exaggerated responses to various contractile agonists are observed and endothelium-dependent relaxations are impaired. Both phenomena are generally associated with the occurrence of endothelium-dependent contractions [47,735,821,1404,1405,1410,1527,1537]. In the rabbit aorta, such endothelium-dependent contractions involve the production of reactive oxygen species, the activation of COX and the stimulation of TP receptors possibly by thromboxane A2, PGH2 and/or 15-hydoxyeicosatetrenoic acid (15-HETE) [1534,1536]. Similar findings were obtained in the aorta of streptozotocin-treated rats [47,209,735,798,1410] and in that of the spontaneously diabetic model of Type 1 diabetes, the “bio bred” rat (BB), i.e., the endothelium-dependent relaxations are impaired, and this involves COX-derived contractile prostanoids [641]. However, in the femoral artery of the former diabetic rat model, the COX-1-derived EDCFs activate both TP and EP receptors [1404].
In rat and rabbit arteries, in vitro incubation with an elevated glucose concentration for just a few hours mimics the endothelial dysfunction created by the induction of diabetes [422,1528,1535]. Again, the detrimental effect of elevated glucose concentration has been attributed to the generation of reactive oxygen species, COX activation and the subsequent stimulation of TP receptors (in the rabbit aorta, possibly by thromboxane A2 and/or PGF2α) [1528,1533,1535,1536].
In rodents, numerous genetic and induced-models of type II diabetes, associated or not with obesity, have been generated. They include the Otsuka Long Evans Tokushima Fatty (OLEFT), the Zucker or the WBN/Kob rats and, the ob/ob, the db/db, the NOD or the Tally Ho mice. In these models, endothelium-dependent relaxations are reduced and are generally associated with oxidative stress, the generation of COX-1- and/or COX-2-derived contractile prostaglandins, and the subsequent activation of TP receptors [244,596,927,1001,1062,1151,1159,1184,1291,1337]. In the OLEFT rats, thromboxane A2 and PGE2 are most likely the predominant COX-1- and COX-2-derived contractile prostaglandins [1001,1006–1008].
Finally, in hypercholesterolemic rats and rabbits as well as in mice fed a high-fat diet or in the ApoE null mice, subjected or not to streptozotocin, endothelium-dependent and TP-dependent contractions are also observed [59,742,1036,1075,1563,1605]. In a subset of New Zealand white rabbits that lacked TP receptors only in the vasculature, when compared to control rabbits, a cholesterol-enriched diet produced a less severe impairment of endothelium-dependent relaxations [1215].
These various experimental models show that metabolic diseases are associated with the enhanced activation of TP receptors and that preventing this activation has a beneficial effect on endothelial function [414].
4.3.2.6.4. Other Vascular Pathologies.
Vascular injury and regeneration of the endothelium is an important mechanism leading to the pathogenesis of atherosclerosis. In remodeled rat femoral arteries following endothelial injury, acetylcholine-induced relaxations are impaired because of the production of COX-1 and COX-2-derived PGH2 and PGF2α, which activate TP and possibly FP receptors [650]. In rats, mice and pigs, both thromboxane A2-dependent endothelial dysfunction and thromboxane A2-dependent platelet activation are implicated in the development of hypoxic pulmonary hypertension [444,445,990,1223]. Additionally, in pulmonary arteries of mice subjected to chronic hypoxia, a decrease in the endothelium-dependent relaxations is associated with endothelium-independent contractions involving the COX-2-dependent production of 8-isoprostanes and the subsequent activation of TP receptors [302]. Finally, in the aorta of the aging hamster, endothelium-dependent contractions are evoked by COX-2-derived PGF2α stimulating TP receptors [1698].
4.3.2.6.5. Endothelium-Dependent Contractions and Estrogens.
The direct action of sex hormones on the vasculature, via genomic and non-genomic effects, partially explains the gender difference in the incidence of cardiovascular diseases in pre-menopausal women and age-matched men [1044,1166]. The modulation of endothelium-dependent contractions by estrogens may contribute to this phenomenon.
In arteries of ovariectomized animals, chronic treatment with estrogens reduces the augmented production of vasoconstrictor prostanoids by endothelial COX-1 and reduces the augmented responsiveness of the vascular smooth muscle cells TP receptor [293,296,1168]. Estrogens also reduce acutely EDCF-mediated responses in an NO-independent way [1794]. The production of endothelium-derived prostanoids is larger in arteries from male than female animals [574,763,795,1550]. Additionally, in female mice, the estrogen-dependent activation of COX-2 has an atheroprotective effect by enhancing the production of prostacyclin and subsequently reducing both oxidant stress and platelet activation [383]. It is tempting to assume that the lesser occurrence of cardiovascular disease in women prior to menopause is related in part to the braking effect of estrogens on EDCF-mediated responses.
However, estrogens have also been involved in vasoconstrictor-induced release of constrictor prostanoids [1391]. In isolated aortic rings taken from ovariectomized rabbits, the estrogen supplementation augments the endothelium-dependent contractions to arachidonic acid, possibly via the endothelial production of prostacyclin, again a contractile prostaglandin in this blood vessel [1046]. Similarly, in aortae from female rats when compared to that of males, the release of prostanoids activating TP receptor explains the observed higher contractile response to either vasopressin or phenylephrine. This estrogen-dependent effect was attributed to the endothelial production of COX-2-derived thromboxane A2 and/or PGH2, associated with a coordinated enhanced expression of COX-2 and thromboxane synthase in both endothelial and vascular smooth muscle cells and increased expression of TP receptor in the smooth muscle cells [487,910,911]. This sex-specific deleterious effect of estrogen on the production of contractile prostaglandins could be related to the occurrence of vascular diseases, involving excessive vasoconstriction such as migraine headache, primary pulmonary hypertension and Raynaud's disease, which are more frequent in pre-menopausal women than in age-matched men or post-menopausal women [1391].
4.3.2.6.6. TP Receptors and Vascular Function: Beyond Endothelium-Dependent Contractions.
TP receptors are expressed not only in vascular smooth muscle cells but also in platelets,endothelial cells and possibly circulating monocytes. Since this receptor is involved in the regulation of vascular tone, platelet aggregation, cell proliferation, immunity and neovascularization, it is likely to play an important role in various cardiovascular diseases [1098,1304,1607]. In atherothrombosis,a leading cause of cardiovascular death, the activation of TP receptor is involved in both the formation of atherosclerotic plaque in the arterial vessel wall (see above) and in the formation of blood clots at the sites of plaque rupture [1608]. The activation of TP receptor has also been implicated in myocardial infarction [1167,1304,1586] and nephropathy [1384,1385,1729] as well as shock, sepsis and associated vascular inflammation [30,116,639,696,782,852,1338,1423,1604].
Taken in conjunction, these results, obtained in various animal models involving different species, indicate that TP receptor activation plays a detrimental role in numerous pathophysiological processes.
4.3.2.6.7. Endothelium-Dependent Contractions in Humans.
Endothelial dysfunction was first reported in human coronary arteries of atherosclerotic patients [956]. Since then, endothelial dysfunction has been associated not only with hypertension or atherosclerosis but also with physiological and pathophysiological processes, including aging, heart and renal failure, coronary syndrome, microalbuminuria, dialysis, thrombosis, intravascular coagulation, preeclampsia, Type I and Type II diabetes, impaired glucose tolerance, insulin resistance, hyperglycemia, obesity, postprandial lipemia, hypercholesterolemia, hyperhomocysteinemia, elevated asymmetric dimethylarginine plasma levels, inflammation, vasculitis, infections, sepsis, rheumatoid arthritis, periodontitis, trauma, transplantation, low birth weight, postmenopause in women, mental stress, sleep apnea syndrome, smoking, nitrate tolerance, glucocorticoids and so on [431]. In the human, reactive oxygen species are elevated in many cardiovascular diseases. An increased production of reactive oxygen species, a decreased antioxidant activity, and a reduced ability to scavenge oxygen-derived free radicals all contribute to oxidative stress [883,1559]. In a number of these conditions, the endothelial dysfunction involves the activation of TP receptors.
Endothelium-dependent vasodilatations to physical (shear stress) and/or pharmacological (e.g., acetylcholine, bradykinin, substance P) stimuli are impaired in the forearm, coronary and renal vasculature as well as in various microcirculatory beds of patients with essential or secondary hypertension [926,1185,1492,1495,1498,1499,1501]. In patients with essential hypertension, but not in those with secondary hypertension (primary aldosteronism or renovascular hypertension), non-specific COX inhibitors partially restore the reduced acetylcholine-induced forearm vasodilatation, suggesting in the former the release of EDCF [1492,1495,1499]. In primary hypertensive patients, selective inhibition of COX-1 partially reverses the impairment of vasodilator responses to acetylcholine, while the selective inhibition of COX-2, which does not produce adverse effects in the forearm of healthy subjects [1611], further reduces the increase in forearm blood flow produced by the muscarinic agonist [157]. These results indicate that COX-1-derived vasoconstrictor prostaglandins contribute to the endothelial dysfunction and that the production of vasodilator prostaglandins by COX-2 is of minor importance in subjects with normal endothelial function, but become relatively more important in hypertensive patients with endothelial dysfunction, presumably playing a beneficial compensatory role. Vitamin C normalizes the impaired endothelium-dependent vasodilatation in the forearm and the coronary circulation in patients with essential hypertension, confirming that the generation of reactive oxygen species contributes to the endothelial dysfunction [1446,1493,1612]. Similarly, during the aging process, progressive endothelial impairment has been demonstrated both in conduit arteries and at the microcirculatory level, in the forearm as well as in the coronary circulation [1498,1499,1787]. In normotensive subjects up to the age of 60 years, the main mechanism responsible for the progressive endothelial dysfunction is a primary defect in the L-arginine–NO pathway, without evidence of a substantial contribution of EDCF [1499]. However, in subjects older than 60 years, the infusion of indomethacin potentiates the vasodilatation to acetylcholine, suggesting that EDCF contributes to the dysfunction [1304]. The production of COX-dependent factors is associated with a further and parallel impairment in the L-arginine–NO pathway [1499]. In essential hypertensive patients on the other hand, the contribution of COX-derived vasoconstrictor substances is already detectable in the age group of 31–45 years, and augments in parallel with increasing age [1499]. This finding certainly supports the conclusion that the production of COX-derived EDCF is a characteristic of the aging blood vessel wall, with essential hypertension merely causing an earlier onset and an acceleration of this endothelial alteration [1600].
In patients with coronary artery disease, the impaired acetylcholine- and flow-induced forearm vasodilatation is restored by the administration of a TP receptor antagonist [85]. The fact that the patients in this latter study were already treated with aspirin suggests that COX-2 activity, rather than COX-1, could be the main source of the vasoconstrictor prostanoids involved in this endothelial dysfunction. Indeed, in patients with severe coronary artery disease, COX-2 inhibition improved flow-mediated dilatation [240]. Additionally, bone morphogenic protein 4 (BMP4) and COX-2 are elevated in the renal arteries of hypertensive patients. By increasing NADPH oxidase-derived reactive oxygen species, BMP4 up-regulates COX-2 resulting in the impairment of endothelium-dependent relaxations and appearance of endothelium-dependent contractions. The BMP4 antagonist, noggin, a selective COX-2 inhibitor, or a TP receptor antagonist normalize the relaxations and/or abolish endothelium-dependent contractions [1304,1699].
In addition, women subjected to ovariectomy/hysterectomy, and therefore to acute estrogen deprivation, show a decrease in the forearm vasodilator response to acetylcholine. In healthy adult women, the vasodilatation is unaffected by COX-inhibitors, but after ovariectomy, indomethacin enhances the response. In these subjects, estrogen replacement therapy restores the proper acetylcholine-induced vasodilatation [1225]. This suggests that estrogen protects endothelial function by preventing the formation of COX-derived EDCFs. COX inhibition also improves the endothelial dysfunction observed in patients with atherosclerosis [687] or with congestive heart failure [792] and in impotent diabetic patients, the reduced endothelium-dependent relaxation of the corpus cavernosum involves the activation of TP receptors [40].
These results indicate that, as has been observed in animal models, COX-1, COX-2 or both isoforms can contribute to endothelial dysfunction. Since in most cases, the activation of TP receptors is the common downstream effector, specific antagonists of this receptor could be of therapeutic interest in the treatment of cardiovascular disorders (Figure 27).
4.3.3. Lipoxygenases
Lipoxygenases are a class of non-heme iron-containing dioxygenases that catalyze the stereo-specific hydroperoxidation of polyunsaturated fatty acids. Mammalian lipoxygenases insert oxygen into the 5-, 8- (mice and rat only), 11-, 12- and 15-positions of arachidonic acid with various stereo-configurations (S or R). In humans, six functional lipoxygenase genes have been identified (and three pseudogenes): 5-lipoxygenase, platelet-type 12-lipoxygenase, 12(R)-lipoxygenase, 15-lipoxygenase type I, 15-lipoxygenase type II and epidermis-type lipoxygenase-3, the former one being located on chromosome 10, while the five others are found clustered on chromosome 17 [492,718] (Figure 28).
12R-Lipoxygenase forms 12R-hydroperoxyeicosatetraenoic acid (12R-HPETE) from arachidonic acid with a high specificity. In the human, the pattern of expression of this enzyme is very restricted and appears limited to the skin and the tonsils. Mice deficient for this lipoxygenase develop normally but die shortly after birth from dehydratation [718]. The catalytic activity of epidermis-type lipoxygenase-3 is not properly defined at present [1366].
4.3.3.1. 5-Lipoxygenases.
5-Lipoxygenase converts arachidonic acid to 5(S)-hydoperoxy-6-trans-8,11,14-cis-eicosatetraenoic acid (5-HPETE) and then further hydratation of 5-HPETE leads to the formation of the allylic epoxide leukotriene A4 (LTA4), which can in turn be converted to the dihydroxic acid LTB4 or the cysteinyl leukotrienes LTC4, LTD4 and LTE4 [1268]. LTB4 and cysteinyl leukotrienes interact with G-coupled, seven transmembrane-spanning domain receptors, BLT1 and BLT2 for the former and CysLT1 and CystLT2 for the latter. 5-Lipoxygenase is a calcium-sensitive enzyme, which needs to be translocated to the nuclear membrane, and to a lesser extent to other organelles, in order to show leukotriene synthesis activity. A 5-lipoxygenase-activating protein (FLAP), once thought to be required for the anchoring of the 5-lipoxygenase to the cell membrane, functions as a scaffold protein and is essential for the activation of the enzyme [1267,1268] (Figure 28). Leukotrienes are involved not only in acute inflammation and asthma but also in the generation of cardiovascular diseases including atherosclerosis, abdominal aortic aneurysm and myocardial infarction and reperfusion injury [1230]. 5-Lipoxygenase knockout mice and rats treated with a FLAP inhibitor develop less right ventricular hypertrophy and less pulmonary hypertension under chronic hypoxic conditions than wild-type animals, suggesting that in rodents, the enzyme also contributes to the generation of chronic pulmonary hypertension [492,1620].
4.3.3.2. 12-Lipoxygenases.
12S-lipoxygenase introduces molecular oxygen into arachidonic acid to form 12S-hydroperoxy-5,8,10,14-eicosatetraenoic acid (12S-HPETE). There are three isoforms of 12S-lipoxygenases named after the cells in which they were first identified: platelet, leukocyte and epidermis [1772]. In the human, only the platelet type 12S-lipoxygenase is expressed. The production of 12S-HPETE and the expression of platelet-type 12-lipoxygenase are increased in hypertension [568]. There is a marked up-regulation of 12-lipoxygenase mRNA in the wall of arteries injured by a balloon catheter, which affects mainly smooth muscle and infiltrating inflammatory cells [1108]. Leukocyte-type 12S-lipoxygenase (rat, mouse, pig) shows higher identity with the human and rabbit 15-lipoxygenase-type I than with the other 12-lipoxygenase enzymes, and they are therefore called 12/15-lipoxygenases [1772].
4.3.3.3. 15-Lipoxygenases.
15-Lipoxygenases are lipid-peroxidizing enzymes that catalyze the stereo-selective introduction of molecular oxygen at carbon 15 of arachidonic acid to form 15S hydroperoxy-5,8,11,13-eicosatetraenoic acid (15S-HPETE). Subsequently, 15S-HPETE becomes the substrate for the synthesis of more complex bioactive lipids such as lipoxins. Two types of 15-lipoxygenases have been cloned in the human. Type I, or reticulocyte-type 15-lipoxygenase, as mentioned above, shares a highly phylogenic relatedness with the rat, murine and porcine leukocyte-type 12-lipoxygenase. Type II, or epidermis-type 15-lipoxygenase, is related to the murine 8-lipoxygenase, which shares only a low degree of homology with type I [855]. Type I is expressed ubiquitously, while the pattern of expression of type II appears to be more restricted (skin, prostate, lung and cornea). Type I has a broad substrate specificity which includes linoleic acid with the introduction of oxygen at carbon 13 giving rise to 13-HODE (13-hydroperoxyoctadecadienoic acid), an inhibitor of platelet adhesion to the endothelial cell surface [1591] (Figures 2 and 28). Liberation of arachidonic acid from the phospholipid stores is not an absolute prerequisite for the enzymatic activity of type I 15-lipoxygenase. This isoform may oxygenate polyenoic fatty acid esterified in the membrane and subsequently hydro(pero)xy derivatives can be released by the phospholipases [207,854]. NO may act as a reversible inhibitor of 15-lipoxygenase and can be effectively consumed during 15-lipoxygenase-catalyzed oxidation of polyenoic fatty acids, suggesting that this enzyme could be considered as a regulator of cellular NO metabolism [668,1141]. The biological and pathophysiological roles of 15-lipoxygenases are multiple and include cell differentiation and maturation, inflammation, hypereactivity of smooth muscle (e.g., asthma), carcinogenesis, apoptosis and atherosclerosis [855]. Mouse knock-out for the leukocyte-type 12-lipoxygenase, the enzyme which is most closely related to human platelet type 15-lipoxygenase, when crossed with other strains susceptible to develop atherosclerosis (apolipoprotein E deficient, low-density lipoprotein deficient), shows a 50% reduction in aortic atherosclerotic lesions [492].
4.3.3.4. Lipoxygenases Metabolites as Endothelium-Derived Vasoactive Factors.
Under physiological and/or pathophysiological conditions, endothelial cells express various lipoxygenases including 5-lipoxygenase, platelet-type 12-lipoxygenase, 12/15 lipoxygenase (rat, mouse, pig) and 15-lipoxygenase type I [593,805,891,1801]. These enzymes metabolize arachidonic acid into vasoactive derivatives (relaxing and contracting substances).
4.3.3.4.1. Endothelium-Dependent Relaxations and Hyperpolarizations.
Following the demonstration of the contribution of metabolites of arachidonic acid to certain endothelium-dependent relaxations [305], the involvement of a short-lived metabolite of arachidonic acid produced through either the cytochrome P450 monooxygenase or the lipoxygenase pathway was proposed to explain certain EDHF-mediated responses [839,1224,1318]. In several arteries, endothelium-dependent responses are blocked by inhibitors of phospholipase A2 [7,629,688,1318], and depending on the species and/or the arteries, cytochrome P450- or lipoxygenase-derived products play a predominant role. Thus, in rabbit aorta and mesenteric arteries as well as various arteries from rodents, dog, pig, and human, the lipoxygenases-dependent metabolization of arachidonic acid can be the predominant pathway(s) involved in the synthesis of endothelium-derived relaxing factors in response to arachidonic acid, neurohumoral substances (e.g., acetylcholine, thrombin, arachidonic acid) or oxidative stress. Some of the derivatives can be considered as endothelium-derived hyperpolarizing factors since they are released by endothelial cells and produce hyperpolarization of the underlying vascular smooth muscle cells [216,523].
4.3.3.4.1.1. 12-Lipoxygenase Metabolites.
In coronary arteries of the rat, the endothelium-dependent vasodilatation, resistant to COX and NOS inhibitors, in response to protease-activated receptor-2 involves such a lipoxygenase-derived eicosanoid [1022]. In the basilar artery of the same species, the arachidonic acid-induced vasodilatation is attributed to the release of 12-(S)-HETE and the subsequent activation of BKCa on the smooth muscle [406]. Similarly, in porcine coronary microvessels, and to a much lesser extent in large conduit coronary arteries, 12-(S)-HETE, released by the endothelial cells in response to oxidative stress, acts as a potent relaxing and hyperpolarizing factor by activating smooth muscle BKCa [1814]. In these coronary arteries the relaxing and hyperpolarizing effects of H2O2 involve the lipoxygenase-dependent activation of BKCa [64] (Figures 28 and 29).
4.3.3.4.1.2. 15-Lipoxygenase Metabolites.
The best characterization of a lipoxygenase derivative as an endothelium-derived hyperpolarizing factor was obtained in rabbit and murine arteries. This NO- and PGI2-independent component of the endothelium-dependent relaxation relies on the activation of smooth muscle potassium channels [1219,1220,1517,1790]. Upon exposure to acetylcholine, arachidonic acid, mobilized by phospholipase A2, is metabolized sequentially by the 15-lipoxygenase type I and hydroperoxide isomerase into an hydroxyepoxyeicosatrienoic acid (HEETA), which is hydrolyzed by a soluble epoxide hydrolase (sEH) to trihydroxyeicosatrienoic acid (11,12,15-THETA) and 11,14,15-THETA, the former possessing relaxing and hyperpolarizing properties while the latter is inactive [187,215,1221]. In the smooth muscle cells of the rabbit aorta, 11,12,15-THETA produces relaxation by activating an apamin-sensitive but charybdotoxin-insensitive KCa of “small” conductance (24 pS) [526] while, in the mesenteric artery, BKCa are involved [1790] (Figures 28 and 29). The synthesis of analogs showed that, in order to observe potassium channel activation and vascular relaxation, specific structures and proper stereochemical configurations were required, suggesting that these compounds interact with a specific binding site(s) or receptor(s) [519]. Furthermore, in rabbit aorta and mesenteric arteries, the combination of 15-lipoxygenase and cytochrome P450 2J2 can convert arachidonic acid into two distinct HEETA metabolites, which are hydrolyzed by soluble epoxide hydrolase into an acid-stable endothelium-derived hyperpolarizing factor, 13-hydroxy-trans-14,15-epoxy-eicosatrienoic acid (13,14,15-THETA) [217].
In rabbits, the endothelial overexpression of 15-lipoxygenase-1 enhances acetylcholine-induced endothelium-dependent relaxation in vitro and acetylcholine induces hypotension in vivo [12]. Similarly, chronic hypoxia enhances expression of 15-lipoxygenase-1 and the generation of 11,12,15-THETA and increases endothelium-dependent relaxations [15]. Conversely, the age-related reduction in the NO and prostaglandin-independent relaxations and the hypotension observed in response to acetylcholine are associated with a reduction in vascular 15-lipoxygenase-1 expression and in THETA and HEETA synthesis [13]. These data suggest that, at least in rabbits, endothelial-derived lipoxygenase metabolites of arachidonic acid contribute to the in vivo regulation of vascular tone under physiological and pathophysiological conditions (Figure 29).
However, this pathway has no or little effect in the regulation of blood pressure, since in normotensive animals, pharmacological inhibition of lipoxygenase, and either the genetic deletion or overexpression of 15-lipoxygenase-1 does not affect heart rate or blood pressure [216]. In fact, in angiotensin II-hypertensive animals, lipoxygenase inhibitors lower arterial blood pressure due to a diminished production of vasoconstrictor HETE(s) [216]. Furthermore, in placental tissue of preeclamptic women, enhanced production of 5-HETE, 12-HETE and 15-HETE metabolites has been observed, suggesting either a compensatory or a pathophysiological role of these eicosanoids in pregnancy-induced hypertension [1204]. Finally, the 12/15-LO inhibitor, baicalein, and its derivatives have favorable effects in animal models of ischemic stroke [216]. Much further work is therefore required to determine whether or not 15-lipoxygenase plays a beneficial role in the regulation of cardiovascular homeostatis or conversely is involved in vascular diseases.
Although there is a strong case for arguing that lipoxygenase derivatives act as endothelium-derived mediators in some arteries, most of the EDHF-mediated responses do not involve an arachidonic acid metabolite from this pathway. Evidence based on the effects observed with inhibitors of lipoxygenases must be taken with caution since they are far from specific. For instance, they can affect guanylyl cyclase as well as ionic channels including potassium channels or voltage-dependent calcium channels [257,1262,1738]. Nevertheless, in guinea-pig carotid and coronary arteries [272,369,1131,1262], rat uterine artery [486], porcine coronary artery [1235,1664] and human resistance arteries [174], the involvement of a lipoxygenase-derivative in the genesis of endothelium-dependent relaxations and hyperpolarizations is extremely unlikely.
4.3.3.4.2. Endothelium-Dependent Contractions.
Hydroperoxyeicosatetraenoic acids (HPETE), hydroxyeicosatetraenoic acids (HETE, see also the next chapter concerning cytochrome P450 derivatives) and cysteinyl-leukotrienes can evoke contractions of vascular smooth muscle [159,892,1561,1630]. Endothelium-dependent contractions involving a lipoxygenase pathway have been reported in arteries and veins in response to arachidonic acid [1217], angiotensin II [290], acetylcholine [748,1015], mechanical stretch [470] and simulated ischemia [866]. In the pulmonary artery of the rabbit, a derivative of the 15-lipoxygenase pathway, most likely 15-HETE, is involved in these endothelium-dependent contractions, this pathway being gender-specific and up-regulated by estrogen [1216] (Figure 29). In the SHR aorta, a 5-lipoxygenase-derived product acting on the CystLT1, possibly LTD4, may contribute modestly to the endothelium-dependent contractions elicited by acetylcholine [892].
However, very often the contractile lipoxygenase derivatives generated in the vascular wall in response to various agonists, such as angiotensin-II, arachidonic acid, acetylcholine or bradykinin, do not originate from the endothelial cells [612,736,987,1293,1451,1452].
4.3.4. Cytochrome P450 Monooxygenases
Cytochrome P450 monooxygenases are expressed ubiquitously in bacteria, plants and animals. More than 2500 cytochrome P450 genes have been identified so far, and they are subdivided into 78 families. In the human, at least 57 cytochrome P450 genes and 58 pseudogenes have been sequenced. Cytochrome P450s are heme-containing membrane-bound enzymes that catalyze NADPH-dependent oxidation. Most of these enzymes are expressed in the liver, but both liver and extra-hepatic enzymes are involved in the biosynthesis and/or metabolism of endogenous substrates such as cholesterol, steroids, bile acids, vitamins and fatty acids including arachidonic acid. They catalyze the breakdown of xenobiotics (drugs, toxic chemicals, carcinogens). Some are constitutively expressed in an organ-, gender-, age-, and species-dependent manner while others are regulated by hormones, cytokines, food intake, disease (e.g., diabetes, hypertension) and intake of xenobiotics [190,1300].
Mammalian cytochrome P450 monooxygenases do not metabolize membrane-bound arachidonic acid and are therefore under the control of phospholipases [191]. They oxidize arachidonic acid by the following reactions (Figure 30).
4.3.4.1. Bis-Allylic Oxidation (Lipoxygenase-Like Reactions).
The products of bis-allylic oxidation are structurally similar to those of lipoxygenases with no evidence that hydroperoxide intermediates are formed. These reactions are catalyzed by cytochrome P450 of the 1A, 3A, 2C and 4F families [1300]. They generate any of the six regio-isomeric hydroxyeicosatetraenoic acids (5-, 8-, 9-, 11-, 12-, and 15-HETE). 12R-HETE is the predominant enantiomer generated by cytochrome P450-catalyzed reactions and is a powerful and enantioselective inhibitor of Na+/K+-ATPase [190,1377].
4.3.4.2. Arachidonic Acid ω/ω Hydroxylase.The ω/ω-1 hydroxylation of arachidonic acid is catalyzed mainly by the cytochrome P450s of the 4A, 4B and 4F families, yielding 16-, 17-, 18-, 19- or 20-HETE.
Cytochrome 4A is expressed in blood vessels, mostly in the smooth muscle cells, and is responsible for the synthesis of 20-HETE [793,1300], an endogenous blocker of BKCa and a potent vasoconstrictor, especially in small arteries [611,1816].
4.3.4.3. Olefin Epoxidation (Arachidonic Acid Epoxygenase).
This reaction, producing the four epoxyeicosatrienoic acids (5,6-; 8,9-; 11,12-; 14,15-EET), is catalyzed by numerous cytochrome P450s, namely, the 1A, 1B, 2B, 2C, 2D, 2E, 2J, 3A and 4A families. Human endothelial cells express the 2C8, 2C9, 2J2, 3A and 2B1 families that all produce EETs. EETs are potent vasodilators. They increase the open probability of BKCa in vascular smooth muscle cells, produce hyperpolarization and a subsequent relaxation [186,533]. Only a very limited number of observations suggest that vascular smooth muscle cells can produce EETs [1300]. EETs are converted by epoxide hydrolases into dihydroxyeicosatetraenoic acids (DiHETE). DiHETEs are, in general, less potent vasodilators than the corresponding EETs [707] with some exceptions, such as the coronary circulation where EETS and DiHETE can be equipotent [882,1160].
Cytochrome P450 monooxygenases can catalyze only one or several type of reactions. For instance, CYP 4A1 catalyzes only the ω/ω-1 hydroxylation while CYP 4A2 and CYP4A3 catalyze both ω/ω-1 hydroxylation and epoxygenation [190,1300]. Additionally, the metabolism of arachidonic acid by cytochrome P450 is a significant source of oxygen-derived reactive species and therefore can contribute to oxidative stress. For instance, in endothelial cells of the porcine coronary artery, CYP2C9, which produces vasodilator EETs, also produces superoxide anions [460].
4.3.4.4. EETs
4.3.4.4.1. EETs and Vascular Smooth Muscle Relaxation and Hyperpolarization.
One of the earliest reports demonstrating the vasodilator action of EETs concerned the intestinal microcirculation [1253]. In fact, EETs directly relax many blood vessels and especially the coronary arteries from many species, including humans [186,369,451,490,533,575,1057,1160]. In some cases, EETs produce endothelium-dependent and NO-dependent relaxation of blood vessels [637].
EETs hyperpolarize arterial smooth muscle cells [186,369,451] and increase the open-state probability of BKCa [186,451,532,533,623,679,914], without affecting their unitary conductance or their calcium sensitivity [485]. The mechanism by which EETs activate BKCa in vascular smooth muscle cells is not completely understood. Although in some cell types, EETs can interact directly with BKCa to enhance the mean open time of this channel [352], as has been previously shown with other fatty acids [1164], they do not directly activate these channels in vascular smooth muscle cells. EETs are likely to interact with a G-protein-coupled “receptor,” which remains to be identified, to activate ADP-ribosyltransferase. This enzyme catalyzes the ADP-ribosylation of Gsα, promoting the phosphorylation and the activation of the Slo1 α subunit of BKCa [912,913]. The observation that some EET analogues act in a stereo-specific manner either as inhibitors of the action of EETs (i.e., “antagonists”), such as 14,15-epoxyeicosa-5(Z)-enoic acid (14,15-EEZE) and 14,15-epoxyeicosa-5(Z)-enoic-methylsulfonylimide (14,15-EEZE-mSI), or as EETS mimetics (i.e., “agonists”), such as 14,15-epoxyeicosatrienoyl-sulfonamides, supports the existence of such receptors [155,235,522,1755]. However, if the presence of such a receptor(s)/high-affinity binding site(s) has been shown in guinea pig mononuclear cells [1697], its existence in vascular cells remains to be demonstrated. In smooth muscle cells of rat small mesenteric arteries, EETs activate KATP and not BKCa. This again is dependent on the ADP-ribosylation of Gsα but, in this artery, involves the stimulation of adenylyl cyclase and the subsequent cyclic-AMP-dependent activation of PKA. PKA in turn phosphorylates and activates vascular KATP channels, producing membrane hyperpolarization and vasodilatation [1763,1764] (Figure 31).
Additionally, EETs can also activate BKCa in much more indirect manners. Indeed, 11,12-EET can increase the activity of heme-oxygenase (HO) and the subsequent generation of carbon monoxide (CO) activates BKCa [1328]. Furthermore, EETs can promote Ca2+ entry into smooth muscle cells by activating cationic channels such as the vanilloid transient receptor potential channel (TRPV4). The Ca2+ influx through TRPV4 preferentially stimulates ryanodine receptors located on the sarcoplasmic reticulum, increasing the frequency of unitary Ca2+ release events (Ca2+ sparks). The EET-induced Ca2+ sparks activate nearby sarcolemmal BKCa and increase the frequency of spontaneous transient outward currents (STOCs). This EET-dependent activation of the Ca2+-signaling complex (TRPV4-ryanodine receptors-BKCa) hyperpolarizes and relaxes vascular smooth muscle cells [363] (Figure 31).
4.3.4.4.2. EETs as Endothelium-Derived Relaxing and Hyperpolarizing Factors.
EETs, derived from cytochrome P450 2C or 2J epoxygenases, play an important role in endothelium-dependent hyperpolarizations and relaxations in various blood vessels [1266]. This is particularly the case in bovine [186,629], porcine [629,1238], canine [1676] and human large coronary arteries [1057].
Inhibitors of cytochrome P450 monooxygenases can inhibit endothelium-dependent vasodilator responses to acetylcholine, bradykinin or arachidonic acid, which are resistant to inhibitors of NO-synthase and cyclooxygenase, for instance: in vivo in the hamster cheek pouch microcirculation and in the canine coronary circulation as well as the perfused heart and kidney of the rat and in isolated arteries including canine, porcine and bovine coronary arteries, small bovine adrenal cortical arteries and the murine skeletal muscle arterioles [44,77,89,488,489,575,629,682,1158,1305,1510,1665,1789]. The inhibition of a given EDHF-mediated response by a cytochrome P450 inhibitor has been frequently interpreted as the signature for the endothelial release of EETs. This may not necessarily be the case. First, cytochrome P450 inhibitors, such as clotrimazole, miconazole and 17-ODYA, especially when studied at high concentrations, are notoriously unspecific and among other effects interfere with the activity of potassium channels [32,210,381,1594]. They inhibit hyperpolarizations and relaxations of vascular smooth muscle induced by mechanisms unrelated to that of EDHF-mediated responses including those evoked by levcromakalim, an activator of KATP [368,381,1587,1588]. The lack of selectivity of the available tools has long made it difficult to determine whether it is the activation of the two endothelial potassium channels IKCa and SKCa or that of EETs that is involved in the mechanism underlying an EDHF-mediated response. For instance, clotrimazole, the reference inhibitor of cytochrome P450 epoxygenases, is also a reference blocker of IKCa [189,739]. Charybdotoxin, the other reference blocker of endothelial cell IKCa, also blocks the smooth muscle BKCa that are opened by EETs.
However, more selective inhibitors of cytochrome P450 [705] and of the cellular targets of EETs, as well as specific blockers of IKCa which are devoid of activity toward either BKCa or cytochrome P450 (TRAM 34 and TRAM 39) [1715,1717], allow the proper definition of the contribution of EET to EDHF-mediated responses. Porcine coronary arterial endothelial cells express several cytochrome P450 epoxygenases and in this artery, as well as in hamster resistance arteries, endothelium-dependent hyperpolarizations and relaxations can be inhibited by antisense oligonucleotides against cytochrome P450-2C8-9 [124,451]. In isolated bovine and porcine coronary arteries and in cultured endothelial cells, receptor-dependent and/or -independent agonists and pulsatile stretch release EETs from the endothelial cells, an effect blocked by inhibitors of cytochrome P450 [186,451,530,1238,1240,1306]. Bradykinin stimulates the release of a transferable factor from isolated bovine and porcine coronary arteries as well as from cultures of human umbilical vein endothelial cells that activates BKCa and hyperpolarizes vascular smooth muscle cells [521,530,623,1238]. Furthermore, in coronary arteries, EDHF-mediated responses are increased by agents that enhance the endothelial expression of cytochrome P450 [451,1238]. Taken in conjunction, these observations support the concept that, in some vascular beds, and particularly but not exclusively in the coronary circulation, EETs act as an endothelium-derived hyperpolarizing factor by eliciting the relaxation of smooth muscle by opening BKCa (Figure 31).
Additionally, in porcine and bovine coronary arteries, the EDHF-mediated hyperpolarizations are inhibited by two antagonists of the EET “receptor,” 14,15-EEZE, a non-specific antagonist of EETs, and by 14,15-EEZE-mSi, a preferential antagonist of 5,6-EET and 14,15-EET, which does not inhibit the effects evoked by 11,12-EET [520,524,614,1669]. Both 5,6-EET and 14,15-EET relax the coronary smooth muscle cells by activating BKCa [521,524,1669,1754]. In bovine coronary arteries, bradykinin releases 14,15-EET from the endothelial cells, strongly suggesting that, as least in this artery, it is an endothelium-derived hyperpolarizing factor [521] (Figure 31).
The fact that in bovine and porcine coronary arteries, an EET, most likely the 14,15-EET, is an endothelium-derived hyperpolarizing factor does not imply that the release of this EET explains all the EDHF-mediated responses in these arteries. For instance, in the porcine coronary artery, the endothelium-dependent hyperpolarizations evoked by substance P involve exclusively the activation of the endothelial potassium channels SKCa and IKCa and the subsequent propagation of the hyperpolarization by myoendothelial gap junctions, while that evoked by bradykinin involves two different mechanisms: the activation of the endothelial potassium channels and, additionally and independently of the activation of the endothelial potassium channels, the release of 14,15-EET with the subsequent activation of BKCa on the smooth muscle [374,1669]. Similarly, in rat cremaster arteries, the NO-independent but endothelium-dependent dilations are attributed to both heterocellular coupling and EET release [1023].
However, in other vascular preparations, such as the guinea-pig carotid and basilar arteries or in the rat hepatic, mesenteric and gracilis arteries, EETs evoke no or minor relaxations and/or hyperpolarization. Furthermore, involvement of an EET in EDHF-mediated responses appears unlikely in these [210,273,483,551,1213,1576,1826] and many other preparations, including guinea-pig coronary and mesenteric arteries [333,1739], rabbit renal and mesenteric arteries [480,760], rat renal and carotid arteries [386,745], murine hindlimb vascular bed [142] and porcine coronary microvessels [998].
Although generally described as vasodilators, EETs have the potential to directly contract vascular smooth muscle cells by activating CaV [402]. Additionally, 5,6-EET is a vasoconstrictor of small intrapulmonary arteries. This involves an endothelium-dependent and a COX-dependent mechanism and the subsequent stimulation of smooth muscle TP receptors and Rho-kinase activation [949,1073,1459].
In conclusion, in some vascular beds and following appropriate stimulation, the endothelial cells can release EETs, which generally act as diffusible endothelium-derived hyperpolarizing factors. In order to dissipate the confusion associated with the involvement, or not, of EETs in EDHF-mediated responses, these responses must thoroughly be analyzed with the most specific tools available in order to properly determine the exact mechanism(s) underlying each endothelium-dependent hyperpolarization in any given artery.
4.3.4.4.3. EETs as Intracellular Second Messengers.
EETs can be involved in EDHF-mediated responses without being a diffusible factor per se. Indeed, the hyperpolarization of the endothelial cells may be partly regulated by the activation of cytochrome P450 since they are involved in the regulation of intracellular calcium homeostasis [185,1218]. They were once thought to be involved in the regulation of endothelial calcium release and the modulation of store-operated Ca2+ channels (SOC) in response to calcium-store depletion [659,660]. However, the predominant role of EETs is the activation of TRP channels. In vascular endothelial cells, the 5,6- and 8,9-EET isomers activate TRPV4 channels resulting in calcium entry and cell hyperpolarization [461,1624,1659]. In murine arteries, both acetylcholine-induced and flow-mediated vasodilatations involve the 5,6-EET-dependent activation of TRPV4, a phenomenon markedly reduced in TRPV4 knockout mice [366,947]. In human endothelial cells, bradykinin rapidly promotes the translocation of TRPC3 and TRPC6 channels toward membrane caveolae and the EET-dependent activation of these channels contributes to the net calcium influx [461] (Figure 31).
The regulation of endothelial [Ca2+]i controls the activation of endothelial K+ channels. In addition, independently of their role in calcium homeostasis, EETs could, as in smooth muscle cells, regulate the activity of KCa [67]. Finally, EETs also cause a biphasic change in gap junctional communication between endothelial cells [1239], suggesting that these arachidonic acid metabolites, provided they produce similar effects on myoendothelial gap junctions, could favor transmission of the endothelial hyperpolarization toward the smooth muscle cells (Figure 31).
EETs and other products of cytochrome P450 can therefore also be classified as intracellular second messengers which in some vascular beds could be crucial for the initiation and transmission of endothelial cell hyperpolarization and, as a consequence, for endothelium-mediated hyperpolarization and relaxation of vascular smooth muscle cells.
4.3.4.4.4. Other Effects of EETs.
EETs can also hyperpolarize platelets and inactivate them by inhibiting the expression of adhesion molecule and platelet adhesion to endothelial cells. This effect is directly linked to the membrane potential of the platelets [845,846]. EETs also possess fibrinolytic properties through the induction of t-PA gene transcription in vascular endothelial cells [1134]. Additionally, EETs decrease the leukocyte-induced endothelial expression of VCAM-1, and to a lesser extent that of ICAM-1 and E-selectin, through a mechanism involving inhibition of the transcription factor NF-kappaB. In human endothelial cells, this effect has been attributed to the activation of cytochrome P450 2J2 [1133]. The prevention by EETs of leukocyte adhesion to the vascular wall is independent of any changes in endothelial cell membrane potential, but could be attributed to the activation of PPARγ. Indeed, EETs and their ω-hydroxylated derivatives directly activate PPARγ and PPARα by binding to their ligand-binding domain, being in essence endogenous ligands of these nuclear receptors [281,937,1702]. Finally, EETs might stimulate lipid metabolism and regulate insulin sensitivity and, in some arteries, could act as antagonists of TP receptors [185,880]. Together, these data indicate that EETs, besides their vasorelaxant properties, can modulate thrombosis, inflammation and metabolic syndrome, and therefore play a beneficial role in cardiovascular diseases [1218]. Furthermore, red blood cells also produce EETs (by the direct oxidation of esterified arachidonic acid to glycerophospholipids followed by the monooxygenase-like activity of hemoglobin) and are reservoirs of EETs. Therefore, in addition to serving as carriers of oxygen, red blood cells, by releasing EETs, regulate vascular resistance and contribute to the beneficial cardioprotective effects of these lipid mediators [746,747].
Additionally, eicosanoids stimulate tyrosine kinase activity in endothelial and smooth muscle cells, including the extracellular signal regulated kinase 1 and 2 (Erk1/2), p38 MAP and protein kinase B/Akt [456,658,660]. The further activation of a complex cascade of phosphorylation stimulates endothelial cell proliferation and angiogenesis by a mechanism involving activation of EGF receptors [231,1024,1034,1241,1788]. Furthermore, EETs inhibit migration of the smooth muscle cells through a cyclic-AMP-PKA pathway [1474], indicating that these cytochrome P450 epoxygenase derivatives play a role not only in angiogenesis but also in vascular remodeling.
These positive effects of cytochrome P450 activation could be blunted by the fact that cytochrome P450 2C9, the isoform most commonly associated with EDHF-mediated responses, is also a significant source of reactive oxygen species in coronary arteries [460]. This suggests that this enzyme could be involved in the generation of oxidative stress and vascular diseases. However, different cytochrome P450 isoforms generate varying amounts of oxygen-derived free radicals and overexpression, in bovine aortic endothelial cells, of cytochrome P450 2J2 protects against oxidative stress [454,1743]. Furthermore, reactive oxygen species can no longer be regarded solely as an indicator of cellular damage or as byproducts of metabolism. They are mediators of physiological and pathophysiological events and are also involved in the regulation of vascular tone [1599].
4.3.4.4.5. EETs in Human Blood Vessels.
The in vitro data available demonstrate that the characteristics of the endothelium-dependent responses observed in isolated human blood vessels are similar to those observed in animal studies [89]. EETs, derived from cytochrome P450 monooxygenase, contribute to the endothelium-dependent relaxations, resistant to inhibitors of nitric oxide synthases and cyclooxygenases, in some isolated arteries including coronary, renal, internal mammary and subcutaneous arteries [44,261,801,881,1056,1058], while other reports indicate that EETs do not appear to be involved in many isolated blood vessels including the human omental, gastroepiploic, subcutaneous, mesenteric, renal interlobar and submucosal intestinal arteries [171,174,621,897,997,1147,1579,1634].
In vivo, in humans, the involvement of cytochrome P450 metabolites in the endothelium-dependent vasodilator responses is difficult to assess. An EDHF-mediated mechanism is often suggested to explain vasodilatations resistant to inhibitors of nitric oxide synthase. Complete blockade of nitric oxide synthase is difficult to obtain (or to demonstrate), many studies do not involve the concomitant administration of an inhibitor of cyclooxygenases and the tools available such as tetraethylammonium, as a blocker of KCa channels, and miconazole, sulphaphenazole or fluconazole, as inhibitors of cytochrome P450, are not very specific (see above) [89,430].
In peripheral muscular resistance artery, by measuring changes in forearm blood flow using venous occlusion plethysmography, the contribution of EETs has been suggested in normotensive subjects [603] and as a compensatory mechanism in aging hypertensive subjects and in patients with primary hyperparathyroidism [1491,1617]. Similarly, in peripheral conduit arteries, by measuring the radial artery diameter (echo tracking) and blood flow (Doppler), it was shown that endothelial-derived EETs regulate basal artery diameter and flow-mediated dilatation [87–90,448]. This pathway, in contrast to the nitric oxide pathway, is preserved in patients suffering from heart failure [448]. Furthermore, during flow variations, arterial wall stiffness is regulated by the endothelium through the release of both NO and cytochrome-related endothelium-derived hyperpolarizing factor(s) [86]. Endothelial EETs could also partially compensate the NO-dependent endothelial dysfunction observed in subcutaneous resistance of preeclamptic women, but this compensatory mechanism was not observed in small myometrial arteries [957,959].
However, in another study also involving occlusion plethysmography in the human forearm, cytochrome P450-derived EETs do not appear to contribute in the baseline blood flow and bradykinin-induced vasodilatation since these responses were insensitive to sulphaphenazole [1196].
4.3.4.4.6. EETs: Therapeutic Potential.
Since EETs are potent vasodilators, possess anti-inflammatory properties and appear in general cardioprotective, this arachidonic acid pathway is likely to contain therapeutic targets (Figure 32).
FIGURE 32
Cytochrome P450 pathway and potential therapeutic targets in cardiovascular diseases. CYP: cytochrome P450; DHETE: dihydroxyeicosatetraenoic acids; EET: epoxyeicosatrienoic acids; HETE: hydroxyeicosatetraenoic acids; sEH: soluble epoxide hydrolase.
The most promising one is the inhibition of soluble epoxide hydrolase, the enzyme that metabolized the EETs in the less active corresponding diols (dihydroxyeicosatrienoic acids; DHETs) [706]. The genetic polymorphisms of cytochrome P450 2C8, 2C9 and 2J enzymes have been associated with hypertension, myocardial infarction and cardiovascular disease and those involving the soluble epoxide hydrolase gene (EPHX2) with coronary artery disease, ischemic stroke and insulin resistance [527,592,704,1148,1815]. Potent and selective inhibitors of this enzyme have been designed and are currently undergoing clinical trials for the treatment of hypertension. The additional anti-inflammatory and cardioprotective properties of soluble epoxide hydrolase inhibitors also make them attractive in patients with heart failure, cardiac arrhythmias, ischemic stroke, atherosclerosis and in the treatment of end-organ damage patients with cardiometabolic syndrome and/or hypertension [704,706].
Metabolically stable analogues of EETs have been designed in order to act as putative agonists of the EET “receptors.” In various animal models of cardiovascular disease and metabolic syndrome, they demonstrate antihypertensive, anti-inflammatory and cardioprotective effects. Similarly, cardioprotective and antidiabetic effects were observed by overexpressing cytochrome P450 2J [587,704,706,1396,1731]. Additionally, existing therapies such as statins, calcium channel blockers and fibrates are potent inducers of cytochrome P450 epoxygenases and the resulting increase in EET generation may contribute to their beneficial therapeutic effects [89].
4.3.4.5. 20-HETE.
In the vascular wall, while endothelial cells preferentially express cytochrome P450 epoxygenase and are the predominant source of EETs, the vascular smooth muscle cells preferentially express cytochrome P450 hydrolase, CYP4A and CYP4F isoforms, and are the predominant source of 20-HETE [1060].
4.3.4.5.1. Physiological Effects of 20-HETE.
20-HETE plays an important role in the regulation of renal function and vascular tone [1681]. 20-HETE is produced in the kidney where it inhibits sodium transport and Na+K+-ATPase in the renal proximal tubule and in the thick ascending loop of Henle and is involved in the tubuloglomerular feedback response. In the glomerulus, it plays a critical role in maintaining the glomerular filtration barrier to albumin. 20-HETE participates in the pressure-natriuretic response and therefore in the long-term control of arterial blood pressure [282,1681].
20-HETE is also a potent vasoconstrictor and plays an important role in regulating vascular tone in the brain, kidney, heart and splanchnic vascular beds. In these vascular beds, the inhibition of cytochrome P450 activity produces vasodilatation [531,611,793]. An increase in the production of 20-HETE mediates the myogenic response of skeletal, renal and cerebral arteries to elevation in transmural pressure and the inhibition of 20-HETE synthesis impairs the in vivo autoregulation of renal and cerebral blood flow. In vascular tissues, the formation of 20-HETE is stimulated by angiotensin II, endothelin-1 or serotonin [1060,1681].
20-HETE-induced vasoconstriction generally involves a direct effect on the smooth muscle cells. Structural analogues act as agonists and antagonists, strongly suggesting that 20-HETE interacts with a specific binding site or with a “receptor” [1777]. For instance, the structural analogues, 5-, 15-, and 19-HETE, antagonize its vasoconstrictor action and could serve as endogenous inhibitors [29] (Figure 30). 20-HETE phosphorylates and inhibits BKCa, possibly via a PKC-dependent or a MAP kinase-dependent pathway, and activates TRPC6 and CaV, thus depolarizing and contracting the vascular smooth muscle cells [71,532,611,874,968,1140,1472,1816]. Additionally, the contraction can be elicited by the activation of Rho-kinase and the increased sensitivity of contractile proteins to calcium [1279].
The vasoconstrictor effect of 20-HETE can also involve indirect effects such as an endothelial and cyclooxygenase-dependent production of thromboxane A2 [1279], or the activation of TRPV1 on C-fiber nerve endings and the release of vasoactive neuropeptides [1379]. In small porcine coronary arteries, the cytochrome P450 4A-dependent production of 20-HETE functionally antagonizes EDHF-mediated relaxations by a protein kinase Cα-dependent mechanism involving the inhibition of the Na+/K+-ATPase [1280].
However, 20-HETE can also act as an endothelium-dependent vasodilator in pulmonary arteries, via the NO pathway and, depending on the species, in basilar and coronary arteries, via the cyclooxygenase pathways [237,401,1249]. Finally, 20-HETE may act as an oxygen sensor in the microcirculation [1300].
4.3.4.5.2. 20-HETE and Cardiovascular Diseases.
Various polymorphisms of the human CYP4A and CY4F genes have been associated with hypertension [1681], and the formation of 20-HETE and the expression of cytochrome P450 enzymes are altered in many models and in some forms of human hypertension [1349]. In humans, an increase in urinary excretion of 20-HETE is associated with endothelial dysfunction, as measured by flow-mediated vasodilatation in the brachial artery [1657]. Cytochrome P450 4A2 overexpression in the blood vessel wall increases the production of 20-HETE and causes hypertension and endothelial dysfunction [1637], and overexpression of CYP4F2 in the proximal tubule of transgenic mice increases the renal formation of 20-HETE and arterial blood pressure [934]. Conversely, specific inhibition of the synthesis of 20-HETE reduces infarct size after ischemic stroke, reverses the delayed vasospasm in models of subarachnoid hemorrhage and decreases cardiac infarct size following ischemia–reperfusion [599,1061,1132,1509]. Androgens increase the expression of CYP4A and the formation of 20-HETE, suggesting that it is a key mediator of androgen-induced endothelial cell activation,vascularresistance and hypertension, possibly by activating the pro-inflammatory nuclear factor NF-κB [241,1703]. The increased expression of this enzyme may explain the gender difference in the regulation of arterial blood pressure and the increased prevalence of hypertension in males [1060,1681].
Taken together, these findings indicate that drugs that target the 20-HETE pathway could be of therapeutic interest. Inhibitors of its synthesis and stable analogues with antagonistic properties are currently being evaluated in various animal models of cardiovascular disease. Conversely, agonists may be valuable in promoting sodium excretion and could oppose salt-sensitive hypertension and prevent proteinuria and glomerular injury [1060,1681] (Figure 32).
4.4. ENDOCANNABINOIDS AND OTHER LIPID MEDIATORS
4.4.1. Endocannabinoids
Endocannabinoids are the endogenous ligands that bind and activate the cannabinoid receptors first identified from the action of (-)-Δ9-tetrahydrocannabinol, the active substance of the recreational drug marijuana, derived from the plant Cannabis sativa. These lipid mediators can be generated in virtually all cell types, including endothelial cells [518] and, along with their cellular receptors and the proteins implicated in their synthesis, release, transport and degradation, are part of a novel signaling system termed the endocannabinoid system. The two most widely studied endocannabinoids are arachidonoyl ethanolamide (anandamide) and 2-arachidonoylglycerol, but several other similar endogenous substances have also been identified. These endogenous lipid mediators are agonists of either one, or both, of the cloned and characterized G-protein-coupled cannabinoid receptor subtypes, CB1 and CB2 [1175]. Based on pharmacological evidence, additional cannabinoid receptor candidates have also been proposed. For instance, anandamide is also an agonist of the elusive non-CB1, non-CB2, termed “abnormal” cannabinoid receptor, possibly the orphan G-protein-coupled receptor GPR55, which may underlie some of the endothelial effects of the lipid mediator [328,556,738]. Furthermore, anandamide stimulates and inhibits different populations of channels/receptors. Anandamide activates the vanilloid receptors TRPV1 on sensory nerves and causes vasodilatation by releasing calcitonin gene-related peptide (CGRP), mimicking the effects of capsaicin, the active ingredient of “hot” red peppers [1829]. Anandamide also inhibits the T-type calcium channel [223] and the K2P potassium channel, TASK-1 [971]. Anandamide is thought to be synthesized and released in a calcium-dependent manner following the enzymatic hydrolysis of the membrane precursor N-acyl-phosphatidylethanolamine by a specific phospholipase D and is a preferential activator of the CB1 receptors [308,312,329].
Additionally, a number of related acylethanolamides, such as oleoylethanolamide and palmitoylethanolamide, have emerged as endogenous lipids with possible signaling and biological regulatory functions. Despite its structural similarity with anandamide, oleoylethanolamide is not a ligand at classical CB receptors but is linked to the activation of the nuclear receptor PPARα. However, some effects of oleoylethanolamide are independent of PPARα and could involve the orphan G-protein-coupled receptor, GPR119. Palmitoylethanolamide is also a ligand for PPARα with analgesic and anti-inflammatory activity. It has very low affinity for CB1 or CB2 receptors but is an agonist for GPR55 [556].
4.4.1.1. Anandamide as an Endothelium-Derived Relaxing and Hyperpolarizing Factor.
In isolated and perfused mesenteric and coronary arterial beds of the rat, anandamide induces a dilatation which mimics the endothelium-dependent vasodilation that could be observed in the presence of inhibitors of NO-synthases and cyclooxygenases [1277]. However, further analysis in isolated blood vessels from various species demonstrated beyond any doubt that anandamide did not fulfill the criteria to be classified as an endothelium-derived hyperpolarizing factor [212,1227,1672,1828]. For instance, in the isolated mesenteric artery of the rat, the acetylcholine-induced endothelium-dependent hyperpolarization involves the activation of both IKCa and SKCa and is reproducible over time, while the endothelium-independent hyperpolarization elicited by anandamide is tachyphylactic and involves the activation of KATP [212]. Furthermore, EDHF-mediated responses are not inhibited by pharmacologically relevant concentrations of the CB1 receptor antagonist [212,1227,1248,1828]. Finally, some of the effects of anandamide are endothelium-dependent, i.e., anandamide, depending on the vascular bed, releases NO, prostacyclin and induces EDHF-mediated responses or conversely inhibits these responses [212,221,390,462,1248,1828].
4.4.1.2. Physiological and Pathophysiological Cardiovascular Effects of CB 1 and CB2 Receptor Activation.
Nevertheless, anandamide and some of the other endocannabinoids, besides their actions in the central and peripheral nervous system, are likely to play an important role in the regulation of the cardiovascular system [648,1275,1278]. In vivo administration of cannabinoids evokes hypotension, bradycardia, and depressed cardiac contractility. Both in isolated blood vessels and in anesthetized animals, endogenous and exogenous cannabinoids usually have vasodilator properties. In vitro, the vasodilatory effect of endocannabinoids and synthetic analogues is tissue- and species-specific and could result from the activation of CB1 receptor, the endothelial “abnormal” cannabinoid receptor and/or TRPV1 channels, and involves NO-mediated or NO-independent mechanisms [1278]. Under numerous pathological conditions (hemorrhagic, endotoxic and septic shock, advanced liver cirrhosis and heart failure), the endocannabinoid system may become over-activated and contribute to hypotension/cardiodepression through the activation of cardiovascular CB1 receptors [1174,1175]. Endocannabinoid-mediated CB1 signaling is also involved in the pathogenesis of atherosclerosis. If, on the one hand, endocannabinoids released from endothelial cells, macrophages or platelets reduce hypertension, on the other hand, they induce CB1-dependent platelet and macrophage activation, endothelial generation of reactive oxygen species, endothelial dysfunction and death as well as smooth muscle cell migration and proliferation [1272,1273,1276,1469]. The endocannabinoid system is also involved in the regulation of food intake and obesity-related metabolic disorders, including alterations in lipid profile and glucose homeostasis [394,996]. Clinical trials have shown the pharmacological CB1 blockade to have multiple therapeutic benefits in obesity and associated cardiometabolic disorders. However, the increased incidence of anxiety and depression in treated patients has halted these clinical trials [754,1354].
Activation of CB2 receptors attenuates endothelial inflammatory response, chemotaxis and adhesion of inflammatory cells to the activated endothelium and the consequent release of a variety of proinflammatory mediators, key processes involved both in the initiation and progression of atherosclerosis and restenosis, and in the mediation of reperfusion-induced tissue damage. CB2 receptor activation also diminishes smooth muscle cell proliferation. The potent anti-inflammatory and anti-mitogenic effects of CB2 receptors could be of interest in ischemia–reperfusion injuries and in atherosclerosis [1174,1175].
4.4.2. Other Lipid Mediators
Endothelial cells can synthesize and release other vasoactive lipid mediators. Adrenic acid (docosatetraenoic acid) is identical to arachidonic acid except for 2 additional carbons on the carboxyl end, and is abundant in the vasculature and especially in the adrenal gland. Adrenic acid is metabolized by cyclooxygenases, cytochrome P450s and lipoxygenases. The cytochrome P450 metabolite of adrenic acid, dihomo-16,17-epoxyeicosatrienoic acid (DH-16,17-EET), activates smooth muscle BKCa channels to cause hyperpolarization and relaxation. In the adrenal cortex and in the coronary arteries, this endothelial- and zona glomerulosa cell-derived metabolite functions as an endogenous hyperpolarizing factor [842,1767].
Sphingolipids, a large class of compounds derived from the aliphatic amino alcohol sphingosine, are structural components of membrane and intracellular signaling molecules but are also circulating compounds, which act as intercellular signaling molecules by interacting with specific cell surface G-protein-coupled receptors (S1P1 to S1P5, the first three being expressed in the vasculature). Sphingomyelin is degraded by sphingomyelinase to form ceramide, which under the action of ceramidase leads to the formation of sphingosine, which is subsequently phosphorylated by sphingosine kinases to yield sphingosine 1-phosphate [1374]. The predominant sources of sphingosine 1-phosphate in the vascular system are the hematopoietic cells, but endothelial cells are also a major contributor to the plasma concentration of this compound, the release of which is stimulated by shear stress [1606]. Sphingosine 1-phosphate is not only a vasoactive molecule but is also involved in endothelial and vascular smooth muscle cell proliferation and migration, in vascular permeability and integrity, and in leukocyte adhesion and inflammation [1374]. By activating S1P2 and S1P3, sphingosine 1-phosphate acts predominantly as a vasoconstrictor but it can cause also endothelium-dependent relaxations by stimulating S1P1 or S1P3 [633,694]. New therapeutic targets for cardiovascular diseases, including atherosclerosis, may be identified within this pathway [1435].
Isoprostanes, the previously mentioned family of prostaglandin isomers generally produced from the oxidative modification of polyunsaturated fatty acids [1081], not only produce vasoconstriction by stimulating TP receptors but can also evoke vascular smooth muscle relaxation. For instance, 8-isoprostaglandin E2 produces relaxation of the murine renal artery by provoking the cAMP-dependent activation of BKCa [1800].
Palmitic acid methyl ester is also a potent vasodilator, and it has been suggested recently that this fatty acid could be the elusive retina-derived relaxing factor first described by the group of Van de Voorde [301,969]. Palmitic acid methyl ester, released from superior cervical ganglion by electrical stimulation and constitutively from retinal tissue, produces relaxation by activating vascular smooth muscle KV channels [890,923]. Whether or not this lipid mediator could be produced and released in the vascular wall has not been yet determined.
4.5. OTHER ENDOTHELIUM-DERIVED VASOACTIVE SUBSTANCES
4.5.1. Adenosine and Purines
Nucleotides and nucleosides are extracellular signaling molecules which are released by many different cells under various physiological and pathophysiological conditions. Adenosine interacts with P1 receptors of which four types have been identified (A1, A2A, A2B and A3), while nucleotides (ATP, ADP, UTP) interact with the P2 receptors divided into two main subgroups: the P2X ligand-gated ion channel (7 subtypes) and the P2Y G-protein-coupled receptor families (8 subtypes) [166]. The A1 and A2A receptor subtypes can form heterodimers with other receptors [474]. In the vascular system, nucleosides and nucleotides contribute to short-term signaling events associated with the moment-to-moment control of vascular tone. However, these molecules are also involved in the regulation of migration, proliferation and apoptosis of vascular smooth muscle and endothelial cells and hence in the long-term normal control of the geometry of the vascular wall, as well as in pathological events such as arteriosclerosis, restenosis, wound healing and the genesis of collateral and tumoral vascularization [165]. In general, ATP and ADP produce endothelium-dependent relaxations and/or hyperpolarizations of smooth muscle cells or evoke direct contractions and/or depolarizations by activating various purinoceptors, P2X and/or P2Y subtypes located in the endothelial and smooth muscle cells [857,1157,1274].
Adenosine is an endogenous vasodilator which is released from contracting skeletal muscle where it contributes to exercise hyperemia, and from the myocardium where it is an important regulator of coronary blood flow especially during ischemia [101,259,1403]. Adenine nucleotides and adenosine are also released by endothelial cells [1416,1421]. The release of adenosine by endothelial cells can be stimulated by chemical and physical stimuli including hypoxia and increases in shear stress [112,164,1047,1112] by neuromediators such as ATP itself [113,1386] and by drugs such as α1-adrenergic agonists [1416]. However, endothelial cells mainly release ATP, but the nucleotide is rapidly transformed into ADP, AMP and adenosine by ectonucleotidases. Thus, CD73, one of the ectonucleotidases that dephosphorylates AMP to adenosine, plays an important role in regulating vascular permeability and leukocyte adhesion and trafficking. Endothelial-specific deletion of Cx40 promotes atherosclerosis by decreasing CD73 expression and activity and the resulting lower adenosine availability [206].
Adenosine can induce both endothelium-dependent relaxations and direct relaxations of vascular smooth muscle cells. The endothelium-dependent relaxations evoked by adenosine are linked to the activation of either A1 or A2A receptor subtypes, while the direct relaxation and hyperpolarization of the vascular smooth muscle cells involve predominantly the stimulation of the A2A adenosine receptor subtype and the subsequent activation of KATP channels [1162,1486]. However, activation of the A2A receptor subtype is also involved in the basal and angiotensin II-dependent reactive oxygen species production by NOX-2 [1541].
4.5.2. Peptides
Endothelial cells can synthesize numerous peptides including endothelins, urotensin, vasoactive intestinal peptide (VIP), substance P, calcitonin gene-related peptide (CGRP), adrenomedullin, neuropeptide Y (NPY), C-type natriuretic peptide (CNP) and possibly angiotensin II [184,1460,1471]. Among these peptides, CNP, VIP, CGRP and adrenomedullin cause relaxation of vascular smooth muscle cells, while endothelins, urotensin, angiotensin II and to a lesser extent NPY are potent vasoconstrictors.
4.5.2.1. Endothelins.
A turning point in the quest to identify endothelium-derived contracting factors was the discovery that, besides superoxide anions, endoperoxides and thromboxane A2, endothelial cells in culture synthesize and secrete a vasoconstrictor peptide [544,645,1313]. A gene coding for this peptide was then cloned and the product of this gene, endothelin-1 (ET-1), a peptide of 21-amino acid residues, was identified as one of the most potent known vasoconstrictors [1741]. Soon after, two other closely related peptides, ET-2 and ET-3, coded by two different genes, were characterized [708]. Nevertheless, ET-1, an autocrine, paracrine and endocrine factor, is the most abundant and important endothelin isoform produced by endothelial cells. However, endothelins are also produced by a wide range of tissues, and indeed cells other than the endothelial cells are likely to be involved in the major role that these peptides play in development and/or physiology [859,1355] (Figure 33).

FIGURE 33
The endothelins. ET: endothelin; ECE: endothelin-converting enzyme.
4.5.2.1.1. Synthesis and Degradation.
ET-1 is synthesized as an inactive pre-proET-1 that undergoes removal of a short signal sequence by a signal peptidase to yield proET-1. In endothelial cells, proET-1 is cleaved by furin or PC7 convertases to form Big-ET-1. The basically inactive Big-ET-1 is then converted to the 21-amino acid ET-1, predominantly by the action of endothelin-converting enzymes (ECEs), integral membrane zinc peptidases belonging to the neprylisin family, which also includes the neutral endopeptidase 24-11 (NEP) and the Kell blood group family of proteins [828,1573]. Two different ECEs encoded by two different genes, ECE-1 and ECE-2 can generate ET-1, ET-2 and ET-3 [393,1727] (Figure 33).
ECE-1 is constituted by 4 isoforms due to alternative splicing, which confers a different cellular localization for each of these different isoenzymes [1582]. In various endothelial cells, ECE-1d is the isoform predominantly expressed [1028,1087]. In endothelial cells, ECE-1a and ECE-1c are preferentially addressed to the cell membrane, while ECE-1b and ECE-1d are preferentially located in endosomal compartments near the trans-Golgi network. Since Big-ET-1 can be present in the plasma, its conversion can occur both extracellularly and intracellularly. However, the ET-1 production by endothelial cells appears to be predominantly an intracellular process [309]. In order to be active, ECE forms homodimers and heterodimers, which regulate the localization of the enzyme [1086]. This sub-cellular ECE-1 distribution is a dynamic process and ECE-1 is permanently recycled between the plasma membrane and the endosomal vesicles. In this latter compartment, ECE degrades various peptides which have been internalized with their receptors, for instance, tachykinins, somatostatin-14 and CGRP. ECE-1 disrupts the complex receptor–β-arrestins and regulates receptor recycling to the membrane [1176,1301,1302].
ECE-2 is a membrane-bound, phosphoramidon sensitive zinc-metalloproteinase with an acidic pH optimum with no activity at pH 7.0, indicating that its activity is intracellular and intravesicular. It shares 60% homology with ECE-1 and also consists of four isomers [393,828]. Besides the conversion of endothelins, ECE-2 and, to a lesser extent, ECE-1 are involved in the degradation of amyloid peptides [1049].
Additionally, some Kell blood group proteins can generate ET-3. However, unlike ECE-1 and ECE-2 that preferentially generate ET-1, Kell proteins have a strong preference for big ET-3 and have much less activity versus big ET-1 and big ET-2 [828,889,1397].
The disruption of ECE-1 is lethal and is associated with craniofacial and gut defects (a phenotype similar to both ET-1/ETA- and ET-3/ETB-deficient mice, see below). However, both ET-1 and ET-2 are still detected in the embryos (50% of the wild type) [1742]. In contrast, the ECE-2 KO mice are healthy and fertile, but the double ECE-2/ECE-1 knockout mice show a more severe phenotype than the ECE-1-/- mice. Again, in these double knockout mice, the ET-1 production is not abolished and still represents 50% of that observed in wild-type animals [1740]. These data indicate that ECE-1 is responsible for the generation of the three peptides and is the predominant, but not the exclusive, enzyme involved in the generation of endothelins. Indeed, chymase and matrix metalloproteinases (Figure 33) are also involved in the production of ET intermediates, such as ET-1-(1–31) and ET-1-(1–32), respectively. Other enzymes, such as NEP, are also responsible for the final production of ET-1 via the hydrolysis of ET-1-(1–31) [342].
Endothelins are degraded at least in part by NEP and deamidase and are rapidly eliminated from the circulation in the lungs, by binding to the endothelin ETB receptor subtype, here acting as a clearance receptor [828,1551].
4.5.2.1.2. Pathophysiological Action of Endothelins
4.5.2.1.2.1. Endothelin Receptors.
Endothelins interact with two G-protein-coupled receptors termed ETA and ETB (Figure 33). ET-1 is the preferential ligand for the ETA receptor subtype, while the three peptides show a similar affinity toward the ETB receptor subtype [43,1334]. The ETA receptor is widely distributed and can be expressed in virtually every cell type of the body, but is especially predominant in vascular smooth muscle cells. The distribution of the ETB receptor is restricted to the cardiovascular and pulmonary systems, neurons, bone, pancreas and kidneys, but its expression is elevated in endothelial cells and renal tubules [828,1662].
ET-1, beyond its function as a vasoactive peptide, also plays a crucial role in the atherogenic process by enhancing mitogenesis, inducing extracellular matrix formation and contributing to the development of inflammation within the vessel wall [1355]. Both ETA and ETB receptors are localized on vascular smooth muscle cells where they induce their vasoconstrictor, proliferative and hypertrophic action, but in arteries, the ETA receptor is the predominant vasoconstrictor receptor [1315] (Figure 34).
The contractions elicited by ETA receptor activation are unusual when compared to those produced by most other agonists as they are slowly developing and long lasting even after washing out the peptide. The almost irreversible binding of the peptide to this receptor and its persistent association even after internalization are likely to explain, at least in part, this prolonged signaling [828]. The signal transduction following ETA receptor stimulation involves several G-protein-dependent and -independent pathways, including phospholipase C, phospholipase A2, adenylate cyclase, Rho kinase, transactivation of receptor tyrosine kinases, beta-arrestin and mitogen activated protein kinase cascades. The G-protein-dependent activation of phospholipase C and phospholipase A (predominantly Gq/11 and G12/13) leads to the usual cascade of events, i.e., on the one hand, the formation of IP3 and diacylglycerol and the resulting activation of protein kinase C, and on the other hand, the metabolism of arachidonic acid. The G-protein-independent pathway involves a direct interaction with β-arrestin, leading to the src/extracellular signal-regulated kinase/mitogen-activated protein kinase pathway (src/ERK/MAPK). These pathways contribute to the increase in [Ca2+]i, linked to the facilitation of calcium influx and calcium mobilization, and the changes in calcium sensitivity, both being essential for ET-1-induced contraction (and proliferation) of vascular smooth muscle cells [719,828,1315,1551].
In the vascular wall, the ETB receptor is predominantly expressed in the endothelial cells and, beyond its role as a clearance receptor, its activation is associated with an increase in [Ca2+]i and leads to the release of the potent vasodilators and anti-proliferative factors, NO and prostacyclin [307,1551]. Both in vivo and in vitro, these endothelial effects of ET-1 counterbalance the effects of ETA stimulation on the vascular smooth muscle cells. However, in numerous cardiovascular diseases, this response is impaired and is associated with endothelial dysfunction, leaving the ETA-dependent responses unimpeded [1551]. In vascular smooth muscle cells, the activation of ETB receptors produces vasoconstriction, the signaling pathway being similar to that described for the ETA receptor (Figure 34).
However, besides the endothelial cells, in the renal collecting duct, the ETB receptor is highly expressed and plays a major role in regulating sodium excretion and therefore arterial blood pressure [828].
Additionally, the ETA receptor can form heterodimers with the ETB receptor and possibly with other receptors, suggesting that the effects of ET-1 may be much more complex than those predicted so far [1551,1662].
4.5.2.1.2.2. Lessons from Genetically Modified Animals.
The total deletion of the endothelin-1 gene is lethal due to craniofacial deformity [859], and the phenotype of the ETA receptor knockout mice is very similar [260]. The disruption of murine ET-3 or the ETB receptor gene produces a phenotype of aganglionic megacolon and pigmentary disorders that are similar to those observed in Hirschsprung's disease, characterized by an absence of enteric ganglia in the distal colon and a failure of innervation in the gastrointestinal tract [673,1256]. As mentioned earlier, mice deleted for ECE-1, which catalyzes the proteolytic activation of big-ET-1 to ET-1 as well as big-ET-3 to ET-3, show the combined phenotype of ETA or ET-1 knockout mice and the phenotype of ETB or ET-3 knockout mice [1742]. Heterozygous knockout mice for ECE-1 develop normally but show an impaired ventilatory response to hypoxia [1290], suggesting that critical levels of endothelins are required for normal foetal development.
Therefore, the extremely severe phenotype of homozygous mice knockout for any component of the endothelin-ETA system does not allow proper appraisal of the role of ET-1 in cardiovascular physiology and pathophysiology. The arterial blood pressure of heterozygous knockout mice for the ETA receptor is normal, while the heterozygous knockout mice for the ETB receptor are hypertensive [102]. Hypertension in these latter mice was initially attributed to a decrease in ET-1 clearance, but specific deletion of the ETB receptor in endothelial cells produced normotensive mice [55]. Since knocking out ET-1 specifically in the cells of the collecting duct causes hypertension and sodium retention [19], ET-1 generated in the kidney may promote natriuresis and diuresis and contribute to the lowering of blood pressure, by activating ETB receptors located most likely on the inner medullary collecting duct cells [827]. Transgenic mice overexpressing ET-1 are normotensive with renal pathology leading to renal fibrosis and fatal kidney disease [656]. Thus, these genetic murine models emphasize the importance of ET-1 in the kidney. Additionally, ET-1 may regulate tubular sodium delivery by controlling blood flow, i.e., vasoconstriction in the afferent arterioles and vasodilatation in the efferent arterioles [828].
Mice with specific conditional deletion of the ET-1 gene in the endothelial cells are hypotensive and provide direct evidence for a paracrine vasoregulatory pathway mediated by endothelial cell-derived ET-1 acting on the vascular smooth muscle ETA receptor [814]. Conversely, transgenic mice overexpressing human ET-1 gene selectively in the endothelium exhibit vascular injury in the absence of blood pressure elevation. This overexpression results in early changes in gene expression associated with enhanced lipid biosynthesis, leading to an acceleration in the progression of atherosclerosis [1426].
4.5.2.1.2.3. Cardiovascular Diseases.
In endothelial cells, ET-1 is stored in Weibel–Palade bodies and in storage vesicles, suggesting that the peptide could be released on demand [615,1325]. Constitutive release of ET-1 maintains basal tone and blood pressure as it has been shown in genetically modified mice [814] and contributes to basal vascular tone in normotensive human subjects [626]. However, attempts to link the peptide to the normal control of moment-to-moment changes in vascular tone were not very conclusive. Nevertheless, it has been recently demonstrated that an acute de novo endothelial synthesis and release of ET-1 in response to thrombin could occur in aortic rings of aging rats and contribute to the contractile response of the underlying smooth muscle cells [557].
A causal or early role of ET-1 in pathologies, such as hypertension, is not obvious [1109,1597]. If in various models of hypertension (such as salt-sensitive hypertension or hypertension associated with insulin resistance and genetic forms of hypertension) endothelin-1 may participate in blood pressure elevation and vascular growth, the endothelial damage caused by a rise in blood pressure may activate the expression of endothelin-1 in blood vessels and in the heart. Thus, the involvement of the endothelin system may be secondary to hypertension rather than primary [1356]. ET-1 could possibly contribute to the late stages of vascular and possibly other diseases [58,69,114,1399]. The availability of endothelin-antagonists has confirmed that endothelin-1 may progressively increase with age [1546,1593] and in diseases such as pulmonary hypertension [114,311,500]. In the latter case, besides the direct vasoconstrictor effect of the peptide [66], the trophic and mitogenic effect of endothelin-1 must play a key role [294]. The production of this peptide is therefore a sign of pathology and, under normal circumstances, it does not appear to play a preponderant role in vascular homeostasis [1598]. Once initiated, the production of ET-1 not only progresses linearly with time (at least in cultured endothelial cells) but can also be up-regulated by a number of factors believed to be involved in vascular disease [298,695,1359]. Thus, under normal physiological conditions, the production and/or the action of endothelins have to be kept under control by a counteracting system [1598].
4.5.2.1.3. Modulation by NO.
In a number of blood vessels, eNOS and ET-1 co-localize, implying interactions between the two mediators [942,1319,1331]. Indeed, stimulation of the production of NO inhibits the expression and the production of endothelin-1 [132,353,1054]. Furthermore, the powerful and sustained vasoconstriction elicited by this peptide, in response to stimulation of ETA (and ETB) receptors, was efficiently blunted by both exogenous and endothelium-derived NO, in a cyclic-GMP-dependent manner [920,1043]. NO attenuates in a cyclic-GMP-dependent manner the activation by ET-1 of the signaling cascade leading to the contraction of vascular smooth muscle [130,236]. Furthermore, when released, endothelin-1 activates the nearby endothelial ETB receptor subtype, which is expressed by healthy endothelial cells and linked to the production of NO [16,132,409,604,1050,1360,1642]. Thus, under normal conditions, any overproduction of ET-1 would be offset by the increased release of NO, which down-regulates its generation and curtails its vasoconstrictor and growth-stimulating effects. In vivo, in different animal models, the sustained increase in arterial blood pressure caused by the acute or chronic administration of various NOS inhibitors is reduced by antagonists of the ETA receptor, elegantly demonstrating the preponderant role of NO in regulating ET-1 release [61,80,291,372,446,511,576,985,1092,1231,1232,1641] (Figure 34).
Besides NO, the feedback inhibition of the production and action of endothelin may also involve the release of prostacyclin and CGRP, via the activation of adenylyl cyclase, and the induction of EDHF-mediated responses, which contributes to the inhibition of its vasoconstrictor properties [569,1102].
In cardiovascular diseases, or during aging, the buffering action of NO on the production of ET-1, as well as the inhibition of the response to ET-1 disappears [23,767,1309,1496]. This peptide thus contributes to vascular abnormalities, such as the elevated vascular tone observed in atherosclerotic human coronary arteries [809].
4.5.2.2. Angiotensin II.
The renin–angiotensin system (RAS) has traditionally been viewed as a circulating system. Kidney-derived renin cleaves liver-derived angiotensinogen to form angiotensin I in circulating blood. Subsequently, angiotensin I is converted into angiotensin II, the main effector peptide of the RAS, by angiotensin-converting enzyme (ACE) predominantly located at the luminal side of the endothelium. Angiotensin II exerts its effects by activating angiotensin II receptors, of which at least two types have been described, AT1 and AT2. However, nearly 25 years ago, it was suggested that, besides the circulating RAS, angiotensin II could be produced locally by a vascular RAS [292,359].
In isolated arteries, a renin substrate, a tetradecapeptide mimetic of angiotensinogen and angiotensin I could be metabolized to form angiotensin II, via renin-dependent and -independent pathways [385,416,426,864]. However, whether or not endothelial cells do express renin remains controversial since renin binds to the vascular wall and can be internalized. Additionally, tissue angiotensin II generation may occur not only on the endothelial cell surface but also within the cells. So angiotensin II-derived from the vasculature could be involved in the autocrine and paracrine control of vascular resistance and the compliance of large conduit arteries. Tissular generation of angiotensin II may also occur in the heart, brain, pancreas and adipose tissue [292,459].
It is now known that the RAS is a much more complex system that overlaps other systems (for instance, the bradykinin/kallikrein system) and includes other enzymatic pathways (ACE-2, NEP, PEP, trypsin, kallikrein, cathepsin, tPA, aminopeptidases), leading to the generation of various other angiotensin peptides (pro-angiotensin 12, angiotensin 1–9, 1–7, 1–5, angiotensin III, angiotensin IV), some acting at other receptor sites (MAS). Furthermore, ACE and the (pro)renin receptor have been identified as membrane receptors able to activate an intracellular signaling cascade [167,458,1063,1118]. The ACE-2-dependent production of angiotensin 1–7 plays a significant role in the regulation of heart and kidney function and acts as a physiological regulator of the classic renin–angiotensin system by counteracting its detrimental effects (Figure 35).
Activation of the AT1 receptor subtype causes renal and systemic vasoconstriction, vascular smooth muscle proliferation and promotes tubular sodium reabsorption directly at the proximal tubule and indirectly via the action of aldosterone at the distal nephron. These combined effects allow the maintenance of extracellular fluid volume in a context of fluid loss and dehydration, but favor the development of hypertension under pathophysiological conditions. Additionally, as mentioned earlier, the stimulation of the renin–angiotensin system is associated with the generation of oxidative stress, mainly through the activation of NAD(P)H oxidase (Figure 13). ROS impairs NO and EDHF-mediated relaxation and enhances COX-dependent contractions causing endothelial dysfunction. Additionally, the generation of ROS, in a positive feedback loop, activates the RAS, leading to a vicious circle exacerbating the end-organ damage and atherosclerosis associated with hypertension [400,431,883,1730].
In conclusion, the renin–angiotensin–aldosterone system plays a predominant role in the control of cardiovascular homeostasis. Extremely useful therapeutic targets have been identified within the classic RAS system, i.e., renin, ACE, AT1 receptor, aldosterone receptor, and the relevance of the new targets recently identified in this expended system is currently being assessed [265,497,966,1199,1596].
4.5.2.3. Urotensin-II and Urotensin-II-Related Peptide.
Urotensin-II and urotensin-II-related peptide are two closely related peptides of 11 and 8 amino acids, respectively, issued from two different genes. They share the same receptor, a G-protein-coupled receptor identified as the orphan receptor GPR14 [34]. Urotensin-II is widely expressed within the cardiovascular system, including by the endothelial cells [1324]. Urotensin-II is one of the most, if not the most, potent vasoconstricting peptide identified to date, but it also produces endothelium- and NO-dependent relaxations. Besides, this peptide promotes vascular smooth muscle cell proliferation, produces inotropic and hypertrophic effect in cardiac muscle, regulates renal vascular tone and glomerular filtration and shows effects outside the cardiovascular system including inhibition of insulin release and regulation of food intake [1307].
The expression of urotensin-II is increased in various cardiovascular diseases, including hypertension, preeclampsia, coronary and renal diseases, heart failure, and type II diabetes [1811]. However, the stimuli leading to the release of urotensin-II have not been properly identified, and whether or not it plays a detrimental or a beneficial role (or both) is not yet completely understood [1324].
4.5.2.4. Neuropeptide Y.
Neuropeptide Y (NPY) is a 36-amino acid peptide belonging to a family which, in mammals, also includes peptide YY (PYY) and pancreatic peptide (PP). They interact, with a variable degree of affinity with 4 to 5 G-protein-coupled receptors, termed NPY Y1, NPY Y2, NPY Y4, NPY Y5 and NPY y6, the latter being functionally expressed only in the mouse and rabbit. They are involved in a variety of physiological functions such as the regulation of appetite, circadian rhythm, anxiety, gastrointestinal motility and cardiovascular system, including heart rate, regional blood flow, vascular smooth muscle cell growth and angiogenesis [349,420,421,423,878]. The NPY activity is modulated by the endothelial ectoenzyme dipeptidyl peptidase IV (DPPIV/cd26), which inactivates the NPY Y1-agonistic activity of NPY but generates the active fragment NPY(3–36), agonist of both NPY Y2 and Y5 receptors [415,1824].
NPY is widely expressed, especially in the brain, the adrenal medulla and peripheral sympathetic nerves where it plays an important role in modulating the contribution of the sympathetic nervous system to the control of vascular tone. NPY is also expressed in the vasculature including in endothelial and endocardial cells, and can act as an autocrine and paracrine mediator [2,726,1425]. In HUVEC, this expression is modulated by inflammatory mediators such as LPS, TNFα or interferon γ, suggesting that NPY could link sympathetic nerves, blood vessels and the immune system [1424,1824].
NPY Y1 receptors are expressed in vascular smooth muscles and potentiate the contractile action of norepinephrine and ATP. This receptor is also responsible for smooth muscle growth and proliferation and could be involved in neointima formation. The NPY Y2 receptor is expressed in sympathetic nerves terminals and modulates the pool of co-transmitters released at the neuroeffector junction. Therefore, NPY plays a dual role as a modulator of sympathetic co-transmission, on the one hand, it facilitates vascular smooth muscle reactivity and, on the other hand, it modulates the presynaptic release of neurotransmitters. Additionally, the angiogenic effect of NPY, as shown by endothelial cell adhesion, migration and proliferation, as well as vessel sprouting and capillary tube formation, is mainly attributed to NPY Y2 and possibly also to Y1 and Y5 receptor stimulation [962,1237,1825].
However, so far, there is little evidence to show that endothelial release of NPY contributes to the moment-to-moment regulation of vascular tone.
4.5.2.5. CNP.
The natriuretic peptide family includes four distinct peptides, atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), C-type natriuretic peptide (CNP) and urodilatin, which are involved in the control of body fluid homeostasis and blood pressure. CNP, a 22-amino acid peptide, was originally isolated from the porcine brain. In contrast to ANP and BNP that are synthesized predominantly in the heart, CNP is produced in the central nervous system but has also been detected in peripheral endothelial cells [835,1099]. Endothelial cells express the transcript of the CNP receptor gene, synthesize and constitutively release CNP. The gene expression and release of CNP are regulated by various cytokines [1111,1460,1468].
CNP evokes relaxation and hyperpolarization of arterial and venous smooth muscle cells, and these vasodilator effects are generally attributed to the activation of natriuretic peptide receptor B subtype (NPR-B) on the smooth muscle, followed by the stimulation of particulate guanylate cyclase, leading to accumulation of cGMP and the subsequent opening of BKCa and KATP channels [669,1303,1468]. In porcine coronary arteries and canine femoral veins, CNP induces the accumulation of cyclic-GMP and opens BKCa, and this peptide has therefore been proposed as a potential endothelium-derived hyperpolarizing factor [60]. Furthermore, in the rat mesenteric artery, it has been suggested that CNP, when released by the endothelial cells, would activate the smooth muscle NPR-C receptor subtype and that the following cGMP-independent activation of a G-protein regulated inward-rectifier potassium channel (GIRK) would provoke the hyperpolarization of the smooth muscle cells [214,1616]. However, in various vascular tissues, this hypothesis has not been confirmed [68,336,903] and in mice deficient for the NPR-C gene, EDHF-mediated responses are not altered [1021].
CNP is therefore unlikely to act as an endothelium-derived relaxing/hyperpolarizing substance or to contribute to moment-to-moment endothelium-dependent regulation of vascular tone. Nevertheless, this peptide plays a key role in preventing smooth muscle proliferation, leukocyte recruitment and platelet reactivity. As such, CNP is likely to exert an anti-atherogenic influence on the blood vessel walls [17,961,1345].
4.5.2.6. Adrenomedullin, CGRP, VIP, PACAP.
Calcitonin gene-related peptides (αCGRP and βCGRP) and adrenomedullin are extremely potent vasodilator peptides belonging to a peptide family that also includes calcitonin and amylin [136,815,1245]. These peptides activate a family of CGRP receptors consisting of a calcitonin receptor-like protein linked to one of the three receptor activity-modifying proteins (RAMPs) which are required for the functional activity of the receptor [136,1245].
Adrenomedullin was originally identified in the extracts of human pheochromocytoma tissue but is expressed constitutively in various other cells including epithelium and cardiac myocytes, fibroblasts, vascular smooth muscle and especially endothelial cells [813,815,1471]. The vasodilator effects of adrenomedullin are predominantly endothelium-dependent, the endothelium-independent relaxations being explained by the increased formation of cAMP. Thus, adrenomedullin is a potent endothelium-dependent vasodilator but is unlikely per se to be a relevant endothelium-derived relaxing factor. Besides, adrenomedullin has a wide range of biological actions such as reduction of oxidative stress and inhibition of endothelial cell apoptosis and could be of potential therapeutic interest in arteriosclerosis. Additionally, adrenomedullin possesses angiogenetic properties [786].
CGRP is expressed mainly in neurons and endocrine cells [136], but can also be expressed in endothelial cells where the peptide is stored in Weibel–Palade bodies [1171,1705]. CGRP is predominantly an endothelium-independent relaxing substance and, in most blood vessels, the cAMP-dependent relaxations are associated with the opening of KATP and, in some instance, BKCa channels. Similarly, endogenous CGRP, released by transmural nerve stimulation or by capsaicin analogues, evokes endothelium-independent relaxations which involve activation of KATP [136]. CGRP receptor antagonists do not inhibit endothelium-dependent responses in any of the studies conducted to date. However, in the hepatic artery of the rat [664] and possibly also in the mesenteric artery of the same species 1382], acetylcholine releases CGRP from sensory nerves producing an endothelium-independent relaxation and hyperpolarization. Thus, CGRP should be considered as a nerve-derived relaxing/hyperpolarizing factor [664].
Vasoactive intestinal peptide (VIP) and pituitary adenylyl cyclase-activating polypeptide (PACAP) belong to the superfamily of structurally related peptides which includes glucagon, glucagon-like peptides, secretin and growth hormone-releasing factor. VIP produces not only endothelium-dependent relaxations by releasing NO and/or prostacyclin but also endothelium-independent relaxations by stimulating adenylyl cyclase and activating KATP, BKCa or KV [636]. PACAP is predominantly an endothelium-independent vasodilator, also via the activation of adenylyl cyclase [1603]. However, whereas VIP and PACAP are well established as neuroendocrine hormones and neurotransmitters, there are only a few anecdotal reports of the expression of these peptides in endothelial cells [826,875]. Therefore, neither VIP nor PACAP can qualify as endothelium-derived mediators. Nevertheless, as both peptides are expressed in nerve endings surrounding arteries and veins, they can be considered, in a similar manner to CGRP, as nerve-derived relaxing/hyperpolarizing factors.
- Endothelium-Dependent Regulation of Vascular Tone - The EndotheliumEndothelium-Dependent Regulation of Vascular Tone - The Endothelium
Your browsing activity is empty.
Activity recording is turned off.
See more...