Introduction
Nitric oxide (NO) is a hydrophobic, highly labile free radical that is catalytically produced in biological systems from the reduction of l-arginine by nitric oxide synthase (NOS) to form l-citrulline, which produces NO in the process. In biological systems NO has long been known to play various roles in physiology, pathology and pharmacology [1]. In 1987, NO was identified as being responsible for the physiological actions of endothelium-derived relaxing factor (EDRF) [2]. Since that discovery, NO has been shown to be involved in numerous biological processes such as: vasodilatation and molecular messaging [2], penile erection [3], neurotransmission [4,5], inhibition of platelet aggregation [6], blood pressure regulation [7], immune response [8], and as a mediator in a wide range of both anti-tumor and anti-microbial activities [9,10]. In addition, NO has been implicated in a number of diseases including: diabetes [11], Parkinson’s and Alzheimer’s diseases [12]. The importance of NO was confirmed in 1992 when Science Magazine declared NO the “Molecule of the Year” and in 1998, when F. Furchgott, Louis J. Ignarro, and Ferid Murad were awarded the Nobel Prize in Physiology and Medicine for unraveling the complex nature of this simple molecule. Despite the obvious importance of NO in so many biological processes, less than 10% of the thousands of scientific publications over the last decade dedicated to the field of NO research involved its direct measurement.
As stated above, NO plays a significant role in a variety of biological processes where its spatial and temporal concentration is of extreme importance. However, the measurement of NO is quite difficult due to its short half-life (~ 5 s) and high reactivity with other biological components such as: superoxide, oxygen, thiols and others. To date, several techniques have been developed for the measurement of NO including: chemiluminescence [13,14], Griess method [15], paramagnetic resonance spectrometry [16], paramagnetic resonance imaging, spectrophotometry [17], and bioassay [18]. Each of these techniques has certain benefits associated with it, but suffer from poor sensitivity and the need for complex and often expensive experimental apparatus. In addition, the above NO sensing techniques are limited when it comes to continuous monitoring of NO concentration in real-time and most importantly in vivo. To date, electrochemical (amperometric) detection of NO is the only available technique sensitive enough to detect relevant concentrations of NO in real-time and in vivo, and suffers minimally from potential interfering species such as: nitrite, nitrate, dopamine, ascorbate, and l-arginine. Also, because electrodes can be made on the micro and nano-scale, these techniques also have the benefit of being able to measure NO concentrations in living systems without any significant effects from electrode insertion.
The first amperometric NO electrode used for direct measurement was described in 1990 [19]. In 1992, World Precision Instruments Inc. (WPI) developed the first commercial NO sensor system called the ISO-NO. Over subsequent years a range of highly specialized and sensitive NO electrodes have been developed offering detection limits for NO ranging from below 1 nM up to 100 μM [20]. Most recently, a unique range of high sensitivity NO sensors based on a membrane coated activated carbon microelectrode, with diameters ranging from 200 μm down to 100 nm, have been developed by this lab. These electrodes exhibit superior performance during NO measurement and feature a detection limit of less than 0.5 nM NO.
NO Microelectrodes
Among the several electrochemical techniques that have been shown to be useful for the measurement of NO, the most popular is amperometry. This technique uses the model set forth by Clark and Lyons in 1962 for continuous gas monitoring during cardiovascular surgery [21]. Generally, this technique involves applying a fixed (poise) voltage potential to a working electrode, vs. a reference electrode, and monitoring the very low redox current produced (e.g., pA’s) by the oxidation of NO. This technique has proven to be very useful for NO detection due to its fast response time, which is less than a few seconds, and its high sensitivity. As a result it is possible to monitor changes in NO concentration on biologically relevant time scales and concentrations, which are typically in the nM range. A multitude of other electrochemical techniques have been used to detect NO including: differential pulse voltammetry (DPV), differential normal pulse voltammetry (DNPV), linear scanning voltammetry (LSV), square wave voltammetry (SWV) and fast scan voltammetry (FSV). These methods typically employ a classical 3-electrode configuration consisting of a working electrode, reference electrode, and a counter electrode. Scanning techniques, with the exception of fast scanning voltammetry, require approximately ten seconds for the voltammogram to be recorded, which precludes its use in most NO research applications. Moreover, since scanning voltammetry-based NO instrumentation is not commercially available, NO researchers typically prefer to use the 2-electrode amperometric technique. Since the amperometric method is so widely used the discussion below will focus on these techniques.
Clark Type NO Microelectrodes
The first described electrochemical NO sensor was based on a classical Clark electrode design, where NO was directly oxidized on the working electrode surface [22]. The NO sensor was composed of a fine platinum wire and a separate silver wire, which were then inserted into a glass micropipette. The micropipette was then filled with 30 mM NaCl and 0.3 mM HCl and sealed at the tip with a chloroprene rubber membrane. The platinum (working) electrode was positioned close to the surface of the membrane. The silver wire was then used as the reference/counter electrode. Although such electrodes could be used to measure NO, their inherent low sensitivity, narrow linear concentration measurement range, and fragility rendered them unsuitable for most research applications. In 1992, utilizing the Clark type design, WPI produced the first commercial electrochemical NO sensor (ISO-NOP) for use with their NO detection meter (ISO-NO). The ISO-NOP sensor consisted of a platinum wire disk working electrode and an Ag/AgCl reference electrode. Both electrodes were encased within a protective Faraday-shielded stainless steel sleeve. The tip of sleeve was covered with a NO-selective membrane and the sleeve itself contained an electrolyte. The rugged design of this sensor made it extremely convenient in many research applications and the sensor became widely used and established in numerous NO measurement research applications. The basic design of this type of NO sensor is illustrated in Figure 21.1 [23].
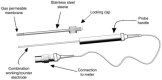
FIGURE 21.1
Illustration of WPI’s ISO-NOP NO sensor. (Reprinted from Zhang, X. J., Front. Biosci., 9, 3434, 2004. With permission.)
In principle the ISO-NOP sensor works as follows. The sensor is immersed in a solution containing NO and a positive potential of ~ 860 mV (vs. Ag/AgCl reference electrode) is applied. NO diffuses across the gas permeable/NO-selective membrane and is oxidized at the working electrode surface producing a redox current. This oxidation proceeds via an electrochemical reaction followed by a chemical reaction. The electrochemical reaction is a one-electron transfer from the NO molecule to the electrode, resulting in the formation of the nitrosonium cation:
NO+ is a relatively strong Lewis acid and in the presence of OH− , it is converted into nitrite (NO2−)
Hence:
Nitrite can then be further oxidized into nitrate.
The amount of NO oxidized is thus proportional to the current flow between the working and reference electrodes, which is measured by an NO meter.
The amount of redox current that is typically generated by the oxidation of NO in biological systems is extremely small, typically on the order of 1–10 pA. As a result of these extremely small currents, the design of an amperometric-based electrode NO detection system requires very sensitive electronics and ultra low noise amplification circuitry. These measurement limitations were overcome by this lab with the development of the low noise and isolated circuit electronic devices ISO-NO and ISO-NO Mark II NO meters. These meters employed a unique electrically isolated low noise circuit that permitted measurement of redox currents as small as 0.1 pA. The design of the instrument also allowed measurement of NO to be performed without the need for special electrical screening, such as a Faraday cage. Recently, the world’s only fully integrated multiple channel electrochemical NO/free radical detection system, the Apollo-4000 was developed at WPI. The detection system is an optically isolated multiple-channel NO/free radical analyzer designed specially for the detection of NO and other free radicals such as oxygen, hydrogen peroxide, hydrogen sulfide, and superoxide [19]. Although the Clark type electrode has enjoyed such great success for NO detection, there have been other significant advances for the detection of NO that deserve mention.
Metalloporphyrin and Metallophthalocyanine Modified NO Microelectrodes
Surface modified NO sensors incorporate an electrode surface that has been modified or treated in some way so as to increase the selectivity of the sensor for NO and promote catalytic oxidation of NO. An early example of such a sensor was presented by Malinski and Taha in 1992 [24]. In this publication a ~ 500 nm diameter carbon fiber electrode was coated with tetrakis (3-methoxy-4- hydroxyphenyl) porphyrin, via oxidative polymerization, and Nafion. This electrode was shown to have a detection limit of ~ 10 nM for NO and great selectivity against common interferences. However, recently it has been shown that this electrode suffers severe interference from H2O2 [25]. Following this publication, Schuhmann showed that pyrrole functionalized porphyrins, containing metals such as Ni, Pd and Mn, can be immobilized on carbon microelectrode surfaces via oxidative polymerization and may be used for NO detection [26,27]. Other researchers have shown that carbon fibers coated with a variety of porphyrins such as iron porphyrin [28], and cobalt porphyrin [24,29–32] are also effective for NO detection. Although metal porphyrin coated electrodes were successfully used to some extent for various applications; [33–35] subsequent studies have shown that carbon fibers modified with unmetallated porphyrins, as well as bare carbon fibers, can detect NO with similar sensitivity [36,37]. The sensitivity and selectivity of porphyrinic NO sensors vary significantly from electrode to electrode and depend not only on the potential at which NO oxidizes, but also on the effects of axial ligation to the central metal in the porphyrin, modification/treatment procedure and other experimental variations. Further-more, because the surface of the electrode remained in direct contact with the measurement medium a variety of biological species were shown to interfere (i.e., give false responses) with the measurement of NO. Adding a Nafion layer to the porphyrin-coated fibers could minimize these interferences. Other practical problems such as easy porphyrin removal and degradation have limited their usefulness for most applications [38]. Phthalocyanines, with a similar structure to porphyrins, containing metals such as Fe, Ni and Co have also been used to modify electrode surfaces for NO sensing [39,40]. Phthalocyanine modified electrodes have comparable detection limits and selectivity to porphyrin modified electrodes with the added benefit of being more stable to degradation.
Combination NO Microelectrodes
During the mid to late 1990s a new range of combination NO sensors with tip diameters between 7 and 200 μm were developed by this lab [41]. These sensors combined a carbon fiber working electrode with a separate integrated Ag/AgCl reference electrode. The resulting combination sensor was then coated with a proprietary gas permeable/NO selective membrane. A high performance Faraday-shielded layer was then added to the sensor outer to minimize susceptibility to environmental noise. This electrode was then operated exactly as outlined above for the Clark type NO sensors. The use of these proprietary diffusion membranes and the novel design allows for NO measurement in small volumes and confined spaces with great selectivity against a wide range of interferences such as ascorbic acid, nitrite and dopamine. This sensor design was elaborated upon by creating L-shaped sensors designed specifically for tissue bath studies [19]. The above design was further elaborated upon by our lab by creating flexible, virtually unbreakable NO sensors designed specifically for use in measuring NO concentrations in arteries and microvessels. This electrode combines a Pt/Ir wire with a separate integrated Ag/AgCl reference electrode [42]. The resulting combination sensor was again coated with a proprietary gas permeable/NO selective membrane and a high performance Faraday-shielded layer and operated as described above. Recently, we developed a novel combination NO nanosensor, which had a tip diameter of just 100 nm [43]. The design of this sensor can be seen in Figure 21.2. This sensor was constructed using a 7 μm carbon fiber that was etched with an Ar ion beam to result in fibers with diameters in the 100 nm range. These 100 nm fibers were then used to construct combination electrodes, as described above. These sensors are capable of making NO measurements on the cellular level. Later in 2000, our group developed a unique “microchip” combination sensor [44]. This sensor is quite unique in its design but its operation principle is the same as for all the other combination electrodes presented in this section. Briefly, a 5000 Å thick carbon film was deposited on a Si wafer by RF sputtering. This carbon layer was then covered with a resist layer. 2 μm diameter carbon electrodes were then exposed by a dry etching process in a 50 × 50 array on the wafer surface, where each carbon electrode is 20 μm away from its nearest neighbor. An Ag/AgCl reference electrode and a Pt counter electrode were added to the wafer surface. The surface was then modified with WPI’s proprietary gas permeable, NO selective membrane and ready for use. The resulting sensor (ISONOP- MC) exhibited an extremely low NO detection limit on the order of 300 pM, great selectivity against ascorbic acid, nitrite and dopamine, and a superior response time.
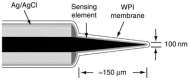
FIGURE 21.2
Illustration of WPI’s ISO-NOPNM 100 nm combination NO nanosensor. (Reprinted from Zhang, X. J. et al., Electrochem. Commun., 4, 11, 2002. With permission.)
Other NO Microelectrodes
Various other types of carbon fiber NO sensors, that utilize a variety of different coatings, have been described. Coatings used for these sensors include: conducting and non-conducting polymers [45–49], multiple membranes [50,51], ruthenium [52], iridium and palladium [53], heated-denatured cytochrome c [54], nafion-CoII-1,10-phenanthroline [55], ferrioxamine [56], a microcoaxial microelectrode was reported for in vivo NO measurement [57], siloxane polymer [58], nafion and cellulose [59], Hb/phosphatidylcholine films [60], hemoglobin–DNA film [61], ionic polymers and α-cyclodextrin [62]. Recently, Mizutani’s group reported the coating of a 10 μm Pt disc electrode with a cross-linked sol–gel Langmuir–Blodgett film of siloxane polymer to render the electrode permselective for NO [49]. Schoenfisch’s group recently reported the addition of a sol–gel film to a Pt electrode for NO detection [63]. Meyerhoff’s group described an improved planar amperometric NO sensor based on a platinized anode [64,65] and its application for measurement of NO release from NO donors. Scheler and co-workers explored using a myoglobin– clay modified electrode for NO detection [66]. Kamei and co-workers fabricated a NO sensing device for drug screening using a polyelectrolyte film [67]. Indium hexacyanoferrate film-modified electrodes were used for NO detection by Casero and co-workers [68]. Schuhmann’s group [69] developed a device for both in situ formation and scanning electrochemical microscopy assisted positioning of NO sensors above human umbilical vein endothelial cells for the detection of nitric-oxide release.
Unfortunately, despite the novelty of the above approaches, none of the sensors have stood the test of time, mostly due to various practical difficulties and/or poor sensitivity/selectivity. Furthermore, the lack of any published data describing the use of these sensors in any biological research applications limits any conclusion that can be made on their individual performance.
Calibration of NO Microelectrodes
Routine calibration of an NO sensor is essential in order to ensure accurate experimental results. One of three calibration techniques is generally used, depending on the sensor type, and will be described in the following section. Each of these methods has already been the subject of several reviews [20,70–72], and will therefore, only be summarized here. NO sensors are typically sensitive to temperature and thus, calibration is usually best performed at the temperature at which the measurements will be made.
Calibration Based on Chemical Generation of NO
This method of calibration generates known concentrations of NO based on the reaction of nitrite with iodide in acid according to the following equation:
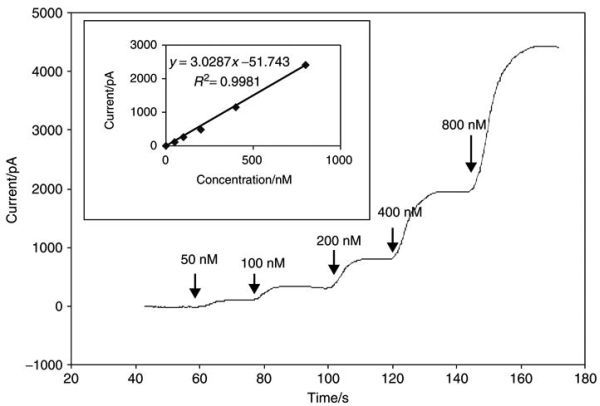
The NO generated from the reaction is then used to calibrate the sensor. Since the conversion of NO2− to NO is stoichiometric (and KI and H2SO4 are present in excess) the final concentration of NO generated is equal to the concentration of KNO2 in the solution. Hence, the concentration of NO can be easily calculated by simple dilution factors. Experiments have demonstrated that NO generated from this reaction will persist sufficiently long enough to calibrate an NO sensor. However, since this technique involves the use of a strong acid, which can damage the delicate selective membrane of most NO microsensors, it is only suitable for use with Clark type stainless steel encased NO sensors such as the ISO-NOP. Figure 21.3 illustrates the amperometric response of a 2.0 mm ISO-NOP sensor following exposure to increasing concentrations of NO. The sensor responds rapidly to NO and reaches steady state current within a few seconds. The data generated from Figure 21.3 is then used to construct a final calibration curve (Figure 21.3, inset). The calibration curve illustrates the good linearity that exists between NO concentration and the current produced by its oxidation.
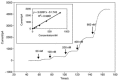
FIGURE 21.3
Response of WPI’s ISO-NOP NO sensor to increasing concentration of chemically generated NO, inset shows the resulting calibration curve. (Reprinted from Zhang, X. J., Front. Biosci., 9, 3434, 2004. With permission.)
Calibration Using an NO Standard Solution
This technique involves the production of an NO stock solution using a supply of compressed NO gas. One advantage of this method is that it allows an NO sensor to be calibrated in a similar environment in which the experimental measurements will be made. However, the major drawback is that it requires a source of compressed NO gas, and since NO gas is toxic, the whole procedure must be performed in a fume hood. This method can be summarized as follows. A vacutainer is first filled with 10 mL deionized water and agitated ultrasonically for 10 min. Purified argon is then passed through an alkaline pyrogallol solution (5% w/v) to scavenge any traces of oxygen before being purged through a deionized water solution for 30 min. NO stock solution is prepared by bubbling compressed NO gas through the argon-treated water for 30 min. The NO gas is first purified by passing it through 5% pyrogallol solution in saturated potassium hydroxide (to remove oxygen) and then 10% (w/v) potassium hydroxide to remove all other nitrogen oxides. The resultant concentration of saturated NO in the water is 2 mM at 22°C [73]. This can be confirmed further by a photometric method based on the conversion of oxyhemoglobin to methemoglobin in the presence of NO [74]. NO standard solutions can then be freshly prepared by serial dilution of saturated NO solution with oxygen-free deionized water prior to each experiment.
Calibration Based on Decomposition of SNAP
For this method S -nitroso-N -acetyl-d, l-penicillamine (SNAP, FW = 220.3) is decomposed to NO in solution in the presence of a Cu(I) catalyst [75]. The resultant NO generated can then be used to calibrate the sensor. The reaction proceeds in accordance to the following reaction:
The stoichiometry of the reaction dictates that the final generated NO concentration will be equal to the concentration of SNAP in the solution. The method can be summarized as follows. Saturated cuprous chloride solution is first prepared by adding 150 mg CuCl to 500 ml distilled water. This solution is then deoxygenated by purging with pure nitrogen or argon gas for 15 min. The final, saturated CuCl solution will have a concentration of approximately 2.4 mM at room temperature. The solution is light sensitive and must therefore, be kept in the dark prior to use.
The SNAP solution is then prepared separately as follows. EDTA (5 mg) is dissolved in 250 mL of pure water (HPLC grade, Sigma) and then adjusted to pH 9.0 using 0.1 M NaOH. The solution is then deoxygenated using the method described above. 5.6 mg of SNAP is then added to the solution to result in a SNAP concentration of ~ 0.1 mM. SNAP solution is also extremely sensitive to light and temperature and must be stored refrigerated and in the dark until required. Under these conditions, and in the presence of the cheating reagent (EDTA), the decomposition of SNAP occurs extremely slowly. This allows the solution to be used to calibrate NO probes throughout the day. In practice, calibration is performed by placing an NO sensor into a vial containing a measured amount of the CuCl solution and known volumes of the SNAP stock solution are then injected into the vial and the final concentration of NO can be calculated using dilution factors.
The concentration of SNAP in the stock solution is calculated as follows:
where C, concentration of SNAP (μM); A, purity of SNAP; W, weight of SNAP in milligrams (mg); M, MW of SNAP (mg/mmole); and V, volume of the solution in liters (L).
If SNAP purity, for example, is 98.5% then the concentration of SNAP is calculated as:
Figure 21.4 shows a typical calibration curve generated using an NO microsensor and the SNAP method described.
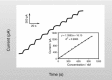
FIGURE 21.4
Response of WPI’s ISO-NOP007 NO sensor to increasing concentration of NO produced by introduction of SNAP to a solution of CuCl. (Reprinted from Zhang, X. J. et al., Electroanalysis, 12, 425, 2000. With permission.)
Characterization of NO Microelectrodes
NO microsensors can be characterized in terms of sensitivity, detection limit, selectivity, response time, stability, linear range, lifetime, reproducibility and biocompatibility. Sensor stability is important especially when measuring low NO concentrations. For example, when measuring low NO concentrations it must be the case that the noise is lower than the anticipated current change upon NO addition. The linear range of an NO sensor is important to know before performing any measurement. For example, the concentration of NO that is being measured must be in the linear range of the sensor in order for the measurement to be accurate. Typical commercially available NO sensors from WPI have a large linear range of 1 nM to 10 μM. Sensor lifetime and reproducibility is important to know when using a sensor and can be determined by frequent calibration, as described above. Biocompatibility is also extremely important when making NO measurements in vivo. Frequently, NO sensors are impaled into living tissue so the reactivity of the tissue toward the NO selective membrane must be minimal and cause a minimal amount of irritation in the tissue. The NO selective membranes used by this lab fulfill the requirements of being unreactive toward biomolecules and do not contribute to tissue inflammation. In most applications detection limit, sensitivity, selectivity and response time are usually the most important requirements, and will be described in further detail.
Sensitivity and Detection Limit
The sensitivity of an NO sensor depends largely on the reactive surface area of the sensor and the electrode materials used in the design and can range from 0.03 to 100 pA/nM NO. Generally speaking, this sensitivity is directly proportional to the electrode size and surface status where an electrode with a small surface area will generally have a lower sensitivity compared to one with a larger surface area. Although a sensor’s sensitivity is clearly important, its detection limit is often more important to the investigator. High sensitivity of a sensor does not necessarily equate to a low detection limit. For example, a highly sensitive NO sensor may have a high background noise level, which at a high NO concentration may not be a problem. However, at lower NO concentrations, measurement can be hindered by excessive noise. Accordingly, in evaluating the performance of an NO sensor, the ultimate detection limit is usually more critical than the sensitivity. Fortunately, most commercial NO sensors can detect NO at levels of 1 nM or less and are therefore well suited for the majority of research applications.
Selectivity
An NO sensor is practically useless unless it is immune to interference from other species likely to be present in the measurement environment. Selectivity is usually controlled by both the voltage applied between the working and reference electrode (poise voltage) and the selective membrane used to coat the sensor. Many species present in a biological matrix are easily oxidized at the poise voltage employed to detect NO (i.e., + 860 mV vs. Ag/AgCl). For example, monoamines such as dopamine (DA), 5-hydroxytryptamine (5-HT), and norepinephrine (NE), as well as their primary metabolites, can be oxidized at 0.3 V (and higher) vs. Ag/AgCl. Ascorbic acid can be oxidized at 0.4 V (and higher). A Clark type NO sensor (e.g., ISO-NOP) is covered with a gas permeable membrane, hence the selectivity of such sensors in biological samples is extremely good. With other types of NO sensors selectivity is usually achieved by coating the sensor surface with Nafion and other gas permeable membranes. Nafion is widely used to eliminate interference caused by anions, such as ascorbate and nitrite, during measurement of catecholamine species. When used for NO detection the negatively charged Nafion layer can stabilize NO + formed upon the oxidation of NO and prevent a complicated pattern of reactions that could lead to the formation of nitrite and nitrate. However, the main drawback with Nafion is that it does not eliminate interference from cationic molecules such as dopamine, serotonin, epinephrine and other catecholamines. Consequently, selectivity of the conventional Nafion coated NO sensors is very poor. Nafion coated NO sensors also exhibit other undesirable characteristics including: unstable background current, continuous drift in the base line, and extended polarization requirements. These problems significantly limit the use of Nafion coated carbon fiber electrodes for measurement of NO. During the late 1990s this lab developed a unique multi-layered proprietary membrane configuration. NO sensors coated with this membrane exhibited increased selectivity and sensitivity for NO, and moreover were shown to be immune from interference caused from a wide range of potentially interfering species [41].
Response Time
Response and recovery times of NO sensors are extremely important for their use in vivo. Theoretically, since the rate of mass transport at a microelectrode is very high it should have a response time on the order of μsec, however the addition of NO selective membranes to the electrode surface decreases this response time significantly. With a membrane present the response time is now dependent on the diffusion rate of NO across the membrane, which is highly dependent on the nature of the membrane as well as its thickness. The response time not only depends on the electrode being used, but also on the electronics being used to read out the current. For example, since the current being read out is typically on the order of pA’s an electronic filter is usually applied, which also slows the system response. Since the half life of NO is ~ 5 s, a sensor response on the order of 3–4 s will work fine.
Selected No Microelectrode Applications
Several hundred research papers have been published over the last decade describing the amperometric detection of NO in biological systems. This is mainly due to the fact that these type of electrochemical sensors are the only way NO can be measured in vivo in biological systems. Because there has been such an explosion in the development of NO microsensors these measurements can now be made in a variety of biological tissues and organs, as well as on the cellular level without significant damage to the system. This section will point out several examples where NO microsensors were used to determine its biological effects [76].
Determinations of NO in a variety of biological systems have been made. For example, measurement of NO has been made in eyes [77–79], gastrointestinal tracts [80,81], brain tissue [45,48,82–85], kidney and kidney tubule fluids [86–91], rat and guinea pig isolated and intact hearts [92,93], rat spinal cords [94], human monocyte cells [95], human endothelial cells [96], mitochondria [97,98], rat penis corpus cavernosum [99], granulocytes [100], invertebrate ganglia and immunocytes [101], choroidal endothelial cells [102], cancer cells [103,104], peripheral blood [105], human blood [106], human leukocytes [107], platelets [108–110], ears [111,112], plants [113–116], and pteropod mollusks [117].
Levine and co-workers first reported on the real-time profiling of kidney tubular fluid nitricoxide concentration in vivo [87,89]. In the 2001 publication, a modified version of a combination NO electrode (WPI, ISONOP007) was successfully used to measure NO concentration profiles along the length of a single nephron of a rat kidney tubular segment. Since it was shown that the electrode is sensitive to NO in the rat tubule, it was used to detect NO concentration differences in rat kidney tubules before and after 5/6 nephrectomy. The results clearly showed that the NO concentration was much higher in nephrectomized rats vs. unnephrectomized rats.
In a recent publication, investigators used a specially customized ISO-NOP sensor to monitor, in real-time, NO production in the stomach and esophagus of human patients [80]. In this method a patient first swallowed two NO electrodes (see Figure 21.5), which were then withdrawn slowly at 1 cm increments every 2 min. The investigators were then able to establish a profile of NO concentration in the upper gastrointestinal tract.
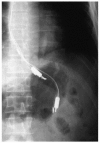
FIGURE 21.5
Abdominal x-ray showing the apparatus consisting of two nitric-oxide sensors, a 4-channel pH catheter, and a Tefion nasogastric tube. (Reprinted from Iijima, K. et al., Gastroenterology, 122, 1248, 2002. With permission.)
Simonsen’s group has performed some elegant work over the years on NO release characteristics from rat superior mesenteric arteries. Initially, Simonsen’s group simultaneously monitored artery relaxation and NO concentration in the artery using a NO microsensor in response to various drugs [118]. NO concentration was monitored via an ISONOP30 electrode, purchased from WPI, that was inserted into the artery lumen using a micromanipulator. The results of this work are shown in Figure 21.6. The figure shows the force (upper traces) and NO concentration (lower traces) in an endothelium intact ( + E) segment of the rat superior mesenteric artery and the same segment after mechanical endothelial cell removal (− E). As can be seen from the traces, if endothelial cells are present the artery is capable of relaxation due to the endothelial cells releasing NO in response to acetylcholine, but if the endothelial cells are removed the artery is insensitive to acetylcholine injection, relaxing upon the introduction of the NO releasing molecule SNAP. In a subsequent publication, Simonsen’s group again monitored artery relaxation and NO concentration in the rat superior mesenteric artery to monitor its hyporeactivity to various endotoxins [119]. In this study an ISONOP30 electrode (WPI) was inserted into the lumen of the artery and the NO concentration, as well as artery relaxation, was measured as a function of endotoxin introduction to determine what effects lipopolysaccharide had on the artery function. The results showed that lipopolysaccharide resulted in induction of iNOS and SOD associated with endotoxin, and NO concentration increased only in response to l-arginine. Simonsen’s group has also experimented with measuring NO concentration in the artery of a hypertensive rat [120] and in isolated human small arteries [121].
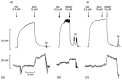
FIGURE 21.6
Simultaneous measurements of force (upper traces) and NO concentration (lower traces) in an endothelium intact (+ E) segment of rat superior mesenteric artery contracted with 0.5 μM noradrenaline (NA) and relaxed with either 10 μM acetylcholine (more...)
Schuhmann’s group has recently published some very interesting results describing the measurement of NO release from human umbilical vein endothelial cells (HUVEC) using a unique array of microelectrodes [122]. Figure 21.7 (top) shows an SEM image of the microelectrode array. Following microelectrode array construction the surface of the electrodes were modified with nickel tetrasulfonate phthalocyanine tetrasodium salt using electrochemically induced deposition. Following this deposition, the HUVEC cells were allowed to grow in the interstitial spaces between the electrodes (Figure 21.7, bottom), and NO release from the cells was monitored as a function of growth and stimulation from bradykinin. This work is unique because the cells are actually being grown on the Si3N4 insulating layer so the cells are not being affected by the microelectrode working potential, which typically causes cell death, allowing for NO concentration to be monitored.
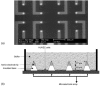
FIGURE 21.7
(a) A representative SEM image of the microelectrode array and (b) a schematic representation of the experimental setup. (Reprinted from Isik, S. et al., Biosens. Bioelectron., 20, 1566, 2005. With permission.)
A recent publication by Millar has shown that NO concentrations can be measured in bovine eyes trabecular meshwork in situ using a ISONOP200 electrode from WPI [77]. For this study, the tip of the 200 μm diameter electrode was inserted into the region of the trabecular meshwork and NO monitored as a function of epinephrine concentration. This study found that NO generation increased as a function of epinephrine addition resulting in a reduction of intraocular pressure. This finding is important for shedding light on treatments for patients with primary open-angle glaucoma. Researchers such as Akeo and Amaki have used a Pt/Ir electrodes, coated with NO selective membranes, for the determination of what effects l-DOPA has on eyes [78,79].
Because NO is a diffusible messenger molecule in the brain, the measurement of NO concentration in vitro is quite important to understand its action. In order to accomplish this general goal, Barbosa’s group recently developed a 8 μm diameter carbon fiber electrode coated with Nafion and o -phenylenediamine [48]. Nafion was added to the electrode surface by dipping the electrode into a 5% solution followed by drying for 10 min at 170° C, followed by o -phenylenediamine electropolymerization on the electrode by holding the potential at + 0.9 V vs. Ag/AgCl reference electrode. These electrodes were then inserted into the CA1 region of the hippocampus, from a Wistar rat, and the NO concentration was monitored as a function of l-glutamate and N -methyl-d-aspartate introduction. The results can be seen in Figure 21.8. The main finding of this study was that these electrodes were sufficiently sensitive to monitor brain NO concentrations with a sensitivity of 954±271 pA/μM and a limit of detection of 6 γ 2 nM. These measurements can also be made with minimal interference from common interferences such as: ascorbate, nitrite, and H2O2. These electrodes can be useful for unraveling pathways for memory and learning processes in the hippocampus.

FIGURE 21.8
NO production from the CA1 region of the hippocampal slice following addition of 5 mM L -glutamate (a) and 10 μM N -methyl-D -aspartate (b). (Reprinted from Ferreira, N. R. et al., Anal. Chim. Acta, 535, 1, 2005. With permission.)
The Mas group recently published results on the measurement of NO release from the corpus cavernosum of the penis and its relation to penile erection [99]. For this study a 30 μm diameter carbon fiber was coated with nickel tetrakis (3-methoxy-4-hydroxyphenyl) porphyrin, via electrodeposition using DPV, and Nafion. This electrode was then inserted into the cavernous bodies of a rat penis. The results showed an increase in NO concentration, and intracavernous pressure, upon cavernous nerve stimulation and subsequently decreased upon introduction of NO synthase isoenzymes. This study was important to facilitate further measurements of NO concentrations in vivo in the penis.
NO measurements have also been made in the ears of a guinea pig. The first of these studies was performed by Nuttall and co-workers in 2002 [111]. For this investigation a ISONOP30 carbon fiber electrode from WPI was inserted into the perilymph of the basal turn of the guinea pig ear to measure changes in NO concentration as a result of noise stimulation. This study showed that guinea pigs exposed to broadband noise for 3 h/day at 120 dBA, for 3 days, exhibited an increase in NO concentration in the perilymph. This result is important in understanding the role that NO plays in hearing loss. A subsequent publication by Nuttall and co-workers used the ISONOP200 from WPI to measure NO concentration in the spiral modiolar artery (SMA) of a guinea pig [112]. This study is important in gaining a better understanding of how NO potentially regulates cochlear blood flow to set a benchmark for pharmacological and pathological evaluations. To perform this study a 3 mm section of the (SMA) was added to a bath solution and the complete tip of the 200 μm electrode was inserted into the bath, parallel to the SMA, to measure basal NO concentration as well as drug induced NO release, and how that relates to cochlear blood flow regulation. The key findings of this study were that the SMA continuously released NO, and that a blockage of this release by l-NAME causes a decrease in NO production and a vasoconstriction. These finding are important in understanding and interpreting future findings.
An interesting study was performed by Kashiwagi and co-workers studying the role that NO plays in tumor vessel morphogenesis and maturation [103]. For this study, B16 tumor cells were injected into mice, and tumor tissue removed from the mice when it reached ~ 8 mm in diameter. The tip of a Nafion polymer coated Au microelectrode was subsequently inserted into the tumor to monitor NO production. The results of this study showed that NO mediates mural cell coverage and vessel branching/longitudinal extension, but does not play a part in the growth of tumor blood vessels. The investigators also used a NOS inhibitor to show that NOS from endothelial cells in tumors is the primary source of NO and mediates tumor growth.
Kishi and co-workers used a commercial NO-selective microelectrode to monitor the effect that exercise has on platelet-derived NO [108]. This study used 23 healthy, male, non-smokers that under-went treadmill exercise. Blood samples were taken from the subjects before and directly following exercise, and blood platelets were isolated. The study showed that NO concentration and platelet levels were increased following exercise. This increase in NO concentration is thought to play a role in the prevention of exercise-induced platelet activation in humans.
Kellogg and co-workers recently reported on the measurement of NO under the human skin in response to heat stress. For this study a flexible 200 μm NO microelectrode from WPI was inserted into the cutaneous interstitial space of the forearm of nine human patients to measure NO concentration while the subjects were at low (34°C) and high (39°C) temperatures. Laser-Doppler flowmetry (LDF) was used to monitor skin blood flow (SkBF) [123]. This publication demonstrated that NO concentration, as well as SkBF increased in the cutaneous interstitial space during heat stress in humans. Figure 21.9 shows the key results of these experiments. As the temperature was increased at ~ 10 min, the data show that the blood flow (top plot) and NO concentration (bottom plot) increased as a function of temperature. Also, as the temperature was decreased again, at ~ 45 min, the blood flow and NO concentration returned to its original value. At ~ 130 min, while the subjects were at low temperature, the investigators injected acetylcholine to show that NO was indeed being detected in the subjects. The same group used a similar experimental design to monitor NO concentration, as well as SkBF, during reactive hyperemia under the human skin [124].
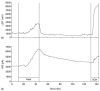
FIGURE 21.9
Laser-doppler flowmetry (a) and NO measurement (b) from one subject. Upon heating the subject to 39°C, at ~ 10 min, NO production and skin blood flow increased, which instantly returned to normal upon cooling the subject at ~ 45 min. After heat (more...)
Thom and co-workers published results on the stimulation of perivascular NO synthesis by oxygen [125]. To perform this study a 200 μm diameter electrode (ISONOP200) was placed between the aorta and vena cava of anesthetized rats and mice (rodents) and then the rodents were placed inside a hyperbaric chamber. Inside the hyperbaric chamber the partial pressure of O2 was regulated/changed as NO concentration was monitored. Figure 21.10 shows that NO concentration increased as a function of O2 partial pressure. This experiment is important in understanding how NO synthesis, by NOS, is altered and regulated by O2
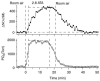
FIGURE 21.10
NO concentration (top) and O 2 concentration (bottom) as a function of O 2 pressure. As can be seen, NO and O 2 concentrations increase significantly when a rat is exposed to pressurized O 2 atmospheres (2.8 atmospheres absolute, ATA). (Reprinted from (more...)
Studies have also been conducted on the NO release from plants and the effects of a plant diet on artherosclerosis and endothelial cell dysfunction. Visioli and co-workers studied the effects that a diet of wild artichoke and thyme had on the release of NO from porcine aortic endothelial cells and cerebral cell membranes [116]. For this study a rat brain was homogenized and cell membranes isolated by ultracentrifugation and NO release was monitored using a 2 mm NO sensor (ISONOP). The cell membranes were then exposed to wild artichoke and thyme extracts. This study showed that NO release was significantly increased following wild artichoke and thyme extract addition. These results suggest that eating a diet rich in phenolic compounds, such as wild artichoke and thyme, contributes to maintenance of a healthy cardiovascular system. Yamasaki and co-workers used an ISONOP from WPI to study the ability of plant nitrate reductase to produce NO [115]. Figure 21.11 and Figure 21.12 show that plant nitrate reductase produces NO in vitro as well as its toxic derivative peroxynitrite.
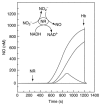
FIGURE 21.11
NO production from the addition of nitrate reductase (NR = 15 mU/mL) to a solution containing 50 μM sodium nitrate and various concentrations of NADH. The curves from top to bottom were obtained in solutions containing 100, 50, 40, and 0 μM (more...)
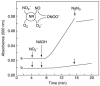
FIGURE 21.12
Absorbance measurement of 2′,7′-dichlorodihydrofluorescein (DCDHF), a peroxynitrite sensitive dye, as a function of nitrite (1 mM) and NADH (1 mM) introduction to a solution containing 100 μM DCDHF and 30 mU/mL NR under ambient (more...)
Concluding Remarks
The use of the electrochemical NO microsensor provides an elegant and convenient way to detect NO in real time and in biological samples. Currently they provide the only means by which to measure NO continuously, accurately and directly within living tissue without significant damage. The increasing acceptance of such sensors and their diversity of use in many NO research applications will help to further the current understanding of the various clinical roles of this interesting and ubiquitous molecule. Continual improvements being made to NO microsensor design and technology will facilitate these studies in the foreseeable future.
Acknowledgments
This research was supported by NIH grants (1 R43 GM62077-01, 2R44 GM62077-02, 5R GM62077-3) to XZ and a WPI R&D priority research funds.
References
- 1.
- Moncada S, Palmer RMJ, Higgs EA. Nitric-oxide—physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109. [PubMed: 1852778]
- 2.
- Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric-oxide. Proc Natl Acad Sci USA. 1987;84:9265. [PMC free article: PMC299734] [PubMed: 2827174]
- 3.
- Ignarro LJ. Nitric-oxide—a novel signal transduction mechanism for transcellular communication. Hypertension. 1990;16:477. [PubMed: 1977698]
- 4.
- Bredt DS, Hwang PM, Glatt CE, Lowenstein C, Reed RR, Snyder SH. Cloned and expressed nitric-oxide synthase structurally resembles cytochrome-P-450 reductas. Nature. 1991;351:714. [PubMed: 1712077]
- 5.
- Feldman PL, Griffith OW, Stuehr DJ. The surprising life of nitric-oxide. Chem Eng News. 1993;71:26.
- 6.
- Radomski MW, Palmer RMJ, Moncada S. An l-arginine nitric-oxide pathway present in human platelets regulates aggregation. Proc Natl Acad Sci USA. 1990;87:5193. [PMC free article: PMC54288] [PubMed: 1695013]
- 7.
- Moncada S, Radomski MW, Palmer RMJ. Endothelium-derived relaxing factor—identification as nitric-oxide and role in the control of vascular tone and platelet-function. Biochem Pharmacol. 1988;37:2495. [PubMed: 3291879]
- 8.
- Akaike T, Yoshida M, Miyamoto Y, Sato K, Kohno M, Sasamoto K, Miyazaki K, Ueda S, Maeda H. Antagonistic action of imidazolineoxyl n -oxides against endothelium-derived relaxing factor: NO through a radical reaction. Biochemistry. 1993;32:827. [PubMed: 8422387]
- 9.
- Hibbs JB, Taintor RR, Vavrin Z, Rachlin EM. Nitric-oxide—a cyto-toxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988;157:87. [PubMed: 3196352]
- 10.
- Anbar M. Hyperthermia of the cancerous breast—analysis of mechanism. Cancer Lett. 1994;84:23. [PubMed: 8076360]
- 11.
- Schmidt H, Warner TD, Ishii K, Sheng H, Murad F. Insulin-secretion from pancreatic B-cells caused by l-arginine derived nitrogen-oxides. Science. 1992;255:721. [PubMed: 1371193]
- 12.
- Moncada S, Palmer RMJ, Higgs EA. Biosynthesis of nitric-oxide from l-arginine—a pathway for the regulation of cell-function and communication. Biochem Pharmacol. 1989;38:1709. [PubMed: 2567594]
- 13.
- Beckman JS, Congert KA. Direct measurement of dilute nitric oxide in solution with an ozone chemiluminescent detector. Methods. 1995;7:35.
- 14.
- Robinson JK, Bollinger MJ, Birks JW. Luminol/H2O2 chemiluminescence detector for the analysis of nitric oxide in exhaled breath. Anal Chem. 1999;71:5131. [PubMed: 10575964]
- 15.
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and N-15-labeled nitrate in biological-fluids. Anal Biochem. 1982;126:131. [PubMed: 7181105]
- 16.
- Wennmalm A, Lanne B, Petersson AS. Detection of endothelial-derived relaxing factor in human plasma in the basal state and following ischemiausing electron-paramagnetic resonance spectrometry. Anal Biochem. 1990;187:359. [PubMed: 2166451]
- 17.
- Bredt DS, Snyder SH. Nitric-oxide mediates glutamate-linked enhancement of cGMP levels in the cerebellum. Proc Natl Acad Sci USA. 1989;86:9030. [PMC free article: PMC298426] [PubMed: 2573074]
- 18.
- Wallace JL, Woodman RC. Detection of nitric oxide by bioassay. Methods. 1995;7:55.
- 19.
- World Precision Instruments Catalog. 2005:42–49.
- 20.
- Zhang X, Broderick M. Amperometric detection of nitric oxide. Mod Aspects Immunobiol. 2000;1:160.
- 21.
- Clark LC, Lyons C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann N Y Acad Sci. 1962;102:29. [PubMed: 14021529]
- 22.
- Shibuki K. An electrochemical microprobe for detecting nitric-oxide release in brain-tissue. Neurosci Res. 1990;9:69. [PubMed: 2175870]
- 23.
- Zhang XJ. Real time and in vivo monitoring of nitric-oxide by electrocehmical sensors—from dream to reality. Front Biosci. 2004;9:3434. [PubMed: 15353368]
- 24.
- Malinski T, Taha Z. Nitric-oxide release from a single cell measured in situ by a porphyrinicbased microsensor. Nature. 1992;358:676. [PubMed: 1495562]
- 25.
- Nagase S, Ohkoshi N, Ueda A, Aoyagi K, Koyama A. Hydrogen peroxide interferes with detection of nitric oxide by an electrochemical method. Clin Chem. 1997;43:1246. [PubMed: 9216468]
- 26.
- Diab N, Schuhmann W. Electropolymerized manganese porphyrin/polypyrrole films as catalytic surfaces for the oxidation of nitric oxide. Electrochim Acta. 2001;47:265.
- 27.
- Diab N, Oni J, Schulte A, Radtke I, Blochl A, Schuhmann W. Pyrrole functionalised metalloporphyrins as electrocatalysts for the oxidation of nitric-oxide. Talanta. 2003;61:43. [PubMed: 18969161]
- 28.
- Hayon J, Ozer D, Rishpon J, Bettelheim A. Spectroscopic and electrochemical response to nitrogen monoxide of a cationic iron porphyrin immobilized in nafion-coated electrodes or membranes. J Chem Soc Chem Commun. 1994;61:9.
- 29.
- Kashevskii AV, Lei J, Safronov AY, Ikeda O. Electrocatalytic properties of mesotetraphenylporphyrin cobalt for nitric oxide oxidation in methanolic solution and in Nafion((R)) film. J Electroanal Chem. 2002;531:71.
- 30.
- Brunet A, Privat C, Stepien O, David-Dufilho M, Devynck J, Devynck MA. Advantages and limits of the electrochemical method using Nafion and Ni-porphyrin-coated microelectrode to monitor NO release from cultured vascular cells. Analysis. 2000;28:469.
- 31.
- Malinski T, Taha Z, Grunfeld S, Burewicz A, Tomboulian P, Kiechle F. Measurements of nitric-oxide in biological-materials using a porphyrinic microsensor. Anal Chim Acta. 1993;279:135.
- 32.
- Bedioui F, Trevin S, Devynck J. Chemically modified microelectrodes designed for the electrochemical determination of nitric oxide in biological systems. Electroanalysis. 1996;8:1085.
- 33.
- Malinski T, Bailey F, Zhang ZG, Chopp M. Nitric-oxide measured by a porphyrinic microsensor in rat-brain after transient middle cerebral-artery occlusion. J Cereb Blood Flow Metab. 1993;13:355. [PubMed: 8478395]
- 34.
- Malinski T, Radomski MW, Taha Z, Moncada S. Direct electrochemical measurement of nitric-oxide released from human platelets. Biochem Biophys Res Commun. 1993;194:960. [PubMed: 8343175]
- 35.
- Zhang ZG, Chopp M, Bailey F, Malinski T. Nitric-oxide changes in the rat-brain after transient middle cerebral-artery occlusion. J Neurol Sci. 1995;128:22. [PubMed: 7536815]
- 36.
- Lantoine F, Trevin S, Bedioui F, Devynck J. Selective and sensitive electrochemical measurement of nitric-oxide in aqueous-solution—discussion and new results. J Electroanal Chem. 1995;392:85.
- 37.
- Bedioui F, Trevin S, Devynck J. The use of gold electrodes in the electrochemical detection of nitric-oxide in aqueous-solution. J Electroanal Chem. 1994;377:295.
- 38.
- Yokoyama H, Mori N, Kasai N, Matsue T, Uchida I, Kobayashi N, Tsuchihashi N, Yoshimura T, Hiramatsu M, Niwa SI. Direct and continuous monitoring of intrahippocampal nitric oxide (NO) by an NO sensor in freely moving rat after N -methyl-d-aspartic acid injection. Denki Kagaku. 1995;63:1167.
- 39.
- Kim KI, Chung HT, Oh GS, Bae HO, Kim SH, Chun HJ. Integrated gold-disk microelectrode modified with iron(II)-phthalocyanine for nitric oxide detection in macrophages. Microchem J. 2005;80:219.
- 40.
- Vilakazi SL, Nyokong T. Voltammetric determination of nitric oxide on cobalt phthalocyanine modified microelectrodes. J Electroanal Chem. 2001;512:56.
- 41.
- Zhang XJ, Cardosa L, Broderick M, Fein H, Lin J. An integrated nitric oxide sensor based on carbon fiber coated with selective membranes. Electroanalysis. 2000;12:1113.
- 42.
- Dickson A, Lin J, Sun J, Broderick M, Harry FA, Zhang XJ. Construction and characterization of a new flexible and nonbreakable nitric oxide microsensor. Electroanalysis. 2004;16:640.
- 43.
- Zhang XJ, Kislyak Y, Lin H, Dickson A, Cardosa L, Broderick M, Fein H. Nanometer size electrode for nitric oxide and S -nitrosothiols measurement. Electrochem Commun. 2002;4:11.
- 44.
- Zhang XJ, Lin J, Cardoso L, Broderick M, Darley-Usmar V. A novel microchip nitric oxide sensor with sub-nM detection limit. Electroanalysis. 2002;14:697.
- 45.
- Fabre B, Burlet S, Cespuglio R, Bidan G. Voltammetric detection of NO in the rat brain with an electronic conducting polymer and Nafion(R) bilayer-coated carbon fiber electrode. J Electroanal Chem. 1997;426:75.
- 46.
- Friedemann MN, Robinson SW, Gerhardt GA. o -Phenylenediamine-modified carbon fiber electrodes for the detection of nitric oxide. Anal Chem. 1996;68:2621. [PubMed: 8694261]
- 47.
- Park JK, Tran PH, Chao JKT, Ghodadra R, Rangarajan R, Thakor NV. In vivo nitric oxide sensor using non-conducting polymer-modified carbon fiber. Biosens Bioelectron. 1998;13:1187. [PubMed: 9871974]
- 48.
- Ferreira NR, Ledo A, Frade JG, Gerhardt GA, Laranjinha J, Barbosa R. Electrochemical measurement of endogenously produced nitric oxide in brain slices using Nafion/o -phenylenediamine modified carbon fiber microelectrodes. Anal Chim Acta. 2005;535:1.
- 49.
- Kato D, Kunitake M, Nishizawa M, Matsue T, Mizutani F. Amperometric nitric oxide microsensor using two-dimensional cross-linked Langmuir–Blodgett films of polysiloxane copolymer. Sens Actuator B-Chem. 2005;108:384.
- 50.
- Ichimori K, Ishida H, Fukahori M, Nakazawa H, Murakami E. Practical nitric-oxide measurement employing a nitric-oxide-selective electrode. Rev Sci Instrum. 1994;65:2714.
- 51.
- Pontie M, Bedioui F, Devynck J. New composite modified carbon microfibers for sensitive and selective determination of physiologically relevant concentrations of nitric oxide in solution. Electroanalysis. 1999;11:845.
- 52.
- Allen BW, Piantadosi CA, Coury LA. Electrode materials for nitric-oxide detection. Nitric Oxide-Biol Chem. 2000;4:75. [PubMed: 10733875]
- 53.
- Xian YZ, Sun WL, Xue JA, Luo M, Jin LT. Iridium oxide and palladium modified nitric oxide microsensor. Anal Chim Acta. 1999;381:191.
- 54.
- Haruyama T, Shiino S, Yanagida Y, Kobatake E, Aizawa M. Two types of electrochemical nitric-oxide (NO) sensing systems with heat-denatured cyt c and radical scavenger PTIO. Biosens Bioelectron. 1998;13:763. [PubMed: 9828370]
- 55.
- He XC, Mo JY. Electrocatalytic oxidation of NO at electrode modified with Nafion-Co-II- 1,10-phenanthroline film and its application to NO detection. Analyst. 2000;125:793.
- 56.
- Smith SR, Thorp HH. Application of the electrocatalytic reduction of nitric oxide mediated by ferrioxamine B to the determination of nitric oxide concentrations in solution. Inorg Chim Acta. 1998;273:316.
- 57.
- Kitamura Y, Uzawa T, Oka K, Komai Y, Ogawa H, Takizawa N, Kobayashi H, Tanishita K. Microcoaxial electrode for in vivo nitric oxide measurement. Anal Chem. 2000;72:2957. [PubMed: 10905334]
- 58.
- Mizutani F, Yabuki S, Sawaguchi T, Hirata Y, Sato Y, Iijima S. Use of a siloxane polymer for the preparation of amperometric sensors: O-2 and NO sensors and enzyme sensors. Sens Actuator B-Chem. 2001;76:489.
- 59.
- Katrlik J, Zalesakova P. Nitric oxide determination by amperometric carbon fiber microelectrode. Bioelectrochemistry. 2002;56:73. [PubMed: 12009447]
- 60.
- Fan CH, Pang JT, Shen PP, Li GX, Zhu DX. Nitric-oxide biosensors based on Hb/phosphatidylcholine films. Anal Sci. 2002;18:129. [PubMed: 11874112]
- 61.
- Fan CH, Li GX, Zhu JQ, Zhu DX. A reagentless nitric-oxide biosensor based on hemoglobin–DNA films. Anal Chim Acta. 2000;423:95.
- 62.
- Kitajima A, Teranishi T, Miyake M. Detection of nitric-oxide on carbon electrode modified with ionic polymers and alpha-cyclodextrin. Electrochemistry. 2001;69:16.
- 63.
- Shin JH, Weinman SW, Schoenfisch MH. Sol–gel derived amperometric nitric oxide microsensor. Anal Chem. 2005;77:3494. [PubMed: 15924380]
- 64.
- Lee Y, Oh BK, Meyerhoff ME. Improved planar amperometric nitric-oxide sensor based on platinized platinum anode. 1. Experimental results and theory when applied for monitoring NO release from diazeniumdiolate-doped polymeric films. Anal Chem. 2004;76:536. [PubMed: 14750844]
- 65.
- Lee Y, Yang J, Rudich SM, Schreiner RJ, Meyerhoff ME. Improved planar amperometric nitric oxide sensor based on platinized platinum anode. 2. Direct real-time measurement of NO generated from porcine kidney slices in the presence of l-arginine, l-arginine polymers, and protamine. Anal Chem. 2004;76:545. [PubMed: 14750845]
- 66.
- Kroning S, Scheller FW, Wollenberger U, Lisdat F. Myoglobin–clay electrode for nitricoxide (NO) detection in solution. Electroanalysis. 2004;16:253.
- 67.
- Kamei K, Haruyama T, Mie M, Yanagida Y, Aizawa M, Kobatake E. The construction of endothelial cellular biosensing system for the control of blood pressure drugs. Biosens Bioelectron. 2004;19:1121. [PubMed: 15018968]
- 68.
- Casero E, Pariente F, Lorenzo E. Electrocatalytic oxidation of nitric oxide at indium hexacyanoferrate film-modified electrodes. Anal Bioanal Chem. 2003;375:294. [PubMed: 12560976]
- 69.
- Pailleret A, Oni J, Reiter S, Isik S, Etienne M, Bedioui F, Schuhmann W. In situ formation and scanning electrochemical microscopy assisted positioning of NO-sensors above human umbilical vein endothelial cells for the detection of nitric oxide release. Electrochem Commun. 2003;5:847.
- 70.
- Magazine HI. Detection of endothelial cell-derived nitric-oxide: Current trends and future directions. Adv Neuroimmunol. 1995;5:479. [PubMed: 8746518]
- 71.
- Kiechle FL, Malinski T. Nitric-oxide—biochemistry, pathophysiology, and detection. Am J Clin Pathol. 1993;100:567. [PubMed: 7504395]
- 72.
- Archer S. Measurement of nitric-oxide in biological models. FASEB J. 1993;7:349. [PubMed: 8440411]
- 73.
- Handbook of Chemistry and Physics. 76th ed. CRC Press; Boca Raton, FL: 1995.
- 74.
- Kelm M, Schrader J. Control of coronary vascular tone by nitric-oxide. Circ Res. 1990;66:1561. [PubMed: 2160870]
- 75.
- Zhang XJ, Cardosa L, Broderick M, Fein H, Davies IR. Novel calibration method for nitric oxide microsensors by stoichiometrical generation of nitric-oxide from SNAP. Electroanalysis. 2000;12:425.
- 76.
- Tristani-Firouzi M, DeMaster EG, Quast BJ, Nelson DP, Archer SL. Utility of a nitricoxide electrode for monitoring the administration of nitric oxide in biologic systems. J Lab Clin Med. 1998;131:281. [PubMed: 9523853]
- 77.
- Millar JC. Real-time direct measurement of nitric-oxide in bovine perfused eye trabecular meshwork using a Clark-type electrode. J Ocular Pharmacol Ther. 2003;19:299. [PubMed: 12964955]
- 78.
- Akeo K, Amaki S, Suzuki T, Hiramitsu T. Melanin granules prevent the cytotoxic effects of l-DOPA on retinal pigment epithelial cells in vitro by regulation of NO and superoxide radicals. Pigment Cell Res. 2000;13:80. [PubMed: 10841029]
- 79.
- Amaki SK, Oguchi Y, Ogata T, Suzuki T, Akeo K, Hiramitsu T. l-DOPA produced nitric-oxide in the vitreous and caused greater vasodilation in the choroid and the ciliary body of melanotic rats than in those of amelanotic rats. Pigment Cell Res. 2001;14:256. [PubMed: 11549108]
- 80.
- Iijima K, Henry E, Moriya A, Wirz A, Kelman AW, McColl KEL. Dietary nitrate generates potentially mutagenic concentrations of nitric oxide at the gastroesophageal junction. Gastroenterology. 2002;122:1248. [PubMed: 11984511]
- 81.
- Stefano GB, Zhu W, Cadet P, Bilfinger TV, Mantione K. Morphine enhances nitricoxide release in the mammalian gastrointestinal tract via the mu 3 opiate receptor subtype: A hormonal role for endogenous morphine. J Physiol Pharmacol. 2004;55:279. [PubMed: 15082884]
- 82.
- Cherian L, Goodman JC, Robertson CS. Brain nitric-oxide changes after controlled cortical impact injury in rats. J Neurophysiol. 2000;83:2171. [PubMed: 10758126]
- 83.
- Buerk DG, Ances BM, Greenberg JH, Detre JA. Temporal dynamics of brain tissue nitric oxide during functional forepaw stimulation in rats. Neuroimage. 2003;18:1. [PubMed: 12507439]
- 84.
- Rocchitta G, Migheli R, Mura MP, Esposito G, Desole MS, Miele E, Miele M, Serra PA. Signalling pathways in the nitric-oxide donor-induced dopamine release in the striatum of freely moving rats: Evidence that exogenous nitric-oxide promotes Ca2+ entry through store-operated channels. Brain Res. 2004;1023:243. [PubMed: 15374750]
- 85.
- Meulemans A. A brain nitric-oxide synthase study in the rat: Production of a nitroso-corn pound NA and absence of nitric oxide synthesis. Neurosci Lett. 2002;321:115. [PubMed: 11872269]
- 86.
- Saito M, Miyagawa I. Real-time monitoring of nitric oxide in ischemia-reperfusion rat kidney. Urol Res. 2000;28:141. [PubMed: 10850639]
- 87.
- Levine DZ, Iacovitti M, Burns KD, Zhang XJ. Real-time profiling of kidney tubular fluid nitric-oxide concentrations in vivo. Am J Physiol-Renal Physiol. 2001;281:F189. [PubMed: 11399660]
- 88.
- Levine DZ, Iacovitti M. Real time microelectrode measurement of nitric oxide in kidney tubular fluid in vivo. Sensors. 2003;3:31.
- 89.
- Levine DZ, Burns KD, Jaffey J, Iacovitti M. Short-term modulation of distal tubule fluid nitric-oxide in vivo by loop NaCl reabsorption. Kidney Int. 2004;65:184. [PubMed: 14675049]
- 90.
- Thorup C, Kornfeld M, Winaver JM, Goligorsky MS, Moore LC. Angiotensin-II stimulates nitric-oxide release in isolated perfused renal resistance arteries. Pflugers Arch. 1998;435:432. [PubMed: 9426302]
- 91.
- Arregui B, Lopez B, Salom MG, Valero F, Navarro C, Fenoy FJ. Acute renal hemodynamic effects of dimanganese decacarbonyl and cobalt protoporphyrin. Kidney Int. 2004;65:564. [PubMed: 14717926]
- 92.
- Fujita S, Roerig DL, Bosnjak ZJ, Stowe DF. Effects of vasodilators and perfusion pressure on coronary flow and simultaneous release of nitric-oxide from guinea pig isolated hearts. Cardiovasc Res. 1998;38:655. [PubMed: 9747433]
- 93.
- Novalija E, Fujita S, Kampine JP, Stowe DF. Sevoflurane mimics ischemic preconditioning effects on coronary flow and nitric-oxide release in isolated hearts. Anesthesiology. 1999;91:701. [PubMed: 10485782]
- 94.
- Schulte D, Millar J. The effects of high- and low-intensity percutaneous stimulation on nitricoxide levels and spike activity in the superficial laminae of the spinal cord. Pain. 2003;103:139. [PubMed: 12749968]
- 95.
- Stefano GB, Prevot V, Beauvillain JC, Fimiani C, Welters I, Cadet P, Breton C, Pestel J, Salzet M, Bilfinger TV. Estradiol coupling to human monocyte nitric-oxide release is dependent on intracellular calcium transients: Evidence for an estrogen surface receptor. J Immunol. 1999;163:3758. [PubMed: 10490972]
- 96.
- Stefano GB, Prevot V, Beauvillain JC, Cadet P, Fimiani C, Welters I, Fricchione GL, Breton C, Lassalle P, Salzet M, Bilfinger TV. Cell-surface estrogen receptors mediate calcium-dependent nitric-oxide release in human endothelia. Circulation. 2000;101:1594. [PubMed: 10747354]
- 97.
- Beltran B, Quintero M, Garcia-Zaragoza E, O’Connor E, Esplugues JV, Moncada S. Inhibition of mitochondrial respiration by endogenous nitric oxide: A critical step in Fas signaling. Proc Natl Acad Sci USA. 2002;99:8892. [PMC free article: PMC124394] [PubMed: 12077295]
- 98.
- Shiva S, Brookes PS, Patel RP, Anderson PG, Darley-Usmar VM. Nitric-oxide partitioning into mitochondrial membranes and the control of respiration at cytochrome c oxidase. Proc Natl Acad Sci USA. 2001;98:7212. [PMC free article: PMC34648] [PubMed: 11416204]
- 99.
- Mas M, Escrig A, Gonzalez-Mora JL. In vivo electrochemical measurement of nitric-oxide in corpus cavernosum penis. J Neurosci Methods. 2002;119:143. [PubMed: 12323418]
- 100.
- Kedziora-Kornatowska KZ, Luciak M, Blaszczyk J, Pawlak W. Effect of aminoguanidine on the generation of superoxide anion and nitric-oxide by peripheral blood granulocytes of rats with streptozotocin-induced diabetes. Clin Chim Acta. 1998;278:45. [PubMed: 9877123]
- 101.
- Stefano GB, Cadet P, Breton C, Goumon Y, Prevot V, Dessaint JP, Beauvillain JC, Roumier AS, Welters I, Salzet M. Estradiol-stimulated nitric-oxide release in human granulocytes is dependent on intracellular calcium transients: Evidence of a cell surface estrogen receptor. Blood. 2000;95:3951. [PubMed: 10845933]
- 102.
- Uhlmann S, Friedrichs U, Eichler W, Hoffmann S, Wiedemann P. Direct measurement of VEGF-induced nitric-oxide production by choroidal endothelial cells. Microvasc Res. 2001;62:179. [PubMed: 11516247]
- 103.
- Kashiwagi S, Izumi Y, Gohongi T, Demou ZN, Xu L, Huang PL, Buerk DG, Munn LL, Jain RK, Fukumura D. NO mediates mural cell recruitment and vessel morphogenesis in murine melanomas and tissue-engineered blood vessels. J Clin Invest. 2005;115:1816. [PMC free article: PMC1143589] [PubMed: 15951843]
- 104.
- Tsatmali M, Graham A, Szatkowski D, Ancans J, Manning P, McNeil CJ, Graham AM, Thody AJ. Alpha-melanocyte-stimulating hormone modulates nitric-oxide production in melanocytes. J Invest Dermatol. 2000;114:520. [PubMed: 10692112]
- 105.
- Rysz J, Luciak M, Kedziora J, Blaszczyk J, Sibinska E. Nitric-oxide release in the peripheral blood during hemodialysis. Kidney Int. 1997;51:294. [PubMed: 8995746]
- 106.
- Rievaj M, Lietava J, Bustin D. Electrochemical determination of nitric-oxide in blood samples. Chem Pap-Chem Zvesti. 2004;58:306.
- 107.
- Larfars G, Lantoine F, Devynck MA, Gyllenhammar H. Electrochemical detection of nitric-oxide production in human polymorphonuclear neutrophil leukocytes. Scand J Clin Lab Invest. 1999;59:361. [PubMed: 10533848]
- 108.
- Kasuya N, Kishi Y, Sakita S, Numano F, Isobe M. Acute vigorous exercised primes enhanced NO release in human platelets. Atherosclerosis. 2002;161:225. [PubMed: 11882336]
- 109.
- De La Cruz JP, Gonzalez-Correa JA, Guerrero A, Marquez E, Martos F, de la Cuesta FS. Differences in the effects of extended-release aspirin and plain-formulated aspirin on prostanoids and nitric-oxide in healthy volunteers. Fundam Clin Pharmacol. 2003;17:363. [PubMed: 12803576]
- 110.
- Freedman JE, Loscalzo J, Barnard MR, Alpert C, Keaney JF, Michelson AD. Nitricoxide released from activated platelets inhibits platelet recruitment. J Clin Invest. 1997;100:350. [PMC free article: PMC508197] [PubMed: 9218511]
- 111.
- Shi XR, Ren TY, Nuttall AL. The electrochemical and fluorescence detection of nitricoxide in the cochlea and its increase following loud sound. Hear Res. 2002;164:49. [PubMed: 11950524]
- 112.
- Jiang ZG, Shi XR, Zhao H, Si JQ, Nuttall AL. Basal nitric-oxide production contributes to membrane potential and vasotone regulation of guinea pig in vitro spiral modiolar artery. Hear Res. 2004;189:92. [PubMed: 14987756]
- 113.
- Sakihama Y, Nakamura S, Yamasaki H. Nitric-oxide production mediated by nitrate reductase in the green alga Chlamydomonas reinhardtii: An alternative NO production pathway in photosynthetic organisms. Plant Cell Physiol. 2002;43:290. [PubMed: 11917083]
- 114.
- Yamasaki H, Sakihama Y, Takahashi S. An alternative pathway for nitric oxide production in plants: New features of an old enzyme. Trends Plant Sci. 1999;4:128. [PubMed: 10322545]
- 115.
- Yamasaki H, Sakihama Y. Simultaneous production of nitric-oxide and peroxynitrite by plant nitrate reductase: In vitro evidence for the NR-dependent formation of active nitrogen species. FEBS Lett. 2000;468:89. [PubMed: 10683447]
- 116.
- Grande S, Bogani P, De Saizieu A, Schueler G, Galli C, Visioli F. Vasomodulating potential of Mediterranean wild plant extracts. J Agric Food Chem. 2004;52:5021. [PubMed: 15291469]
- 117.
- Moroz LL, Norekian TP, Pirtle TJ, Robertson KJ, Satterlie RA. Distribution of NADPH-diaphorase reactivity and effects of nitric-oxide on feeding and locomotory circuitry in the pteropod mollusc, Clione limacina. J Comp Neurol. 2000;427:274. [PubMed: 11054693]
- 118.
- Simonsen U, Wadsworth RM, Buus NH, Mulvany MJ. In vitro simultaneous measurements of relaxation and nitric oxide concentration in rat superior mesenteric artery. J Physiol-Lond. 1999;516:271. [PMC free article: PMC2269215] [PubMed: 10066940]
- 119.
- Hernanz R, Alonso MJ, Zibrandtsen H, Alvarez Y, Salaices M, Simonsen U. Measurements of nitric-oxide concentration and hyporeactivity in rat superior mesenteric artery exposed to endotoxin. Cardiovasc Res. 2004;62:202. [PubMed: 15023567]
- 120.
- Stankevicius E, Martinez AC, Mulvany MJ, Simonsen U. Blunted acetylcholine relaxation and nitric-oxide release in arteries from renal hypertensive rats. J Hypertens. 2002;20:1571. [PubMed: 12172319]
- 121.
- Buus NH, Simonsen U, Pilegaard HK, Mulvaney NJ. Nitric-oxide, prostanoid and non-NO, non-prostanoid involvement in acetylcholine relaxation of isolated human small arteries. Br J Pharmacol. 2000;129:184. [PMC free article: PMC1621136] [PubMed: 10694219]
- 122.
- Isik S, Berdondini L, Oni J, Blochl A, Koudelka-Hep M, Schuhmann W. Cell-compatible array of three-dimensional tip electrodes for the detection of nitric-oxide release. Biosens Bioelectron. 2005;20:1566. [PubMed: 15626610]
- 123.
- Kellogg DL, Zhao JL, Friel C, Roman LJ. Nitric-oxide concentration increases in the cutaneous interstitial space during heat stress in humans. J Appl Physiol. 2003;94:1971. [PubMed: 12679350]
- 124.
- Zhao JL, Pergola PE, Roman LJ, Kellogg DL. Bioactive nitric-oxide concentration does not increase during reactive hyperemia in human skin. J Appl Physiol. 2004;96:628. [PubMed: 14715681]
- 125.
- Thom SR, Fisher D, Zhang J, Bhopale VM, Ohnishi ST, Kotake Y, Ohnishi T, Buerk DG. Stimulation of perivascular nitric-oxide synthesis by oxygen. Am J Physiol Heart Circ Physiol. 2003;284:H1230. [PubMed: 12505879]
Publication Details
Author Information and Affiliations
Authors
Michael J. Serpe and Xueji Zhang.Copyright
Publisher
CRC Press/Taylor & Francis, Boca Raton (FL)
NLM Citation
Serpe MJ, Zhang X. The Principles, Development and Application of Microelectrodes for the In Vivo Determination of Nitric Oxide. In: Michael AC, Borland LM, editors. Electrochemical Methods for Neuroscience. Boca Raton (FL): CRC Press/Taylor & Francis; 2007. Chapter 21.