NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004-2013.
| Chemical name: | N-Methyl-[11C]-2-(4’-methylaminophenyl)-6-hydroxybenzothiasole |
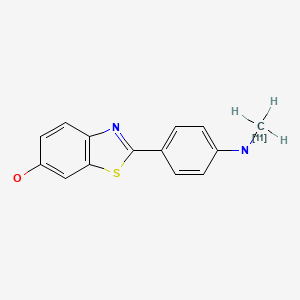
|
| Abbreviated name: | [11C]6-OH-BTA-1, [11C]PIB | |
| Synonym: | [11C]Pittsburgh Compound-B | |
| Backbone: | Compound | |
| Target: | Aggregates of Amyloid-beta peptide | |
| Mechanism: | Acceptor binding | |
| Method of detection: | PET | |
| Source of signal: | 11C | |
| Activation: | No | |
| Studies: |
| Click on the above structure for additional information in PubChem. |
Background
[PubMed]
Alzheimer's disease (AD) is a form of dementia with a gradual memory loss and a progressive decline in mental functions overtime (1, 2). It is characterized pathologically by neuronal loss, extracellular senile plaques (aggregates of amyloid-beta peptides consisting of 40 to 42 amino acids) and intracellular neurofibrillary tangles (filaments of microtubule-binding hyper-phosphorylated protein tau) in the brain, especially in the hippocampus and associative regions of the cortex (3, 4). β-amyloid peptides and tau protein are implicated as the main causes of neuronal degeneration and cell death (5, 6).
Early diagnosis of AD is important for treatment consideration and disease management (7). Various β-amyloid imaging agents have been developed for magnetic resonance imaging (MRI), single photon emission computed tomography (SPECT), and positron emission tomography (PET) (8-13). The binding of different derivatives of Congo red, thioflavin, stibene, and aminonaphthalene has been studied in human post-mortem brain tissue and in transgenic mice. Out of these analogues, 2-(1-(6-[(2-[18F]fluoroethyl)(methyl)amino]-2-naphthyl)ethylidene)malono nitrile ([18F]FDDNP) was studied in humans, showing more binding in the brains of patients with AD than in those of healthy people (14). However, [18F]FDDNP showed low signal-to-noise ratios for PET imaging, because it is highly lipophilic. N-Methyl-[11C]-2-(4’-methylaminophenyl)-6-hydroxybenzothiasole, a β-amyloid binding compound based on a series of neutral thioflavin-T derivatives (15), was radiolabeled with the positron-emitting radionuclide 11C ([11C]6-OH-BTA-1 or [11C]PIB). [11C]6-OH-BTA-1 was found to be a promising imaging agent for the senile plaques in the brain (16).
Synthesis
[PubMed]
[11C]6-OH-BTA-1 was readily synthesized by standard 11C-methylation of 2-(4’-aminophenyl)-6-methoxymethoxybenzothiazole with [11C]methyl iodide, followed by hydrolysis (15). The radiochemical yield averaged 12.1% at the end of synthesis based on [11C]methyl iodide, and the specific activity averaged 85 GBq/μmol (2.3 Ci/μmol) at the end of synthesis. Radiochemical and chemical purities were >95% as determined by high-performance liquid chromatography (HPLC).
In Vitro Studies: Testing in Cells and Tissues
[PubMed]
6-OH-BTA-1 has a binding affinity (Ki) of 4.5 nM for aggregated amyloid-beta(1-40) fibrils in competition with [N-methyl-3H]BTA-1 (15). The Ki Value for thioflavin-T was found to be 580 nM. Saturation binding studies with [N-methyl-3H]6-OH-BTA-1 to amyloid-beta(1-40) peptide showed a Kd value of 4.7 nM and a Bmax value of 2.7 pmol of 6-OH-BTA-1 per nmol of amyloid-beta(1-40) peptide. 6-OH-BTA-1 showed no inhibition in an array of neurotransmitter receptor and transporter assays. Saturation binding studies with [N-methyl-3H]6-OH-BTA-1 to homogenates of frontal cortex from postmortem advanced AD brain showed a KD value of 1.4 nM and a Bmax value of 1900 pmol per g tissue. There was no specific binding of [N-methyl-3H]6-OH-BTA-1 to homogenates of frontal cortex from age-matched control brain as well as to homogenates of cerebellum from AD brain or control brain. Klunk et al (16) showed that [11C]6-OH-BTA-1 bound specifically to frontal cortex of postmortem AD brain slices, and its binding could be blocked by excess unlabeled BTA-1. There was little binding of [11C]6-OH-BTA-1 to frontal cortex of normal elderly control brain slices.
Animal Studies
Rodents
[PubMed]
[11C]-labeled BTA derivatives (0.37-3.7 MBq, 10-100 μCi) were injected intravenously into mice to study their accumulation into brain of normal mice (15). 6-OH-BTA-1 has an apparent lipophilicity (log Pc18) of 1.2. It is expected to enter the brain readily. The radioactivity in excised brain samples was measured at 2 and 30 min after injection. [11C]6-OH-BTA-1 showed the lowest 30-min brain value of 0.018% injected dose (ID)-kg/g, with the highest 2 min-to-30 min ratio of 12. [11C]BTA-1 was the second best tracer, with a 2 min-to-30 min ratio of 7.6. The peripheral metabolism of [11C]6-OH-BTA-1 in normal mice was rapid, with intact [11C]6-OH-BTA-1 composing 73% of the total plasma activity at 2 min after injection and 6% at 30 min. All [11C]6-OH-BTA-1 metabolites were polar and are not expected to cross the blood-brain barrier because the brain homogenates at 2 and 10 min contained >95% unmetabolized [11C]6-OH-BTA-1.
Non-Human Primates
[PubMed]
PET studies were performed in six adult baboons after injection of 185 MBq (5 mCi) of [11C]6-OH-BTA-1 or [11]BTA-1 and co-registered with MRI anatomical brain images (15). A dynamic series of PET scans were acquired over 60 min. At early time points (0-9 min), radioactivity was uniformly distributed throughout the baboon brains, whereas at the later time points, radioactivity became more heterogeneously distributed. Regions of brain containing higher levels of white matter (such as pons) exhibited 20-30% higher accumulation of radioactivity than regions that were mainly gray matter (such as the temporal, mesial-temporal, and occipital cortex). In the baboon brains, radioactivity concentrations of [11C]BTA-1 and [11C]6-OH-BTA-1 at 30 min were 0.45 and 0.27% ID-kg/g, respectively. These values were similar to those in mice. [11C]6-OH-BTA-1 showed a faster rate of clearance of radioactivity than [11C]BTA-1.
Human Studies
[PubMed]
The first human study with [11C]6-OH-BTA-1 in 16 patients with mild AD and 9 healthy people was reported (16). The subjects were given an intravenous injection of 300 MBq (8.1 mCi) of [11C]6-OH-BTA-1. Plasma metabolism of [11C]6-OH-BTA-1 was rapid and similar in the AD patients and normal subjects. The amount of intact [11C]6-OH-BTA-1 was 7.2 ± 3.6% (normal control subjects) and 9.8 ± 3.0% (AD) at 60 min after injection. The dynamic PET data showed that [11C]6-OH-BTA-1 retention is 1.52-1.94-fold greater in brain regions (such as frontal, parietal, temporal, and occipital cortex and the striatum) that are known to contain amyloid plaques in AD patients than in controls. The frontal cortex had the highest tracer uptake. There was a similar retention of [11C]6-OH-BTA-1 in the cerebellum and white matter containing little amyloid deposits in AD and controls. There is an inverse correlation of [11C]6-OH-BTA-1 retention with cerebral FDG metabolism in the cortical regions. However, the differences between AD and controls were greater with [11C]6-OH-BTA-1 than with FDG.
References
- 1.
- Forstl H., Kurz A. Clinical features of Alzheimer's disease. Eur Arch Psychiatry Clin Neurosci. 1999;249(6):288–90. [PubMed: 10653284]
- 2.
- Heininger K. A unifying hypothesis of Alzheimer's disease. IV. Causation and sequence of events. Rev Neurosci. 2000;11(Spec No):213–328. [PubMed: 11065271]
- 3.
- Mirra S.S., Heyman A., McKeel D., Sumi S.M., Crain B.J., Brownlee L.M., Vogel F.S., Hughes J.P., van Belle G., Berg L. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41(4):479–86. [PubMed: 2011243]
- 4.
- Hardy J.A., Higgins G.A. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256(5054):184–5. [PubMed: 1566067]
- 5.
- Hardy J. The relationship between amyloid and tau. J Mol Neurosci. 2003;20(2):203–6. [PubMed: 12794314]
- 6.
- Brandt R., Hundelt M., Shahani N. Tau alteration and neuronal degeneration in tauopathies: mechanisms and models. Biochim Biophys Acta. 2005;1739(2-3):331–54. [PubMed: 15615650]
- 7.
- de Leon M.J., DeSanti S., Zinkowski R., Mehta P.D., Pratico D., Segal S., Clark C., Kerkman D., DeBernardis J., Li J., Lair L., Reisberg B., Tsui W., Rusinek H. MRI and CSF studies in the early diagnosis of Alzheimer's disease. J Intern Med. 2004;256(3):205–23. [PubMed: 15324364]
- 8.
- Bacskai B.J., Klunk W.E., Mathis C.A., Hyman B.T. Imaging amyloid-beta deposits in vivo. J Cereb Blood Flow Metab. 2002;22(9):1035–41. [PubMed: 12218409]
- 9.
- Nordberg A. PET imaging of amyloid in Alzheimer's disease. Lancet Neurol. 2004;3(9):519–27. [PubMed: 15324720]
- 10.
- Mathis C.A., Wang Y., Klunk W.E. Imaging beta-amyloid plaques and neurofibrillary tangles in the aging human brain. Curr Pharm Des. 2004;10(13):1469–92. [PubMed: 15134570]
- 11.
- Klunk W.E., Engler H., Nordberg A., Bacskai B.J., Wang Y., Price J.C., Bergstrom M., Hyman B.T., Langstrom B., Mathis C.A. Imaging the pathology of Alzheimer's disease: amyloid-imaging with positron emission tomography. Neuroimaging Clin N Am. 2003;13(4):781–9. [PubMed: 15024961]
- 12.
- Wang Y., Klunk W.E., Debnath M.L., Huang G.F., Holt D.P., Shao L., Mathis C.A. Development of a PET/SPECT agent for amyloid imaging in Alzheimer's disease. J Mol Neurosci. 2004;24(1):55–62. [PubMed: 15314250]
- 13.
- Kung M.P., Hou C., Zhuang Z.P., Skovronsky D., Kung H.F. Binding of two potential imaging agents targeting amyloid plaques in postmortem brain tissues of patients with Alzheimer's disease. Brain Res. 2004;1025(1-2):98–105. [PubMed: 15464749]
- 14.
- Shoghi-Jadid K., Small G.W., Agdeppa E.D., Kepe V., Ercoli L.M., Siddarth P., Read S., Satyamurthy N., Petric A., Huang S.C., Barrio J.R. Localization of neurofibrillary tangles and beta-amyloid plaques in the brains of living patients with Alzheimer disease. Am J Geriatr Psychiatry. 2002;10(1):24–35. [PubMed: 11790632]
- 15.
- Mathis C.A., Wang Y., Holt D.P., Huang G.F., Debnath M.L., Klunk W.E. Synthesis and evaluation of 11C-labeled 6-substituted 2-arylbenzothiazoles as amyloid imaging agents. J Med Chem. 2003;46(13):2740–54. [PubMed: 12801237]
- 16.
- Klunk W.E., Engler H., Nordberg A., Wang Y., Blomqvist G., Holt D.P., Bergstrom M., Savitcheva I., Huang G.F., Estrada S., Ausen B., Debnath M.L., Barletta J., Price J.C., Sandell J., Lopresti B.J., Wall A., Koivisto P., Antoni G., Mathis C.A., Langstrom B. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004;55(3):306–19. [PubMed: 14991808]
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review 2-(1-{6-[(2-[(18)F]Fluoroethyl)(methyl)amino]-2-naphthyl}ethylidene)malononitrile.[Molecular Imaging and Contrast...]Review 2-(1-{6-[(2-[(18)F]Fluoroethyl)(methyl)amino]-2-naphthyl}ethylidene)malononitrile.Leung K. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review (E)-4-(2-(6-(2-(2-(2-([(18)F]-fluoroethoxy)ethoxy)ethoxy)pyridin-3-yl)vinyl)-N-methylbenzenamine.[Molecular Imaging and Contrast...]Review (E)-4-(2-(6-(2-(2-(2-([(18)F]-fluoroethoxy)ethoxy)ethoxy)pyridin-3-yl)vinyl)-N-methylbenzenamine.Leung K. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review Trans-4-(N-Methylamino)-4´-{2-[2-(2-[(18)F]fluoro-ethoxy)-ethoxy]-ethoxy}-stilbene.[Molecular Imaging and Contrast...]Review Trans-4-(N-Methylamino)-4´-{2-[2-(2-[(18)F]fluoro-ethoxy)-ethoxy]-ethoxy}-stilbene.Leung K. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review 2-(4-Aminophenyl)-6-(2-([(18)F]fluoroethoxy))quinoline.[Molecular Imaging and Contrast...]Review 2-(4-Aminophenyl)-6-(2-([(18)F]fluoroethoxy))quinoline.Leung K. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review 2-(4-(2-[(18)F]Fluoroethyl)piperidin-1-yl)benzo[4,5]imidazo[1,2-a]pyrimidine.[Molecular Imaging and Contrast...]Review 2-(4-(2-[(18)F]Fluoroethyl)piperidin-1-yl)benzo[4,5]imidazo[1,2-a]pyrimidine.Leung K. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- N-Methyl-[11C]-2-(4'-methylaminophenyl)-6-hydroxybenzothiasole - Molecular Imagi...N-Methyl-[11C]-2-(4'-methylaminophenyl)-6-hydroxybenzothiasole - Molecular Imaging and Contrast Agent Database (MICAD)
Your browsing activity is empty.
Activity recording is turned off.
See more...

 In vitro
In vitro