NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Arvin A, Campadelli-Fiume G, Mocarski E, et al., editors. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge: Cambridge University Press; 2007.
Congenital cytomegalovirus infection and the placenta
Congenital CMV infection
Human cytomegalovirus (CMV) is a ubiquitous virus that causes asymptomatic infections in healthy individuals (for review see Pass, 2001). Because breast feeding (Stagno et al., 1980), exposure to young children (Pass et al., 1987) and sexual contact (Fowler and Pass, 1991) are major risk factors for infection, most adults are seropositive. Diverse organs and specialized cells, including polarized epithelial cells (Tugizov et al., 1996) and endothelial cells (Fish et al., 1998; Maidji et al., 2002), are susceptible to CMV infection. CMV establishes latent infection in granulocyte-macrophage progenitors (Kondo et al., 1996) and reactivates upon cellular differentiation (Hahn et al., 1998; Soderberg-Naucler et al., 1997). Congenital CMV infection is estimated to affect 1 to 3% of infants in the United States annually and remains an important public health problem causing significant morbidity and mortality (for review see Britt, 1999).
It has long been appreciated that maternal neutralizing antibodies reduce the risk of symptomatic congenital disease in the fetus (Ahlfors et al., 1984; Boppana and Britt, 1995; Fowler et al., 2003; Stagno et al., 1982). The importance of adaptive immunity to CMV is apparent in women with primary infection, often with low-avidity neutralizing antibodies (Boppana and Britt, 1995; Lazzarotto et al., 1998; Revello et al., 2002). Approximately 15% of these women spontaneously abort in early gestation (Griffiths and Baboonian, 1984). Examination of placentas infected with CMV in vitro and in utero has suggested potential routes of virus transmission from the uterus to the placenta (Fisher et al., 2000; Pereira et al., 2003). Importantly, these studies suggest that placental involvement precedes virus transmission and infection of the embryo/fetus. Progression of infection hinges on maternal immunity to CMV, the mechanics of cytotrophoblast development and the presence of other pathogens at the maternal–fetal interface. In this chapter, we describe patterns of CMV infection in early gestation, routes of viral transmission at the maternal-fetal interface and dysregulation of cytotrophoblast differentiation and function secondary to CMV infection in vitro.
CMV infects specialized cells in the placenta
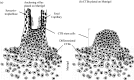
Numerous reports indicate that placentas from pregnancies complicated by congenital CMV infection contain viral DNA and proteins (Benirschke and Kaufmann, 2000; Muhlemann et al., 1992; Nakamura et al., 1994; Sinzger et al., 1993). Later in pregnancy, CMV infection is associated with premature delivery and, in 25% of affected infants, intrauterine growth retardation (Istas et al., 1995), outcomes that are often associated with placental pathologies. CMV replicates in cytotrophoblasts isolated from early and late gestation placentas in vitro (Fisher et al., 2000; Halwachs-Baumann et al., 1998; Hemmings et al., 1998). The routes of virus transmission and the types of immune responses elicited are likely linked to the unusual nature of cytotrophoblast interactions with maternal cells at the uterine-placental interface. A diagram of the maternal-fetal interface midway through gestation with potential sites of CMV infection is shown in Fig. 45.1.
Placental development in early gestation
Diverse cell types in the uterus
Immunologically competent cells are detected in the uterine endometrium and decidua (Kamat and Isaacson, 1987). Early studies of leukocyte antigens by using immunohistologic approaches suggested that cells in the endometrium of cycling women in the mid-secretory phase (7–10 days after ovulation) resemble leukocytes in early gestation decidua (King et al., 1989). Granulated lymphocytes with an unusual antigenic phenotype (CD56+ high, CD16–), known as natural killer (NK) cells, constitute a substantial proportion of these cells (Bulmer et al., 1991; Starkey et al., 1988), increasing from the proliferative endometrium to the late secretory endometrium. Macrophages increase prior to menstruation (Kamat and Isaacson, 1987). CD83+ dendritic cells in the uterine stratum basale are present in the non-pregnant and pregnant uterus (Kammerer et al., 2000; Soilleux et al., 2002).
In response to implantation, the uterine lining develops into the decidua, which is maintained by progesterone (Norwitz et al., 2001). Interglandular tissues increase in quantity, and the cytoplasm of resident stromal cells is distended with glycogen, lipid and vimentin-type intermediate filaments (Fig. 45.1, Zone Ⅲ). Temporal and spatial expression of growth factors and cytokines (e.g., insulin-like growth factor 1 and its binding protein) suggests that these molecules may influence decidualization (Crossey et al., 2002). Decidual granular leukocytes intermingle with resident maternal cells and invasive fetal cells (Zone Ⅲ) (Drake et al., 2001; Kamat and Isaacson, 1987; Red-Horse et al., 2001; Starkey et al., 1988). These immune cells are involved in innate pattern recognition, mostly NK cells with some macrophages, dendritic cells and T-lymphocytes. Novel patterns of cytokine/chemokine expression in the decidua, as well as specialized adhesion molecules on uterine vessels (Kruse et al., 1999), probably attract this unusual leukocyte population, which functions in immunity and cytotrophoblast differentiation. Dendritic cell protein ICAM-3-grabbing non-integrin (DC-SIGN+) cells, occasionally found in the endometrium, are abundant in the decidua and associate with NK cells (Kammerer et al., 2003, 1999; Pereira et al., 2003; Soilleux et al., 2002). The unusual immune cell population in the decidua suggests that when macrophage/dendritic cell progenitors (Mϕ/DC) latently infected with CMV are attracted to the endometrium and early gestation decidua, CMV could be reactivated in the presence of inflammatory stimuli.
Development of the hemochorial human placenta
The embryo’s acquisition of a supply of maternal blood is a critical hurdle in pregnancy maintenance. The mechanics of this process are accomplished by the placenta’s specialized epithelial cells, termed cytotrophoblasts. The histology of the maternal-fetal interface is diagrammed in Fig. 45.1. The placenta is composed of individual units termed chorionic villi, each with a connective core that contains fetal blood vessels and numerous macrophages (Hofbauer cells) that often lie under a thick basement membrane (Fig. 45.1, Zone I). Placentation is a stepwise process whereby cytotrophoblast progenitor cells, attached to the basement membrane as a polarized epithelium, leave the membrane to differentiate along one of two independent pathways depending on their location. In floating villi, they fuse to form a multinucleate syncytial covering attached at one end to the tree-like fetal portion of the placenta (Zone I). The rest of the villus floats in a stream of maternal blood, which optimizes exchange of substances between the mother and fetus across the placenta. In the pathway that gives rise to anchoring villi, which attach the placenta to the uterine wall (Zone Ⅱ), cytotrophoblasts aggregate into cell columns of non-polarized mononuclear cells that attach to and then penetrate the uterine wall. The ends of the columns terminate within the superficial endometrium, where they give rise to invasive cytotrophoblasts. During interstitial invasion a subset of these cells, either individually or in small clusters, comingles with resident decidual, myometrial and immune cells. During endovascular invasion, masses of cytotrophoblasts open the termini of uterine arteries and veins they encounter, then migrate into the vessels, thereby diverting maternal blood flow to the placenta (Zone Ⅲ). In arterioles, cytotrophoblasts replace the endothelial lining and partially disrupt the muscular wall, whereas in veins, they are confined to the portions of the vessels near the inner surface of the uterus. Together, the two components of cytotrophoblast invasion anchor the placenta to the uterus and permit a steady increase in the supply of maternal blood that is delivered to the developing fetus.
Invasive cytotrophoblasts modulate the expression of stage-specific antigens
During uterine remodeling, cytotrophoblasts switch from an epithelial to a mesenchymal type (Table 45.1). Cytotrophoblasts express novel adhesion molecules and proteinases that enable the cells’ attachment and invasion, as well as immune modulating factors that play a role in maternal tolerance of the hemiallogeneic fetus (Cross et al., 1994; Norwitz et al., 2001). Interstitial invasion requires downregulation of integrins characteristic of epithelial cells (α6β4) and novel expression of α1β1, α5β1 and αVβ3 (Damsky et al., 1994). Endovascular cytotrophoblasts that remodel maternal blood vessels transform their adhesion receptor phenotype to resemble the endothelial cells they replace (Fig. 45.1, site 1) turning on the expression of VE-(endothelial) cadherin, platelet-endothelial adhesion molecule-1 and vascular endothelial adhesion molecule-1 (Damsky and Fisher, 1998; Zhou et al., 1997).
Table 45.1
Selected differentiation molecules expressed by placental trophoblasts.
Degradation of the basement membrane and extracellular matrix of the uterine stroma is precisely regulated during placentation (Fisher et al., 1985). Cytotrophoblasts upregulate urokinase-type plasminogen activator (uPA) (Queenan et al., 1987; Solberg et al., 2003), matrix metalloproteinase-9 (MMP-9) (Librach et al., 1991) and inhibitors such as tissue inhibitor of metalloproteinases-3, a likely regulator of proteolytic activity and invasion depth (Bass et al., 1997) (Table 45.1). Molecules that may function in maternal immune tolerance are also produced, such as the non-classical major histocompatibility complex (MHC) class Ib molecule HLA-G (Kovats et al., 1990; McMaster et al., 1995) and interleukin-10 (IL-10) (Roth et al., 1996; Roth and Fisher, 1999). This remarkable transformation, evidenced by novel expression of differentiation molecules and invasiveness, underscores the extraordinary plasticity of cytotrophoblasts.
CMV infects the placenta in vitro and in utero
Potential routes for CMV transmission
The cellular organization of the placenta suggests potential routes by which CMV infection spreads from the uterus, first to the placenta and then to the embryo/fetus (Fig. 45.1) (Fisher et al., 2000; Pereira et al., 2003). One likely site of transmission is within the uterine wall (sites 1 and 2). Interstitial invasive cytotrophoblasts could encounter infected endometrial glands, uterine blood vessels and decidual granular leukocytes. Endovascular cytotrophoblasts could encounter infected endothelial and vascular smooth muscle cells, as well as maternal immune cells. Once cytotrophoblasts within the uterine wall become infected (site 2), CMV might spread in a retrograde manner through the cell columns to the anchoring chorionic villi (site 3). In the villus stromal cores, virus could be transmitted from infected cytotrophoblasts to fibroblasts, fetal macrophages and possibly endothelial cells that line chorionic vessels. This conjecture is based on focal patterns of CMV protein expression in the placenta (Fisher et al., 2000; Muhlemann et al., 1992). Infected Mϕ/DC and sloughed endothelial cells seem likely candidates for entering the venous circulation of the placenta and subsequently carrying the infection via the placental circulation to the fetus. Another likely site of transmission is across the syncytiotrophoblast layer that covers floating chorionic villi (site 4). These placental cells, in direct contact with maternal blood, express the neonatal Fc receptor (FcRn), a molecule that facilitates maternal IgG transfer and passive immunization of the fetus (Simister et al., 1996). The syncytium may allow passage of CMV virions complexed with maternal IgG to the underlying layer of cytotrophoblast progenitor cells that could become infected in the presence of virus-binding antibodies with low avidity (Boppana and Britt, 1995; Fisher et al., 2000; Lazzarotto et al., 1997ȉ; Pereira et al., 2003; Revello et al., 2002). Accordingly, adaptive and innate immune responses that reduce infectious virions at the uterine–placental interface likely play a central role in preventing transmission.
CMV protein expression in placental cells in chorionic villi infected in vitro and in utero
Clues about potential routes of prenatal CMV infection emerged from a model tissue culture system (Fig. 45.2(a)). Chorionic villi are plated on filters coated with Matrigel, an extracellular matrix, infected with virus and then cultured from 2 to 4 days. Experiments that used this model revealed an unexpected pattern of CMV infection (Fisher et al., 2000). Briefly, tissue sections of villus explants that were infected for several days were double-stained with anti-cytokeratin to identify trophoblast cells and with a monoclonal antibody to CMV immediate-early (IE) proteins to identify infected cells. Notably, syncytiotrophoblasts that cover the villus surface were not infected and failed to stain for CMV IE proteins, whereas nuclear staining of small, isolated clusters of underlying cytotrophoblast progenitor cells was observed (Fig. 45.1, site 4). In some tissues, CMV IE protein expression was also detected in cytotrophoblasts in the cell columns of anchoring villi (Fig. 45.1, site 3). The staining patterns observed when placentas were infected in utero had remarkable similarities to and differences from those observed after infection in vitro. Sometimes, patterns of CMV-infected cells were virtually indistinguishable from those found after infection in vitro; in some locations, isolated clusters of cytotrophoblasts underlying the syncytium were the only cells infected (Fig. 45.3(b)). At other times, nearly all the cytotrophoblast progenitor cells, in highly infected tissues, expressed CMV IE proteins (Fig. 45.3(c), (d)). Comparatively fewer syncytial nuclei stained, but numerous cells within the villus cores stained for viral proteins, including fibroblasts, endothelial cells and macrophages. These studies suggested that in vitro infection is a model for the initial steps in placental infection, whereas in utero infection shows virus transmission from trophoblasts to other cell types in the villus core. The interplay between pathogens and immune responses in other tissues suggest that CMV infection might often occur in the context of the microbial ecology of female reproductive tissues.
Pathogenic microorganisms at the placental–decidual interface
In a study using PCR-based strategies, the presence of viral and bacterial DNA was assessed in biopsy specimens of the decidua and adjacent placentas of 282 healthy pregnancies (McDonagh et al.; Pereira et al., 2003). Overall, CMV DNA was detected in 69% of specimens, and CMV with bacteria was detected in 38%. When found in isolation, CMV was detected in 27% of placental samples. Other pathogens included herpes simplex virus type 1 (HSV-1) in 3%; HSV-2 in 9%, and more than one bacterium in 15%. Sixteen percent of placental samples were negative for these pathogens. These findings suggest that early gestation placentas frequently contain DNA from viral and bacterial pathogens.
Detailed analysis of paired first-trimester decidual and placental biopsy specimens from individual pregnancies showed that some pathogens were present in both. CMV DNA was detected in 89% of the decidual samples and 63% of the placentas. When CMV was found in isolation in the decidua (40%), virus was also sometimes present in the placenta (26%). In contrast, when bacterial DNA was detected in the placenta (11%), signals were less frequently found in the decidual samples (6%). Together these results suggest that CMV can be selectively transferred from the decidua, a potential reservoir, to the adjacent placenta. When the effects of gestational age were examined, CMV DNA, with or without other pathogens, was detected in 63% of first-trimester placentas and 74% of second-trimester placentas. Together, samples with both CMV and bacterial DNA increased from 31% in the first trimester to 44% in the second trimester, whereas CMV alone was reduced in the second trimester. Fewer second-trimester placentas were negative for all pathogens. These studies suggested that (a) CMV is commonly present at the uterine-placental interface together with pathogenic bacteria, (b) infection tends to increase in the second trimester, and (c) virus is selectively transmitted to the adjacent placenta.
Neutralizing antibodies to CMV gB in placental syncytiotrophoblasts
Development of neutralizing antibodies is delayed when primary CMV infection occurs shortly before or during gestation (Boppana and Britt, 1995; Lazzarotto et al., 1997; Revello et al., 2002), whereas high titers indicate resolution of acute infection and/or reactivation. Antibody responses to CMV in the group of donors from whom paired biopsy specimens were obtained showed that, with one exception, the donors were seropositive with a range of neutralizing activity. Briefly, neutralizing activity in IgG purified from the conditioned medium of biopsy specimens was evaluated. Ten women had low neutralizing titers (0% to 32%), nine had moderate titers (43% to 67%) and four had high titers (70 to 98%).
Some serologic evidence suggests that reinfection with new CMV strains in seropositive women might be associated with symptomatic fetal infection (Boppana et al., 1999). To determine whether multiple strains colonize the placental-decidual interface, a region of the gB gene with characteristic nucleotide differences was sequenced. Sequence analysis of a small number of CMV-positive samples revealed that the gB genotypes were similar to variants in groups 1, 2 and group 3 (Chou and Dennison, 1991). Paired decidua and adjacent placenta from a seropositive donor without detectable neutralizing antibodies contained a mixture of gB genotypes, suggesting that different CMV strains could infect the maternal-fetal interface early in the course of maternal infection.
Patterns of CMV-infected-cell proteins in the decidua and placenta
Decidual biopsy specimens that contained CMV DNA were studied by immunofluorescence confocal microscopy to determine whether viral proteins could be detected (McDonagh et al., 2004; Pereira et al., 2003). Tissue sections of decidual biopsy samples were incubated with a pool of monoclonal antibodies to CMV-infected-cell proteins and to gB, an abundant virion envelope glycoprotein. Staining revealed islands of infected resident uterine and fetal cells, as well as innate immune cells among much larger uninfected areas. Several common staining patterns emerged. In the most highly affected samples, CMV-infected-cell proteins were found in the nuclei and cytoplasm of glandular epithelium (Fig. 45.4(a), a–c), vascular endothelium and endovascular cytotrophoblasts (Fig. 45.4(a), d–f). Resident decidual cells positive for insulin growth factor binding protein 1 (IGFBP-1) also stained brightly (Fig. 45.4(a), g–l). These data indicate that CMV infects a diverse population of maternal cells within the uterine wall and fetal invasive cytotrophoblasts. Innate immune cells showed a staining pattern that was distinctly different from that of CMV-infected cells, suggesting phagocytosis of enveloped virions. Macrophages (CD68+) contained cytoplasmic vesicles that stained strongly for CMV gB (Fig. 45.4(b), a–c). Some gB-positive cells also stained for DC-SIGN (Kammerer et al., 2003; Soilleux et al., 2001) (Fig. 45.4(b), d–f). NK (CD56+) cells were often dispersed among Mϕ/DC that were filled with gB-positive vesicles (Fig. 45.4(b), g). Occasionally, striking numbers of NK cells and Mϕ/DC intermingled (Fig. 45.4(b), h and i). Additionally, neutrophils inside uterine blood vessels were found near endothelial cells and decidual cells that expressed CMV-infected-cell proteins, suggesting phagocytosis (Fig. 45.4b, j–l). These observations suggested that the uterus serves as a reservoir for CMV virions that could potentially infect the placenta.
Different patterns of CMV infection in the decidua mirrored in the adjacent placenta
Examination of CMV proteins in paired decidual and placental biopsy specimens showed three staining patterns (Pereira et al., 2003). In the first, islands in both decidual and placental compartments stained strongly for CMV-infected-cell proteins. This pattern predominated in samples from donors with low neutralizing titers and a few with intermediate titers and other pathogens. In the decidua, cytokeratin-positive glandular epithelial cells, endovascular cytotrophoblasts in remodeled uterine blood vessels, and interstitial cytotrophoblasts were sometimes positive. Resident decidual cells strongly stained for viral proteins, suggesting that these cells were permissive for viral replication. In the adjacent portions of the placenta, floating villi contained syncytiotrophoblasts and cytotrophoblast progenitor cells expressing CMV-infected-cell proteins that localized to the nuclei and cytoplasm. Abundant vesicles amassed close to the plasma membrane of the villus surface and contained gB. In regions with infected syncytiotrophoblasts, fibroblasts and fetal capillaries in the villus core expressed infected-cell proteins. Invasive cytotrophoblasts in developing cell columns that anchor the placenta to the uterine wall also stained. In contrast, Mϕ/DCs within the villus stromal cores contained infected-cell proteins in cytoplasmic vesicles but not in the nuclei, suggesting phagocytosis.
In the second group of paired biopsy specimens, the number of cells that stained for CMV-infected-cell proteins was reduced in the decidua, and occasional focal infection was found in the placenta. This pattern predominated in samples from donors with low to intermediate neutralizing titers, several of which contained other pathogens. In the decidua, CMV replication was detected in some glandular epithelial cells and decidual cells. In the interstitium, Mϕ/DCs were abundant throughout, especially near infected glands and blood vessels. These cells contained gB-positive cytoplasmic vesicles but were not infected. Sometimes the adjacent placentas contained small clusters of cytotrophoblast progenitor cells that expressed CMV-infected-cell proteins. Isolated gB-containing vesicles were present in the overlying syncytiotrophoblast layer. In the villus core, Mϕ/DCs containing CMV gB-positive vesicles were often observed. In other placental biopsies, only gB-containing vesicles were detected in syncytiotrophoblasts and villus core Mϕ/DCs without infection.
In the last group of paired biopsy specimens, few cells stained for CMV-infected-cell proteins in the decidua, and none were found in the placenta. This pattern predominated in samples from donors with intermediate to high neutralizing titers, several of which contained other pathogens.
In the decidua, neutrophils with viral proteins were found in uterine blood vessels near infected cells. In the adjacent portions of the placenta, syncytiotrophoblasts contained numerous CMV gB-positive vesicles but were not infected. In villus core Mϕ/DCs, gB accumulated in large cytoplasmic vesicles. When placentas were stained for IgG, syncytiotrophoblasts contained many positive vesicles, and gB colocalized with a small subset of them. In villus core Mϕ/DCs, some gB-staining vesicles colocalized with the more abundant IgG-positive vesicles. FcRn-positive vesicles at the apical and basolateral membranes suggested IgG transcytosis in syncytiotrophoblasts (Simister and Story, 1997). In some cases, the presence of viral nucleocapsids in syncytiotrophoblasts was confirmed by electron microscopy.
These studies concluded that CMV is commonly present at the maternal-fetal interface, one possible explanation for why pregnant women shed virus from the cervix (Collier et al., 1995; Shen et al., 1993; Stagno et al., 1975). Bacteria were often found in donors with intermediate to high neutralizing titers whose uninfected placentas contained virion proteins suggesting limited CMV replication in the decidua. Reactivation from decidual Mϕ/DCs might occur as a consequence of inflammatory responses to pathogenic bacteria and could depend on the number of latently infected Mϕ/DCs infiltrating the uterus (Cook et al., 2002; Hahn et al., 1998; Soderberg-Naucler et al., 2001). Placentas from healthy pregnant donors contained isolated areas of infection that were a small part of the whole tissue. Since these tissues were from normal pregnancies, placental infection that leads to fetal transmission likely involves the decidual and placental components that stained for infected-cell proteins, i.e., an exacerbation of the situation found in samples from women with the lowest neutralizing titers and some with intermediate titers as well as bacterial pathogens.
Coordinated immune responses suppressed CMV infection of the placenta in women with intermediate to high neutralizing titers, one explanation for a correlation between high-avidity IgG and protection against vertical transmission (Boppana and Britt, 1995; Revello et al., 2002). The most remarkable result is that women with uncomplicated pregnancies had suppressed infection in the decidua. Virion-IgG complexes may be transported to the placenta without infection, a process that demonstrates the efficacy of innate and adaptive immunity. CMV infection of the decidua is a novel paradigm and further illustrates how this virus utilizes host immunity (Mocarski, 2002) by exploiting maternal hyporesponsiveness.
Analysis of CMV DNA and proteins expressed in placentas from uncomplicated deliveries by PCR and immunohistochemistry showed evidence of transplacental transmission (McDonagh et al., 2006). CMV DNA was detected in 62% of term placentas examined. In biopsy specimens from placentas with high levels of CMV DNA and low maternal neutralizing titers, fetal blood vessels contained leukocytes with viral replication proteins. Some cord blood samples contained CMV DNA, confirming viral replication. In placentas with low levels of viral DNA and high neutralizing titers, villus core macrophages and dendritic cells contained CMV gB, comparable to infection in early gestation, suggesting virion uptake without transmission. Together the results showed that CMV infection spreads from villus cytotrophoblasts to stromal fibroblasts, placental blood vessels and fetal leukocytes in late gestation. Over 5% of uncomplicated deliveries contained CMV replication proteins, suggesting a higher incidence of transplacental transmission and asymptomatic congenital infection than previously thought.
Complexes of IgG and CMV virions transcytosed from maternal circulation across syncytiotrophoblasts to underlying cytotrophoblasts
Immunohistochemical analysis of early gestation biopsy specimens showed an unusual pattern of CMV replication proteins in underlying cytotrophoblast progenitor cells. Whereas syncytiotrophoblasts were spared in placentas with low to moderate CMV neutralizing titers, cytotrophoblasts were infected, suggesting virion transcytosis from maternal blood (Pereira et al., 2003). Early steps of CMV infection and the role of FcRn were examined using the villus explant model (Fig. 45.2(a)) and polarized epithelial cells (Tugizov et al., 1996). The results showed that (ⅰ) maternal IgG modulates CMV infection in chorionic villi, (ⅱ) FcRn transcytoses IgG–virion complexes that retain infectivity with low neutralizing antibodies, (ⅲ) villus core macrophages capture transcytosed IgG-virions, (ⅳ) IgG and gB accumulate in caveolae and (ⅴ) CMV DNA is present in syncytiotrophoblasts without viral replication. Receptor-mediated transport and caveolar endocytosis explain the infection patterns in villus cytotrophoblasts and CMV virion gB accumulation in vesicular compartments in utero (Pereira et al., 2003). The rapid kinetics of receptor-mediated transport of IgG–virions was similar in syncytiotrophoblasts and polarized T-84 intestinal epithelial cells expressing FcRn: transcytosed immune complexes were detected in villus core macrophages (explants), underlying cytotrophoblasts or the basal medium (cells). In villus explants, IgG–virion transcytosis and macrophage uptake were blocked with trypsin treatment and soluble protein A. The results suggest that CMV virions could disseminate to the placenta by co-opting the receptor-mediated transport pathway for IgG. It was recently reported that passive immunization with hyperimmune IgG at midgestation prevents congenital disease and growth restriction in infected infants of mothers with primary CMV infection (Nigro et al., 2005). This remarkable outcome suggests once CMV replication has been interrupted, functional villi develop that transport neutralizing IgG to the fetus, reducing dissemination.
Receptors for CMV virions are developmentally regulated in cytotrophoblasts
CMV replication in distinct cytotrophoblast populations suggests that virion receptors could be developmentally regulated as these specialized cells proceed along the differentiation pathway from the fetal to the maternal compartment (Fig. 45.1). Function-blocking methods and immunohistochemical analysis were used to correlate infection with expression of CMV receptors in cytotrophoblasts in situ and in vitro (Maidji et al., 2006). In placental villi, syncytiotrophoblasts express a virion receptor, epidermal growth factor receptor (EGFR) (Wang et al., 2003), but lack integrin coreceptors, and endocytosis occurs without replication. IgG–CMV virion complexes transcytosed by FcRn reach underlying cytotrophoblasts (Maidji et al., 2006). Some EGFR-expressing cells selectively initiate expression of a coreceptor, αV integrin (Feire et al., 2004; Wang et al., 2005), and focal infection can occur. In cell columns, proximal cytotrophoblasts lack receptors, and distal cells express integrins α1β1 and αVβ3 but remain uninfected. In the uterine decidua, invasive cytotrophoblasts expressing integrin coreceptors upregulate EGFR, thereby dramatically increasing susceptibility. These findings indicate that virion engagement with receptors in the placenta (ⅰ) changes as cytotrophoblasts differentiate and (ⅱ) correlates with spatially distinct sites of CMV replication in maternal and fetal compartments in utero.
CMV infection dysregulates cytotrophoblast differentiation/invasion in vitro
CMV replicates in placental cytotrophoblasts in vitro
Several groups have reported that cytotrophoblasts isolated from early gestation (Fisher et al., 2000; Hemmings et al., 1998) and term placentas (Halwachs-Baumann et al., 1998) are susceptible to CMV infection. A detailed examination of the viral life cycle was done using an in vitro model of progenitor cytotrophoblasts from chorionic villi plated as a monolayer on Matrigel and cultured from 2 to 4 days after infection (see Fig. 45.2(b)) (Fisher et al., 2000; Librach et al., 1991). Under these conditions the cells form aggregates, analogous to cell columns, and differentiate along the invasive pathway. In CMV-infected cells, nuclear staining for IE proteins was detected by 24 hours and cytoplasmic staining for gB was detected by 72 hours in 20 to 40% of the cells. There was an increase in the titers of intracellular and progeny virions released during the culture period, establishing that differentiating cytotrophoblasts are fully permissive for CMV replication.
Investigation of the effects of viral infection in cytotrophoblasts showed considerable changes, evidenced by dysregulated expression of stage-specific adhesion and immune molecules, as well as metalloproteinases and their inhibitors (Fisher et al., 2000; Maidji et al., 2002; Yamamoto-Tabata et al., 2004). Importantly, the cells’ central function, invasion, was significantly impaired following infection.
CMV infection in vitro downregulates cytotrophoblast expression of HLA-G
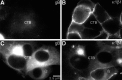
In healthy placentas, the non-classical MHC class Ib molecule HLA-G is expressed in differentiating cytotrophoblasts, particularly cells in anchoring villi with an increasing gradient of expression in the distal columns that is maintained once the cells enter the uterine wall (Kovats et al., 1990; McMaster et al., 1995). Immunolocalization experiments showed that CMV infection of differentiating cytotrophoblasts in vitro downregulates expression of HLA-G (Fisher et al., 2000). At late times when high levels of CMV gB were detected (Fig. 45.5(a)), staining for HLA-G was either greatly reduced or lost (Fig. 45.5(b)). This was in contrast to cells that were not infected with CMV and stained with anti-HLA-G.
Several CMV genes downregulate expression of classical MHC class Ia molecules (for review see Ploegh, 1998). Studied in the context of cytotrophoblasts infected with CMV mutants in which all of the genes known to downregulate cell surface expression of MHC class Ia molecules are deleted, Jones and Muzithras, (1992), showed that HLA-G expression was not rescued (Fisher et al., 2000). Others reported that HLA-G is resistant to the effects of CMV protein US11, which binds to class Ⅰ heavy chains and mediates their dislocation to the cytosol and subsequent proteasomal degradation (Schust et al., 1998). Subsequent analyses using chimeric molecules of MHC class Ia and Ib showed that the degradation efficiency depended on sequences in the heavy-chain cytosolic tail that HLA-G lacks (Barel et al., 2003). Since the mechanism of HLA-G downregulation does not involve CMV glycoproteins that alter class Ia expression, it is most likely novel.
CMV infection in vitro downregulates α1β1 integrin expression and impairs cytotrophoblast invasion
Congenital CMV infection is associated with abnormal placentation at a morphological level and intrauterine growth restriction (Benirschke et al., 1974), likely related to impaired remodeling of uterine arterioles by invasive cytotrophoblasts. This prompted examination of the expression of the laminin/collagen receptor integrin α1β1 in the context of CMV infection. This extracellular matrix receptor is both a stage-specific antigen whose expression is preferentially associated with cytotrophoblasts inside the uterine wall (Damsky et al., 1992) and an adhesion molecule that mediates invasion in vitro (Damsky et al., 1994). Co-localization of CMV gB (Fig. 45.6(a) and (c)) and integrin α1 expression (Fig. 45.6(b) and (d)) showed that cells that did not stain for gB (Fig. 45.6(a)) expressed integrin α1 in a plasma membrane-associated pattern (Fig. 45.6(b)). Diffuse cytoplasmic staining for gB in infected cytotrophoblasts was also correlated with integrin α1 expression (see cell marked with an asterisk in Fig. 45.6(c) and (d)), but accumulation of gB in vesicles (Fig. 45.6(c)) at late times after infection was associated with the absence of staining for integrin α1 (Fig. 45.6(d)). In contrast, immunostaining for another integrin whose expression is upregulated as the cells invade, the fibronectin receptor α5 that functions to inhibit invasion, was not affected.
Flow cytometric analysis and RT-PCR were used to quantify proteins expressed on the surface of freshly isolated cytotrophoblasts from term placentas and changes in differentiating cells infected with VR1814, a pathogenic clinical strain (Tabata et al., 2006). Significant downregulation of HLA-G at the protein level shown by immunohistochemistry was confirmed, and transcription was reduced in infected cells. Likewise, infected cytotrophoblasts significantly dysregulated integrin α1 and α5 proteins. Integrin α9 and VE-cadherin, which promote cell-cell adhesion, were also reduced by infection. Cytotrophoblasts isolated from placentas with CMV DNA and virion gB in syncytiotrophoblasts and in villus core macrophages were uninfected and showed similar expression of the differentiation molecules studied.
The impact of CMV infection on cytotrophoblast invasion was examined using an in vitro assay (see Fig. 45.2(b)) (Fisher et al., 2000). This functional assay tests the ability of isolated cytotrophoblasts plated on the upper surfaces of Matrigel-coated filters to penetrate the surface, pass through pores in the underlying filter, and emerge on the lower surface of the membrane (Damsky et al., 1994; Librach et al., 1991). Invasion is quantified by determining the number of cytokeratin-positive cell processes that emerge through the filter pores. The invasion ability of cells infected with CMV was dramatically impaired, as compared with control uninfected cells, suggesting that functional defects could result from a constellation of virus-induced changes that impair cell–matrix and cell–cell adhesion. Interestingly, the effect on invasion was greater than could be accounted for by the number of CMV-infected cells, suggesting that the presence of infected cells in the invading aggregates influences the behavior of the population as a whole.
CMV infection downregulates MMP activity altering cell-cell and cell–matrix interactions
MMPs are a family of degradative enzymes that remodel the extracellular matrix during many processes, including cell migration, vascularization, and invasion (Chang and Werb, 2001). MMPs are highly regulated during translation and post-translationally by activation and secretion. Invasive cytotrophoblasts secrete relatively large amounts of MMP-9 in early gestation, when invasion peaks; later, when invasion is complete, MMP-9 levels fall (Librach et al., 1991). Accordingly, cytotrophoblast invasion is also regulated by factors controlling MMP activation. The inactive proenzyme is activated by cleavage and removal of an inhibitory domain. Activated MMP-9 is absolutely required for invasion, whereas pro-MMP-9 is associated with noninvasive cells (Fisher et al., 1989; Librach et al., 1991).
Examination of cytotrophoblasts and endothelial cells infected with CMV in vitro showed altered MMP protein and activity (Yamamoto-Tabata et al., 2004). Infection with VR1814, an endothelial cell-tropic CMV strain, but not AD169, a laboratory strain, reduced MMP-9 activity, thereby decreasing the cells’ capacity to degrade the extracellular matrix. Likewise, MMP-2 activity in uterine microvascular endothelial cells was reduced. Since VR1814-infected endothelial cells transmit infection to cocultured differentiating cytotrophoblasts in vitro (Maidji et al., 2002), infection could undermine contacts between endothelial cells and cytotrophoblasts (Fig. 45.2, site 1). The observation that uterine arterioles are infected by CMV in utero (Pereira et al., 2003) suggests that virus could spread to cytotrophoblasts and in a retrograde direction to the placenta proper (i.e., floating chorionic villi) and to fetal blood vessels in the villus core.
CMV IL-10 dysregulates MMP activity
Several cytokines and growth factors regulate MMP expression and activity. For example, IL-1β is an autocrine stimulator of MMP-9 secretion and cytotrophoblast invasion of Matrigel in vitro (Librach et al., 1994). In contrast, human IL-10 (hIL-10) downregulates these processes and impairs cytotrophoblast invasion (Roth et al., 1996; Roth and Fisher, 1999). Recent reports indicated that CMV IL-10 (cmvIL-10) (Kotenko et al., 2000) binds the hIL-10 receptor 1 (hIL-10R1) with affinity similar to that of natural ligand (Jones et al., 2002) and has comparable immunosuppressive activity (Spencer et al., 2002). Analysis of the cmvIL-10 genes from several strains showed very high sequence conservation, suggesting conserved functions (Kotenko et al., 2000; Spencer et al., 2002). Like other intracellular pathogens that infect macrophages, CMV exploits the IL-10 signaling pathway, expressing an IL10 homologue and upregulating the cell’s production of the cytokine (Kotenko et al., 2000; Redpath et al., 2001). Although cmvIL-10 shares only 27% sequence identity with hIL-10, the proteins have essentially identical affinity for the receptor, IL-10R1, and similarly reorganize the cell surface receptor complex (Jones et al., 2002).
Both cytotrophoblasts and endothelial cells express IL-10R1, suggesting possible autocrine and paracrine regulation by its ligand (Cattaruzza et al., 2003; Roth and Fisher, 1999). hIL-10 in cytotrophoblasts’ culture medium can suppress allogeneic lymphocyte reactivity (Roth et al., 1996), an important link between immune protection of the fetus and cytotrophoblast invasion of the uterus. Likewise, recombinant cmvIL-10 can inhibit proliferation of mitogen-stimulated peripheral blood mononuclear cells and production of proinflammatory cytokines at a level comparable to that of hIL-10 (Spencer et al., 2002). Together these findings suggest that cmvIL-10 might, like the cellular molecule, impair invasion of differentiating cytotrophoblasts. MMP activity was examined in uterine microvascular endothelial cells and differentiating cytotrophoblasts in vitro treated with purified recombinant cmvIL-10 or hIL-10 (Yamamoto-Tabata et al., 2004). Culture medium and cell lysates of treated endothelial cells contained less MMP activity than untreated contols, suggesting that the viral cytokine inhibits proteinase production in the absence of infection and that cmvIL-10-and hIL-10 have comparable effects. Likewise, levels of MMP-9 activity in differentiating cytotrophoblasts treated with these cytokines were significantly reduced in a dose-dependent fashion, confirming previous results (Roth and Fisher, 1999).
CMV IL-10 impairs endothelial cell migration and cytotrophoblast invasiveness in vitro
Having shown that cmvIL-10 downregulates MMP-2 and MMP-9 activity, the effect of reduced proteinase activity on fibroblast and endothelial cell function was examined in cell wound healing assays. Briefly, subconfluent cells were scratched (“wounded”) and then incubated until control cells closed the wound. Infection with VR1814 or treatment with hIL-10 and cmvIL-10 had impaired endothelial cell wound closure but had no inhibitory effect on fibroblast migration. To assess the effect on cytotrophoblasts, the frequency with which the cells passed through narrow pores in a Matrigel-coated filter was quantified. Treatment with cmvIL-10 alone impaired invasion to a level comparable to that of hIL-10-treated cells, and significantly fewer cells traversed the filter pores after treatment with cmvIL-10 as compared with control untreated cells. Together these results indicated that, like hIL-10, cmvIL-10 impairs endothelial cell migration in wound closure assays and cytotrophoblast invasion as previously observed in CMV-infected cells in vitro (Fisher et al., 2000). These studies suggest that CMV exploits an immune mechanism to dysregulate endothelial cell migration and cytotrophoblast invasion (Yamamoto-Tabata et al., 2004).
Concluding remarks
We are just beginning to appreciate how the unusual anatomy of the maternal-fetal interface is advantageous for CMV spread to the placenta and how innate and adaptive immunity often precludes transplacental transmission. These studies open the door to testing a variety of hypotheses regarding CMV infection of placental tissues. Numerous questions and challenges remain. What is the functional significance of the static picture we obtained of immune defenses and viral proteins at the placental-decidual interface? Does coinfection with viruses and pathogenic bacteria in the decidua and adjacent placenta correlate with fetal transmission in early and late gestation? Additionally it will be interesting to decipher the network of cytokines and chemokines that regulates trafficking of immune cells in the infected decidua. Finally, identifying the molecules used for virion attachment and entry into cytotrophoblasts and syncytiotrophoblasts is crucial to the development of therapeutic strategies. One key to the puzzle of resolving infection in utero will be the capacity to gauge the threshold for maternal hyporesponsive. Onset of inflammation could trigger CMV reactivation and processes whereby NK cells, macrophages and dendritic cells control infection in the decidua. We theorize that detailed studies will resolve the serious dichotomy between the devastating consequences of congenital CMV infection and our lack of knowledge, at the molecular level, of the mechanisms involved.
References
- Ahlfors K., Ivarsson S. A., Harris S., et al. Congenital cytomegalovirus infection and disease in Sweden and the relative importance of primary and secondary maternal infections. Preliminary findings from a prospective study. Scand. J. Infect. Dis. 1984;16:129–137. [PubMed: 6330880]
- Barel M. T., Pizzato N., Leeuwen, Bouteiller P. L., Wiertz E. J., Lenfant F. Amino acid composition of alpha1/alpha2 domains and cytoplasmic tail of MHC class Ⅰ molecules determine their susceptibility to human cytomegalovirus US11-mediated down-regulation. Eur. J. Immunol. 2003;33:1707–1716. [PubMed: 12778489]
- Bass K. E., Li H., Hawkes S. P., et al. Tissue inhibitor of metalloproteinase-3 expression is upregulated during human cytotrophoblast invasion in vitro. Dev. Genet. 1997;21:61–67. [PubMed: 9291581]
- Benirschke K., Kaufmann P. 2000Pathology of the Human Placenta. 4th edn. New York: Springer.
- Benirschke K., Mendoza G. R., Bazeley P. L. Placental and fetal manifestations of cytomegalovirus infection. Virchow’s Arch. B Cell Pathol. 1974;16:121–139. [PubMed: 4373898]
- Boppana S. B., Britt W. J. Antiviral antibody responses and intrauterine transmission after primary maternal cytomegalovirus infection. J. Infect. Dis. 1995;171:1115–1121. [PubMed: 7751685]
- Boppana S. B., Fowler K. B., Britt W. J., Stagno S., Pass R. F. Symptomatic congenital cytomegalovirus infection in infants born to mothers with preexisting immunity to cytomegalovirus. Pediatrics. 1999;104:55–60. [PubMed: 10390260]
- Britt W. J., Hitchcock, MacKay, Wasserheit 1999). Congenital cytomegalovirus infection. In Sexually Transmitted Diseases and Adverse Outcomes of Pregnancy, P. , eds., pp. 269–281. Washington, DC: ASM Press.
- Bulmer J. N., Morrison L., Longfellow M., Ritson A., Pace D. Granulated lymphocytes in human endometrium: histochemical and immunohistochemical studies. Hum. Reprod. 1991;6:791–798. [PubMed: 1757516]
- Cattaruzza M., Slodowski W., Stojakovic M., Krzesz R., Hecker M. Interleukin-10 induction of nitric oxide synthase expression attenuates CD40-mediated interleukin-12 synthesis in human endothelial cells. J. Biol. Chem. 2003;11:11. [PubMed: 12857749]
- Chang C., Werb Z. 2001The many faces of metalloproteases: cell growth, invasion, angiogenesis and metastasis Trends Cell Biol. 11S37–S43. [PMC free article: PMC2788992] [PubMed: 11684441]
- Chou S. W., Dennison K. M. Analysis of interstrain variation in cytomegalovirus glycoprotein B sequences encoding neutralization-related epitopes. J. Infect. Dis. 1991;163:1229–1234. [PubMed: 1709960]
- Collier A. C., Handsfield H. H., Ashley R., et al. Cervical but not urinary excretion of cytomegalovirus is related to sexual activity and contraceptive practices in sexually active women. J. Infect. Dis. 1995;171:33–38. [PubMed: 7798680]
- Cook C. H., Zhang Y., Guinness B. J., Lahm M. C., Sedmak D. D., Ferguson R. M., Mc2002Intra-abdominal bacterial infection reactivates latent pulmonarycytomegalovirus in immunocompetent mice J. Infect. Dis. 1851395–1400. [PubMed: 11992273]
- Cross J. C., Werb Z., Fisher S. J. Implantation and the placenta: key pieces of the development puzzle. Science. 1994;266:1508–1518. [PubMed: 7985020]
- Crossey P. A., Pillai C. C., Miell J. P. Altered placental development and intrauterine growth restriction in IGF binding protein-1 transgenic mice. J. Clin. Invest. 2002;110:411–418. [PMC free article: PMC151083] [PubMed: 12163461]
- Damsky C. H., Fisher S. J. Trophoblast pseudo-vasculogenesis: faking it with endothelial adhesion receptors. Curr. Opin. Cell Biol. 1998;10:660–666. [PubMed: 9818178]
- Damsky C. H., Fitzgerald M. L., Fisher S. J. Distribution patterns of extracellular matrix components and adhesion receptors are intricately modulated during first trimester cytotrophoblast differentiation along the invasive pathway, in vivo. J. Clin. Invest. 1992;89:210–222. [PMC free article: PMC442839] [PubMed: 1370295]
- Damsky C. H., Librach C., Lim K. H., et al. Integrin switching regulates normal trophoblast invasion. Development. 1994;120:3657–3666. [PubMed: 7529679]
- Drake P. M., Gunn M. D., Charo I. F., et al. Human placental cytotrophoblasts attract monocytes and CD56(bright) natural killer cells via the actions of monocyte inflammatory protein 1alpha. J. Exp. Med. 2001;193:1199–1212. [PMC free article: PMC2193324] [PubMed: 11369791]
- Feire A. L., Koss H., Compton T. Cellular integrins function as entry receptors for human cytomegalovirus via a highly conserved disintegrin-like domain. Proc. Natl. Acad. Sci. USA. 2004;101:15470–15475. [PMC free article: PMC524452] [PubMed: 15494436]
- Fish K. N., Soderberg-Naucler C., Mills L. K., Stenglein S., Nelson J. A. Human cytomegalovirus persistently infects aortic endothelial cells. J. Virol. 1998;72:5661–5668. [PMC free article: PMC110233] [PubMed: 9621025]
- Fisher S., Genbacev O., Maidji E., Pereira L. Human cytomegalovirus infection of placental cytotrophoblasts in vitro and in utero: implications for transmission and pathogenesis. J. Virol. 2000;74:6808–6820. [PMC free article: PMC112198] [PubMed: 10888620]
- Fisher S. J., Leitch M. S., Kantor M. S., Basbaum C. B., Kramer R. H. Degradation of extracellular matrix by the trophoblastic cells of first-trimester human placentas. J. Cell. Biochem. 1985;27:31–41. [PubMed: 3980589]
- Fisher S. J., Cui T. Y., Zhang L., et al. Adhesive and degradative properties of human placental cytotrophoblast cells in vitro. J. Cell. Biol. 1989;109:891–902. [PMC free article: PMC2115717] [PubMed: 2474556]
- Fowler K. B., Pass R. F. Sexually transmitted diseases in mothers of neonates with congenital cytomegalovirus infection. J. Infect. Dis. 1991;164:259–264. [PubMed: 1649872]
- Fowler K. B., Stagno S., Pass R. F. Maternal immunity and prevention of congenital cytomegalovirus infection. J. Am. Med. Assoc. 2003;289:1008–1011. [PubMed: 12597753]
- Griffiths P. D., Baboonian C. A prospective study of primary cytomegalovirus infection during pregnancy: final report. Br. J. Obstet. Gynaecol. 1984;91:307–315. [PubMed: 6324849]
- Hahn G., Jores R., Mocarski E. S. Cytomegalovirus remains latent in a common precursor of dendritic and myeloid cells. Proc. Natl. Acad. Sci. USA. 1998;95:3937–3942. [PMC free article: PMC19941] [PubMed: 9520471]
- Halwachs-Baumann G., Wilders-Truschnig M., Desoye G., et al. Human trophoblast cells are permissive to the complete replicative cycle of human cytomegalovirus. J. Virol. 1998;72:7598–7602. [PMC free article: PMC110014] [PubMed: 9696860]
- Hemmings D. G., Kilani R., Nykiforuk C., Preiksaitis J., Guilbert L. J. Permissive cytomegalovirus infection of primary villous term and first trimester trophoblasts. J. Virol. 1998;72:4970–4979. [PMC free article: PMC110059] [PubMed: 9573266]
- Hoang V. M., Foulk R., Clauser K., Burlingame A., Gibson B. W., Fisher S. J. Functional proteomics: examining the effects of hypoxia on the cytotrophoblast protein repertoire. Biochemistry. 2001;40:4077–4086. [PubMed: 11300788]
- Istas A. S., Demmler G. J., Dobbins J. G., Stewart J. A. Surveillance for congenital cytomegalovirus disease: a report from the National Congenital Cytomegalovirus Disease Registry. Clin. Infect. Dis. 1995;20:665–670. [PubMed: 7756493]
- Jones B. C., Logsdon N. J., Josephson K., Cook J., Barry P. A., Walter M. R. Crystal structure of human cytomegalovirus IL-10 bound to soluble human IL10R1. Proc. Natl. Acad. Sci. USA. 2002;99:9404–9409. [PMC free article: PMC123153] [PubMed: 12093920]
- Jones T. R., Muzithras V. P. A cluster of dispensable genes within the human cytomegalovirus genome short component: IRS1, US1 through US5, and the US6 family. J. Virol. 1992;66:2541–2546. [PMC free article: PMC289055] [PubMed: 1312642]
- Kamat B. R., Isaacson P. G. The immunocytochemical distribution of leukocytic subpopulations in human endometrium. Am. J. Pathol. 1987;127:66–73. [PMC free article: PMC1899604] [PubMed: 3565538]
- Kammerer U., Marzusch K., Krober S., Ruck P., Handgretinger R., Dietl J. A subset of CD56+ large granular lymphocytes in first-trimester human decidua are proliferating cells. Fertil. Steril. 1999;71:74–79. [PubMed: 9935119]
- Kammerer U., Schoppet M., Lellan A. D., et al. , Mc2000Human decidua contains potent immunostimulatory CD83(+) dendritic cells Am. J. Pathol. 157159–169. [PMC free article: PMC1850207] [PubMed: 10880386]
- Kammerer U., Eggert A. O., Kapp M., et al. Unique appearance of proliferating antigen-presenting cells expressing DC-SIGN (CD209) in the decidua of early human pregnancy. Am. J. Pathol. 2003;162:887–896. [PMC free article: PMC1868095] [PubMed: 12598322]
- King A., Wellings V., Gardner L., Loke Y. W. Immunocytochemical characterization of the unusual large granular lymphocytes in human endometrium throughout the menstrual cycle. Hum. Immunol. 1989;24:195–205. [PubMed: 2925453]
- Kondo K., Xu J., Mocarski E. S. Human cytomegalovirus latent gene expression in granulocyte-macrophage progenitors in culture and in seropositive individuals. Proc. Natl Acad. Sci. USA. 1996;93:11137–11142. [PMC free article: PMC38297] [PubMed: 8855322]
- Kotenko S. V., Saccani S., Izotova L. S., Mirochnitchenko O. V., Pestka S. Human cytomegalovirus harbors its own unique IL-10 homolog (cmvIL-10). Proc. Natl Acad. Sci. USA. 2000;97:1695–1700. [PMC free article: PMC26498] [PubMed: 10677520]
- Kovats S., Main E. K., Librach C., Stubblebine M., Fisher S. J., Mars R., and De1990A class Ⅰ antigen, HLA-G, expressed in human trophoblasts Science 248220–223. [PubMed: 2326636]
- Kruse A., Hallmann R., Butcher E. C. Specialized patterns of vascular differentiation antigens in the pregnant mouse uterus and the placenta. Biol. Reprod. 1999;61:1393–1401. [PubMed: 10569981]
- Lazzarotto T., Spezzacatena P., Pradelli P., Abate D. A., Varani S., Landini M. P. Avidity of immunoglobulin G directed against human cytomegalovirus during primary and secondary infections in immunocompetent and immunocompromised subjects. Clin. Diag. Lab. Immunol. 1997;4:469–473. [PMC free article: PMC170552] [PubMed: 9220166]
- Lazzarotto T., Varani S., Spezzacatena P., et al. Delayed acquisition of high-avidity anti-cytomegalovirus antibody is correlated with prolonged antigenemia in solid organ transplant recipients. J. Infect. Dis. 1998;178:1145–1149. [PubMed: 9806047]
- Librach C. L., Werb Z., Fitzgerald M. L., et al. 92-kD type IV collagenase mediates invasion of human cytotrophoblasts. J. Cell Biol. 1991;113:437–449. [PMC free article: PMC2288933] [PubMed: 1849141]
- Librach C. L., Feigenbaum S. L., Bass K. E., et al. Interleukin-1 beta regulates human cytotrophoblast metalloproteinase activity and invasion in vitro. J. Biol. Chem. 1994;269:17125–17131. [PubMed: 8006017]
- Maidji E., Percivalle E., Gerna G., Fisher S., Pereira L. Transmission of human cytomegalovirus from infected uterine microvascular endothelial cells to differentiating/invasive placental cytotrophoblasts. Virology. 2002;304:53–69. [PubMed: 12490403]
- Maidji, E., Genbacev, O., Chang, H. T., and Pereira, L. (submitted). Developmental regulation of human cytomegalovirus receptors in cytotrophoblasts correlates with distinct replication sites in the placenta. [PMC free article: PMC1900158] [PubMed: 17314173]
- Maidji E., Donagh S., Genbacev O., Tabata T., Pereira L., Mc2006Maternal antibodies enhance or prevent cytomegalovirus infection in the placenta by neonatal Fc receptor-mediated transcytosis Am. J. Pathol. 1681210–1226. [PMC free article: PMC1606573] [PubMed: 16565496]
- Donagh S., Maidji E., Ma W., Fisher S., Pereira L.Mc, Chang, H.-T., 2004Viral and bacterial pathogens at the maternal-fetal interface J. Infect. Dis. 190826–834. [PubMed: 15272412]
- Donagh S., Maidji E., Chang H.-T., Pereira L.Mc2006Patterns of human cytomegalovirus infection in term placentas: a preliminary analysis J. Clin. Virol. 35210–215. [PubMed: 16386950]
- Master M. T., Librach C. L., Zhou Y., et al. Mc1995Human placental HLA-G expression is restricted to differentiated cytotrophoblasts J. Immunol. 1543771–3778. [PubMed: 7706718]
- Mocarski E. S. Immunomodulation by cytomegaloviruses: manipulative strategies beyond evasion. Trends Microbiol. 2002;10:332–339. [PubMed: 12110212]
- Muhlemann K., Miller R. K., Metlay L., Menegus M. A. Cytomegalovirus infection of the human placenta: an immunocytochemical study. Hum. Pathol. 1992;23:1234–1237. [PubMed: 1330874]
- Nakamura Y., Sakuma S., Ohta Y., Kawano K., Hashimoto T. Detection of the human cytomegalovirus gene in placental chronic villitis by polymerase chain reaction. Hum. Pathol. 1994;25:815–818. [PubMed: 8056423]
- Nigro G., Adler S. P., Torre, Best A. M. Passive immunization during pregnancy for congenital cytomegalovirus infection. N. Engl. J. Med. 2005;353:1350–1362. [PubMed: 16192480]
- Norwitz E. R., Schust D. J., Fisher S. J. Implantation and the survival of early pregnancy. N. Engl. J. Med. 2001;345:1400–1408. [PubMed: 11794174]
- Knipe, HowleyPass, B. F. (2001). Cytomegalovirus. In Fields Virology, eds., 4th edn. Vol. 2, pp. 2675–2705. New York: Lippincott-Raven.
- Pass R. F., Little E. A., Stagno S., Britt W. J., Alford C. A. Young children as a probable source of maternal and congenital cytomegalovirus infection. N. Engl. J. Med. 1987;316:1366–1370. [PubMed: 3033505]
- Pereira L., Maidji E., Donagh S., Genbacev O., Fisher S., Mc2003Human cytomegalovirus transmission from the uterus to the placenta correlates with the presence of pathogenic bacteria and maternal immunity J. Virol 7713301–13314. [PMC free article: PMC296088] [PubMed: 14645586]
- Ploegh H. L. Viral strategies of immune evasion. Science. 1998;280:248–253. [PubMed: 9535648]
- Queenan J. T., Kao L. C., Arboleda C. E., et al. Regulation of urokinase-type plasminogen activator production by cultured human cytotrophoblasts. J. Biol. Chem. 1987;262:10903–10906. [PubMed: 3301845]
- Red-Horse K., Drake P. M., Gunn M. D., Fisher S. J. Chemokine ligand and receptor expression in the pregnant uterus: reciprocal patterns in complementary cell subsets suggest functional roles. Am. J. Pathol. 2001;159:2199–2213. [PMC free article: PMC1850586] [PubMed: 11733370]
- Redpath S., Ghazal P., Gascoigne N. R. Hijacking and exploitation of Il-10 by intracellular pathogens. Trends Microbiol. 2001;9:86–92. [PubMed: 11173248]
- Revello M. G., Zavattoni M., Furione M., Lilleri D., Gorini G., Gerna G. Diagnosis and outcome of preconceptional and periconceptional primary human cytomegalovirus infections. J. Infect. Dis. 2002;186:553–557. [PubMed: 12195384]
- Roth I., Fisher S. J. IL-10 is an autocrine inhibitor of human placental cytotrophoblast MMP- 9 production and invasion. Dev. Biol. 1999;205:194–204. [PubMed: 9882507]
- Roth I., Corry D. B., Locksley R. M., Abrams J. S., Litton M. J., Fisher S. J. Human placental cytotrophoblasts produce the immunosuppressive cytokine interleukin 10. J. Exp. Med. 1996;184:539–548. [PMC free article: PMC2192727] [PubMed: 8760807]
- Schust D. J., Tortorella D., Seebach J., Phan C., Ploegh H. L. Trophoblast class Ⅰ major histocompatibility complex (MHC) products are resistant to rapid degradation imposed by the human cytomegalovirus (HCMV) gene products US2 and US11. J. Exp. Med. 1998;188:497–503. [PMC free article: PMC2212475] [PubMed: 9687527]
- Shen C. Y., Chang S. F., Yen M. S., et al. Cytomegalovirus excretion in pregnant and nonpregnant women. J. Clin. Microbiol. 1993;31:1635–1636. [PMC free article: PMC265594] [PubMed: 8391026]
- Simister N. E., Story C. M. Human placental Fc receptors and the transmission of antibodies from mother to fetus. J. Reprod. Immunol. 1997;37:1–23. [PubMed: 9501287]
- Simister N. E., Story C. M., Chen H. L., Hunt J. S. An IgG-transporting Fc receptor expressed in the syncytiotrophoblast of human placenta. Eur. J. Immunol. 1996;26:1527–1531. [PubMed: 8766556]
- Sinzger C., Plachter B., Jahn G., Müntefering, H., Löning, T., Stöss, H., 1993Cell types infected in human cytomegalovirus placentitis identified by immunohistochemical double staining Virchows Archiv. A Pathol. Anat. Histopathol. 423249–256. [PubMed: 8236822]
- Soderberg-Naucler C., Fish K. N., Nelson J. A. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell. 1997;91:119–126. [PubMed: 9335340]
- Soderberg-Naucler C., Streblow D. N., Fish K. N., Allan-Yorke J., Smith P. P., Nelson J. A. Reactivation of latent human cytomegalovirus in CD14(+) monocytes is differentiation dependent. J. Virol. 2001;75:7543–7554. [PMC free article: PMC114989] [PubMed: 11462026]
- Soilleux E. J., Morris L. S., Lee B., et al. Placental expression of DC-SIGN may mediate intrauterine vertical transmission of HIV. J. Pathol. 2001;195:586–592. [PubMed: 11745695]
- Soilleux E. J., Morris L. S., Leslie G., et al. Constitutive and induced expression of DC-SIGN on dendritic cell and macrophage subpopulations in situ and in vitro. J. Leukoc. Biol. 2002;71:445–457. [PubMed: 11867682]
- Solberg H., Rinkenberger J., Dano K., Werb Z., Lund L. R. A functional overlap of plasminogen and MMPs regulates vascularization during placental development. Development. 2003;130:4439–4450. [PMC free article: PMC2775444] [PubMed: 12900459]
- Spencer J. V., Lockridge K. M., Barry P. A., et al. Potent immunosuppressive activities of cytomegalovirus-encoded interleukin-10. J. Virol. 2002;76:1285–1292. [PMC free article: PMC135865] [PubMed: 11773404]
- Stagno S., Reynolds D., Tsiantos A., et al. Cervical cytomegalovirus excretion in pregnant and nonpregnant women: suppression in early gestation. J. Infect. Dis. 1975;131:522–527. [PubMed: 165244]
- Stagno S., Reynolds D. W., Pass R. F., Alford C. A. Breast milk and the risk of cytomegalovirus infection. N. Engl. J. Med. 1980;302:1073–1076. [PubMed: 6245360]
- Stagno S., Pass R. F., Dworsky M. E., et al. Congenital cytomegalovirus infection: The relative importance of primary and recurrent maternal infection. N. Engl. J. Med. 1982;306:945–949. [PubMed: 6278309]
- Starkey P. M., Sargent I. L., Redman C. W. Cell populations in human early pregnancy decidua: characterization and isolation of large granular lymphocytes by flow cytometry. Immunology. 1988;65:129–134. [PMC free article: PMC1385031] [PubMed: 3181993]
- Tabata, T., McDonagh, S., Kawakatsu, H., and Pereira, L. (2006). Cytotrophoblasts infected with a pathogenic human cytomegalovirus strain dysregulate cell-matrix and cell-cell adhesion molecules: a quantitative analysis. Placenta, July 3. (Epub ahead of print) [PubMed: 16822542]
- Tugizov S., Maidji E., Pereira L. Role of apical and basolateral membranes in replication of human cytomegalovirus in polarized retinal pigment epithelial cells. J. Gen. Virol. 1996;77:61–74. [PubMed: 8558129]
- Wang X., Huang D. Y., Huong S. M., Huang E. S. Integrin alphavbeta3 is a coreceptor for human cytomegalovirus. Nat. Med. 2005;11:515–521. [PMC free article: PMC1904494] [PubMed: 15834425]
- Wang X., Huong S. M., Chiu M. L., Raab-Traub N., Huang E. S. Epidermal growth factor receptor is a cellular receptor for human cytomegalovirus. Nature. 2003;424:456–461. [PubMed: 12879076]
- Yamamoto-Tabata T., Donagh S., Fisher S., Pereira L., Mc, Chang, H.-T., 2004Human cytomegalovirus interleukin-10 downregulates matrix metalloproteinase activity and impairs endothelial cell migration and placental cytotrophoblast invasiveness in vitro J. Virol. 782831–2840. [PMC free article: PMC353759] [PubMed: 14990702]
- Zhou Y., Damsky C. H., Chiu K., Roberts J. M., Fisher S. J. Preeclampsia is associated with abnormal expression of adhesion molecules by invasive cytotrophoblasts. J. Clin. Invest. 1993;91:950–960. [PMC free article: PMC288047] [PubMed: 7680671]
- Zhou Y., Fisher S. J., Janatpour M., et al. Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? J. Clin. Invest. 1997;99:2139–2151. [PMC free article: PMC508044] [PubMed: 9151786]
- Review Virus entry into host, establishment of infection, spread in host, mechanisms of tissue damage.[Human Herpesviruses: Biology, ...]Review Virus entry into host, establishment of infection, spread in host, mechanisms of tissue damage.Britt W. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. 2007
- Congenital cytomegalovirus infection in fraternal twins: a longitudinal case study examining neurocognitive and neurobehavioral correlates.[Appl Neuropsychol Child. 2012]Congenital cytomegalovirus infection in fraternal twins: a longitudinal case study examining neurocognitive and neurobehavioral correlates.Llorente AM, Castillo CL. Appl Neuropsychol Child. 2012; 1(1):63-73.
- Review Persistence in the population: epidemiology and transmisson.[Human Herpesviruses: Biology, ...]Review Persistence in the population: epidemiology and transmisson.Boppana SB, Fowler KB. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. 2007
- [Influence of congenital asymptomatic cytomegalovirus infection on development of infants].[Zhonghua Er Ke Za Zhi. 2008][Influence of congenital asymptomatic cytomegalovirus infection on development of infants].Shan RB, Wang XL, Fu P. Zhonghua Er Ke Za Zhi. 2008 Sep; 46(9):658-61.
- Review Molecular basis of persistence and latency.[Human Herpesviruses: Biology, ...]Review Molecular basis of persistence and latency.Jarvis MA, Nelson JA. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. 2007
- HCMV persistence in the population: potential transplacental transmission - Huma...HCMV persistence in the population: potential transplacental transmission - Human Herpesviruses
Your browsing activity is empty.
Activity recording is turned off.
See more...