NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Bast RC Jr, Kufe DW, Pollock RE, et al., editors. Holland-Frei Cancer Medicine. 5th edition. Hamilton (ON): BC Decker; 2000.
Mammography is the primary imaging modality for breast cancer screening and diagnosis. Improvements over the last decade in the quality of performance and the reporting of mammography studies rank among the most important advances in breast imaging. Performance improvements can be attributed to programs established by professional societies as well as by governmental agencies. The introduction of the American College of Radiology (ACR) Mammography Accreditation Program in 19871 and the Mammography Quality Standards Act of 19942 are among the most important contributory developments. In addition, the ACR Breast Imaging and Reporting System (BI-RADS)3 has improved the communication of mammography results, monitoring and tracking of patients, and quality assurance through medical audits. In view of its demonstrated utility and widespread application, the BI-RADS standardized lexicon will be used in this section.
Ultrasonography is the most important adjunctive imaging modality for breast cancer diagnosis. Over the years, it has also undergone significant improvements that have extended its utility for breast imaging. Other imaging techniques that are undergoing development and clinical trials at this time include digital mammography, magnetic resonance imaging (MRI), and radionuclide imaging.
Types of Mammography Examinations
There are two basic types of mammographic examinations: screening mammography and diagnostic mammography. Screening mammography refers to examinations of asymptomatic women to detect clinically occult breast cancers.4 The standard screening examination includes two views of the breast, sometimes referred to as the “standard views”: a mediolateral oblique view and a craniocaudal view (Fig. 30F.1).5 The effectiveness of screening mammography in reducing breast cancer mortality has been confirmed through randomized clinical trials.6 While there is general agreement that screening mammography reduces mortality from breast cancer in women over 50 years of age, there has been considerable debate over the effectiveness of screening mammography in women who are aged 40 to 49.7 On the basis of evidence of benefit for younger women that has been developed from a meta-analysis of several studies.8 the American Cancer Society and most major professional societies have continued to recommend mammography screening for women in their forties. Two reports from recent clinical trials also support the use of screening mammography for women in this age group. A 14-year follow-up in the Edinburgh trial has shown a mortality reduction of 21% for women aged 45 to 49 years who were screened with mammography.9 A 16-year follow-up in the UK Trial of Early Detection of Breast Cancer revealed a 27% decrease in mortality in women who were screened with mammography, and there was no evidence that women who were aged 45 to 46 years at the start of screening received less benefit than older women.10 No reduction in mortality could be attributed to breast self-examination, however.

Figure 30F.1
Standard mammography views. A. Mediolateral oblique (MLO) view includes more breast tissue than any other single view. The X-ray beam is directed perpendicular to the body of the pectoral muscle. B. Craniocaudal (CC) view provides a second projection (more...)
Diagnostic mammography, also called “consultative” or “problem-solving” mammography, is the type of study preferred when there are clinical findings, such as a palpable lump or an abnormal screening mammogram, requiring additional study.11 Accordingly, each diagnostic mammography examination is tailored to the individual patient who has symptoms or abnormal findings. Diagnostic mammography may entail additional views of the breast, such as spot compression and magnification, a correlative clinical examination, and ultrasonography. With some exceptions, a radiologist should be present during the performance of a diagnostic mammography study.
Diagnostic mammography should also be performed when a biopsy is being considered for a palpable lump in a woman over 30 years of age. The purposes of doing a mammogram prior to biopsy are to define the nature of the clinical abnormality better and to find other (unexpected) lesions, including multi-focal carcinomas or intraductal extensions of an invasive carcinoma. To correlate the clinical with the imaging findings, a radiopaque marker (“BB”) should be placed over any area(s) of clinical concern prior to performing a diagnostic mammogram (Fig. 30F.2).

Figure 30F.2
Radiopaque BB (arrow) placed directly over a palpable mass prior to performance of the mammogram. The mammogram shows irregular density directly behind the BB, confirming that this highly suspicious density mass is the palpable abnormality.
Standardized Mammography Reporting
A standardized BI-RADS mammography report should include the reason(s) for doing the examination, the observed composition of the breast tissue (see below), a description of the mammographic findings using the standardized lexicon, and a final assessment with a management recommendation.3
Breast Tissue Composition
The overall density of a patient’s breast tissue will help determine the sensitivity of mammography. Since breast cancers are radiodense (e.g., white on mammograms), radioluscent fat (dark gray-to-black on mammograms) provides an excellent background in which to see small cancers. On the other hand, dense fibroglandular breast tissue can obscure small cancers. Therefore, the standardized mammography report must include a statement about the overall composition of the breast in terms of the relative amounts of fatty and dense tissue. The breast composition can be characterized as one of four types: (1) the breast is almost entirely fat; (2) there are scattered islands of fibroglandular tissue (Fig. 30F.3A); (3) the breast tissue is heterogeneously dense (which may lower the sensitivity of mammography); and (4) the breast tissue is extremely dense (Fig. 30F.3B) (which will always lower the sensitivity of mammography).

Figure 30F.3
Different types of breast tissue composition. A. Primarily fatty breast with scattered islands of fibroglandular tissue. Mammography has high sensitivity in this type of breast. B. Extremely dense breast. The sensitivity of mammography is limited, due (more...)
Findings
The standard lexicon for reporting abnormal findings on mammograms will include descriptors to indicate the likelihood of malignancy. Masses and calcifications are the most common abnormalities encountered on mammograms, and the radiographic appearances of these abnormalities are important clues to their etiology.
Overall Impression and Assessment Category
The standardized report will also include one of six assessment categories, indicating the likelihood of malignancy and the radiologist’s recommendation(s) for management (Table 30F.1). Category “0”, or “Incomplete: Need additional imaging evaluation,” is usually used for a screening examination in which a definitive recommendation cannot be made until more information is obtained. The additional information that is needed might be an earlier comparison mammogram or a subsequent study, such as a problem-solving (diagnostic) mammogram and/or ultrasonogram. Once the individual patient’s work-up has been completed, the resulting examination will then be assigned to one of five other assessment categories as listed in Table 30F.1.
Table 30F.1
Mammography Final Assessment Categories.
The Final Regulations of the Mammography Quality Standards Act12 requires that the assessment category be included in the mammography report to identify patients who need further tracking and monitoring. Since some of the required follow-up steps will not necessarily be done by individuals with a detailed understanding of medical terminology, the standardized reporting mechanism should be user-friendly for the office staff of referring physicians and other institutions.
The Normal Mammogram
The mammographic appearance of the normal breast can be quite variable (see Fig. 30F.3). Since younger women usually have more fibroglandular tissue, their breasts normally tend to be more “dense.” However, there is wide variation in normal patterns, and dense normal tissue may predominate on the mammograms of some older women, while some younger women may have relatively fatty breasts. An increase in breast density has been reported in postmenopausal women, in conjunction with exogenous hormone replacement.
The Abnormal Mammogram
Masses
The most significant features that indicate whether a mass may be benign or malignant are its shape and the character of its margins (Fig. 30F.4). The shape can be round, oval, lobulated, or irregular. Circumscribed oval and round masses are usually benign. An irregular shape suggests a greater likelihood of malignancy. The margins can be described as circumscribed, microlobulated, obscured (partially hidden by adjacent tissue), indistinct, or spiculated. The likelihood of malignancy with a circumscribed mass is very low, but additional work-up may be necessary to verify that the margins are completely circumscribed. Typical examples of benign circumscribed masses are cysts (Fig. 30F.5) and fibroadenomas (Fig. 30F.6). Ultrasonography can be used to establish whether a circumscribed mass is cystic or solid. If the mass is a simple cyst, no further work-up is necessary. If solid, the shape and margins should be evaluated carefully, possibly with the help of magnification mammography. Unless there are previous mammograms to establish that it is a new finding, a solitary, completely circumscribed, nonpalpable, solid mass is often managed by ordering a 6-month follow-up examination to establish that the mass is stable (not growing).13 If it is stable, continued mammographic surveillance is recommended for at least 2 more years. The presence of multiple circumscribed masses is even stronger evidence of benign etiologies, such as cysts, fibroadenomas, intramammary lymph nodes, or papillomas, and annual surveillance is usually sufficient. If a circumscribed mass is directly adjacent to fibroglandular tissue of similar density, the margins of the mass may be obscured, and spot compression may be used in an attempt to show the margins of the mass more completely.

Figure 30F.4
Terminology used to describe masses.

Figure 30F.5
Cyst. Mediolateral oblique mammogram shows a round circumscribed mass (arrow) in the subareolar area. Ultrasonography revealed a simple cyst.

Figure 30F.6
Fibroadenomas. Mediolateral mammogram shows a lobular, circumscribed, low density mass (arrow). Biopsy revealed fibroadenoma.
Masses with irregular shapes and ill-defined or spiculated margins have a higher likelihood of malignancy. Indistinct margins (Fig. 30F.7) are generally suspicious for malignancy, and spiculated margins (Fig. 30F.8) are highly suggestive of malignancy.

Figure 30F.7
Invasive ductal carcinoma. Close-up of mammogram shows a mass (arrow) with an irregular shape and ill-defined margins.

Figure 30F.8
Invasive ductal carcinoma. Close-up of mammogram shows a mass with irregular shape and spiculated margins.
A small number of cancers may exhibit a round shape and relatively circumscribed margins. Some of the subtypes of ductal carcinomas, such as medullary, papillary or colloid carcinomas, are likely to have at least partially circumscribed margins. The majority of “circumscribed” carcinomas turn out to be the “usual,” or “not otherwise specified,” type of ductal carcinoma. Microlobulated margins are not common and can occur with either benign or malignant masses.
The density of a mass may provide at least some clue to its etiology. The density can be designated as low, intermediate (see Figs. 30F.5 and 6), or high (see Fig. 30F.7) by comparing it with an area of normal breast tissue on the mammogram. While benign masses in general tend to be lower in density than carcinomas, density is not reliable as a distinguishing mammographic sign.14
Calcifications
In the BI-RADS lexicon, calcifications are divided into categories of typically benign, intermediate-concern, and higher probability of malignancy (Fig. 30F.9). Typically benign calcifications on mammograms include cutaneous, vascular, coarse, rod-like, round, egg-shell, and milk-of-calcium types (Fig. 30F.10). Many calcifications are so typical of a benign lesion that additional work-up is unnecessary. However, if there is any doubt, magnification mammography should be performed to depict the calcifications better. Intermediate-concern calcifications are tiny, amorphous or indistinct (Fig. 30F.11). Higher probability of malignancy calcifications can be “pleomorphic, heterogeneous” or “fine, linear and branching (casting)” (Fig. 30F.12). Malignant calcifications may occur with or without an associated mammographic mass.15 Calcifications on a screening study are often the only evidence of an intraductal carcinoma or “ductal carcinoma in situ” (DCIS).16 In noncomedo DCIS the calcifications are of varying sizes and are caused by an active secretory process that produces aggregates of calcifications in cribriform spaces or tumor excrescences within the ducts. In comedo DCIS, typical linear, branching calcifications form within necrotic debris in the centers of ducts that are filled with tumor cells.

Figure 30F.9
Terminology used to describe calcifications.

Figure 30F.10
Typically benign calcifications. A. Rod-like calcifications (arrows) of benign secretory disease (duct ectasia). B. 90° lateral mammograms revealed calcifications (arrows) that layer at the bottom of fluid-filled microcysts. These are sometimes (more...)

Figure 30F.11
Barely visible intermediate (amorphous, indistinct) calcifications (arrows).

Figure 30F.12
Calcifications with higher probability of malignancy. A. This cluster manifests pleomorphic calcifications (arrows) of varying sizes and shapes. Biopsy revealed intraductal cribriform carcinoma. B. The fine, linear and branching calcifications (arrow (more...)
Calcifications can also be characterized by their distribution: grouped or clustered calcifications refer to groups of more than five within a small tissue volume (<2 cc). These can be benign or malignant (Fig. 30F.12a). Fine, linear, branching calcifications are arranged in a line that may have small branch points (Fig. 12B), a distribution that is suspicious for malignancy. Segmental calcifications, (Fig. 30F.13) which are distributed in a duct and its branches, also suggest malignancy. Regional calcifications occupy a larger volume of breast tissue and can be associated with benign or malignant conditions. Diffuse/scattered calcifications are distributed randomly through the breast and are almost always benign.

Figure 30F.13
Segmental distribution of fine, linear, branching calcifications (arrows) of intraductal comedocarcinoma.
Indirect Signs of Malignancy
Other “indirect” or subtle signs that may suggest a malignancy include a neodensity, architectural distortion of the breast tissue, or an asymmetric density (see below).17 Identifying such subtle signs may require careful comparisons with a patient’s previous examinations. Any new or evolving density that is identified by comparison with previous mammograms should lead to further evaluation. Ultrasonography may be indicated if the neodensity has features that are suggestive of a cyst. Additional views to evaluate the margins of a solid neodensity may help to determine the probability of malignancy, but biopsy is still indicated for most patients in whom a new breast mass has been determined to be solid.
Architectural distortion is the appearance on a mammogram of tissue spicules without an associated mass (Fig. 30F.14). The differential diagnosis of an area of architectural distortion includes scarring from previous surgery, radial scar, and carcinoma.

Figure 30F.14
Invasive carcinoma presenting as a subtle architectural distortion. Biopsy revealed an 8-mm invasive ductal carcinoma.
Asymmetric breast tissue is one of the most difficult mammographic findings to evaluate. The breasts tend to be relatively symmetrical in their distribution of fibroglandular tissue. For this reason, mammograms should be viewed so that the right and left breasts can be compared back-to-back on the viewbox. Minor asymmetries in the distribution of fibroglandular tissue between the two breasts are a common normal variant, but moderate asymmetry may warrant further investigation. Further study of a suspicious asymmetric density should include a correlative clinical breast examination (with careful palpation of the area of concern). Depending on the degree of suspicion, follow-up steps might include additional mammographic views, an ultrasound scan, or 6-month follow-up mammography. When asymmetry is associated with architectural distortion, calcifications, or an underlying mass, the probability of malignancy is greater and a biopsy should be considered.
Mammography of the Postsurgical Breast
Postsurgical changes on mammograms may include skin thickening or retraction, architectural distortion, asymmetry, calcification, and fat necrosis.18 Any of these changes can mimic a carcinoma. Therefore, knowledge of the sites of previous breast surgery or biopsy is important when interpreting a mammogram. When patients have undergone breast conservation surgery for carcinoma, it is particularly important to know the location of the “lumpectomy” scar and to compare the current study with a previous baseline study. In general, a new scar should decrease in size over time.
Breast conservation therapy for extensive intraductal carcinoma, as manifested by calcifications (see Fig. 30F.13), should entail a robust management protocol prior to and after treatment (Fig. 30F.14). Multiple localization needles may be required preoperatively to guide excision of malignant calcifications. Radiography of the excised breast tissue specimens should then be performed to determine whether all the calcifications have been removed. If calcifications extend to the margins of a breast specimen, re-excision can be performed prior to wound closure. Before radiotherapy is begun, magnification mammography should also be performed directly over the surgical site to rule out any residual malignant calcifications. If calcifications are still present, local re-excision or a mastectomy may be considered.19
Mammography for Staging
In addition to a careful clinical examination and diagnostic blood work, the pretreatment staging protocol for breast cancer should include chest radiography and bilateral mammography. Mammography prior to biopsy a palpable abnormality is useful for excluding bilateral and/or multi-focal lesions, but not for evaluating ipsilateral axillary nodes: while normal-sized or enlarged axillary nodes can be visualized in the mediolateral oblique (MLO) views, there are no mammographic criteria that will effectively exclude nodal involvement.20 Therefore, histologic assessment of the nodes is still essential to stage invasive carcinomas.
Ultrasonography
The normal breast exhibits some typical features on ultrasonograms (Fig. 30F.16). Sonographic images depict the skin as double parallel lines, below which is a layer consisting of hypoechoic lobules of subcutaneous fat. Fibroglandular tissue in the breast is more echogenic than fat. Hypoechoic fat may also be visible deep to the glandular tissue and adjacent to the pectoral muscle, which lies at the base of the breast overlying the ribs and chest wall.
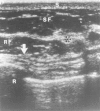
Figure 30F.16
Ultrasonography of the normal breast. The skin (S) lies between two bright echogenic lines on the surface of the breast. The subcutaneous fat (SF) is hypoechoic. The fibroglandular tissue (FG) is relatively echogenic. There is also retroglandular fat (more...)
The role of ultrasonography in breast imaging has evolved over many years. The traditional role of ultrasonography was to differentiate cysts from solid masses.21–23 Characteristic ultrasonographic features of a cyst are its round or oval shape, circumscribed margins, anechoic interior, and enhanced distal echoes posterior to the cyst (Fig. 30F.17). Continuing improvements in ultrasonography equipment have led to the identification of additional features that may help to differentiate benign from malignant solid masses.24 These include the shape, margins, and echogenicity of a breast mass (Figs. 30F.18). It remains to be seen whether these signs are reliable enough to avoid the need for biopsy ofsolid masses that have “benign” ultrasound features. Ultrasonography also plays an important role in guiding interventional procedures, such as needle aspirations, core-needle biopsies, and prebiopsy needle localizations of breast masses or calcifications.25,26

Figure 30F.17
Ultrasonogram of a cyst. The cyst (arrow) is characterized by an oval or round shape, circumscribed margins, absence of internal echoes (anechoic interior), clearly defined posterior wall, and enhancement of distal echoes (*).

Figure 30F.18
Ultrasonogram of carcinoma. This mass (arrows) has features suggesting malignancy: ill-defined margins, height equal to or greater than width, and low-level heterogeneous internal echoes.
Imaging-Guided Needle Biopsy of the Breast
The positive predictive value of breast biopsies that have been prompted by mammographic abnormalities, or the number of cancers that are detected divided by the number of biopsies that have been done, has been reported to be in the range of 10 to 40%,27 with a desirable range being 25 to 40%.4 Concerns about the positive predictive value of imaging studies relate to the cost and morbidity of biopsies that are performed for what later prove to be benign conditions. Some reports suggest that the costs of such biopsies may exceed the costs of screening, with a large proportion of the biopsies that are done yielding benign results.28
Percutaneous needle biopsies offer an alternative to open breast biopsies. They result in lower costs, less morbidity, no breast scarring, and no changes in subsequent mammograms. In Europe, fine-needle aspiration cytology (FNAC) has largely replaced excisional biopsy, thus reducing the overall costs of mammographic screening.29 In the United States, barriers to wider acceptance of FNAC for nonpalpable breast abnormalities have included inadequate numbers of skilled cytopathologists, variability in the reported accuracy of these procedures, high rates of insufficient sampling, and the current medicolegal environment.30 Even when adequate specimens are obtained by this method, a definitive diagnosis is not always possible. For example, infiltrating carcinoma cannot be differentiated from in situ carcinoma by FNAC.
In recent years, core-needle biopsy (CNB) of the breast has been adopted widely as a substitute for excisional biopsy.31 Stereotactic equipment can be used to guide CNB for the evaluation of calcifications and masses that have been identified only on mammography. Stereotactically guided core-needle biopsies are performed with an automated Tru-cut 14G core biopsy gun or a vacuum-assisted directional biopsy device with a 14- or 11-gauge needle. Ultrasound-guided CNB is another method for the biopsy of breast masses.32 Compared with FNAC, CNB can offer a more specific diagnosis, greater accuracy, and fewer inadequate specimens, without the requirement for specially trained cytopathologists.
Imaging–pathology correlation is essential after a needle biopsy has been performed.33 If the results of an imaging study and a CNB are discordant, an excisional biopsy is still indicated. Furthermore, because of the possibility of a sampling error, some benign results on CNB may still require a follow-up excisional biopsy to rule out a coexistent carcinoma. These include atypical ductal hyperplasia and radial scar.34
Other Breast Imaging Modalities
Digital Mammography
With digital mammography, an image of the breast is recorded electronically rather than on film. The acquired digital image can be stored in computer memory and printed out later as a film or displayed and interpreted on a high-definition monitor. The potential advantages of digital mammography include the ability to postprocess images and emphasize areas of interest, to transmit images via teleradiology for remote consultations and/or interpretations, and to employ computer-aided detection and diagnostic techniques.35 Digital mammography may eventually eliminate or reduce some of the other problems that have been associated with conventional film-screen mammography, such as the physical storage space requirements and lost films. The digital images can also be viewed on diagnostic workstations within seconds. They have already proven to be useful for the stereotactic guidance of needle biopsies. Current problems that need to be overcome before digital mammography can be applied more widely include the high cost of the equipment (2 to 3 times the cost of conventional mammography units), a lag in the development of viewing-station technology (which makes routine interpretations of large numbers of images still somewhat cumbersome), and delays in the approval processes by the U.S. Food and Drug Administration (FDA).
Magnetic Resonance Imaging of the Breast
Magnetic resonance imaging (MRI) has been used successfully to evaluate silicone breast implants for suspected intracapsular or extracapsular ruptures.36 However, when initial studies were carried out in the 1980s to determine the potential value of MRI of the breast,37 the technique was felt to be unreliable for detecting or diagnosing breast cancer. Later investigations using intravenous contrast agents and the more advanced instrumentation that is now available have confirmed a high sensitivity for MRI in detecting breast cancer,38–41 but high cost and low specificity have continued to limit the use of MRI as a screening tool. Another problem is that MRI cannot identify malignant calcifications reliably. Nevertheless, there appear to be several potential roles for contrast-enhanced MRI of the breast: (1) determining the size and extent of known invasive cancers; (2) identifying multi-centric lesions; (3) evaluating the ipsilateral breast of a woman who comes initially to attention with axillary metastases; and (4) identifying a recurrent carcinoma in a conservatively treated breast. Multi-center clinical trials are currently underway to determine the exact role(s) of MRI in breast cancer management.
Radionuclide Imaging
Another area of imaging that is under active investigation is breast scanning after the injection of radionuclide-labeled substances which concentrate in areas of high metabolic activity, including some tumors. 99mTc methoxy isobutyl isonitrile (MIBI) breast scintigraphy (“scintimammography”) has been under investigation for several years. Early reports indicated a high sensitivity (over 90%) and specificity (slightly less than 90%) for breast cancer with this technique.42 However, more recent studies have shown a relatively low sensitivity for cancers that are <1 cm in size (39%) and for lesions that had been identified only on screening mammography (56%).43,44 Therefore, the ultimate role of 99mTc MIBI scintigraphy in breast cancer still needs to be determined. The technique ultimately may be helpful for avoiding unnecessary biopsies of palpable but benign masses that are >1 cm in size, and which have equivocal mammographic or ultrasonographic features.
Focal radionuclide uptake in breast cancers has also been identified with positron emission tomography (PET) after the injection of fluorine-18 2-deoxy-2-fluoro-D-glucose (FDG).45 This labeled compound may also accumulate in abnormal axillary nodes. PET scanning requires additional research to determine its sensitivity, specificity, and cost-effectiveness in breast cancer.
Nuclear scans with 99mTc sulfur colloid have shown early promise in clinical practice for the identification of the so-called “sentinel nodes” in the axilla prior to surgery.46 This labeled compound is injected interstitially by the surgeon near a biopsy-proven breast cancer. The injected material may then track along potential routes of tumor spread in the lymphatic vessels. At subsequent surgery, an axillary sentinel node which drains the primary cancer site and which contains the radioactive tracer is then identified using a radionuclide probe, and the node is removed and evaluated histologically. If the sentinel node is negative for tumor, axillary node dissection and its potential complications may be avoided. Further studies will be needed to determine with greater accuracy what the false-negative rate is for sentinel node biopsies in oncologic practice.

Figure 30F.15
Protocol for managing extensive suspicious calcifications.
References
- 1.
- McLelland R, Hendrick R E, Zinninger M D, Wilcox P A. The American College of Radiology mammography accreditation program. AJR Am J Roentgenol. 1991;157:473–479. [PubMed: 1872231]
- 2.
- Mammography Quality Standards Act of 1992. Public Law 102539.
- 3.
- American College of Radiology (ACR). Breast imaging reporting and data system (BI-RADS), 3rd ed. Reston, VA: ACR; 1998. [PubMed: 17164078]
- 4.
- Bassett, LW, Hendrick RE, Bassford TL, et al. Quality determinants of mammography. Clinical practice guideline, N. 13. AHCPR Publication No. 95-0632. Rockville, MD: Agency for Health Care Policy and Research, Public Health Service, U.S. Department of Health and Human Services; October 1994.
- 5.
- American College of Radiology (ACR). Standards for the performance of screening mammography [Adopted by the ACR Council 1990, Revised 1994]. In: ACR eigest of official actions. Reston, VA: ACR; 1994.
- 6.
- Tabár L, Fagerberg C J, Gad A. et al. Reduction in mortality from breast cancer after mass screening with mammography. Randomized trial from the Breast Cancer Screening Working Group of the Swedish National Board of Health and Welfare. Lancet. 1985;1:829–832. [PubMed: 2858707]
- 7.
- National Institutes of Health Consensus Development Panel. Breast cancer screening for women ages 40-49, January 21-23, 1997. J Natl Cancer Inst. 1997;89:1015–1026. [PubMed: 9230883]
- 8.
- Smart C R, Hendrick R E, Rutledge J H, Smith R A. Benefit of mammography screening in women ages 40 to 49 years. Current evidence from randomized controlled trials. Cancer. 1995;75:1619–1626. [PubMed: 8826919]
- 9.
- Alexander F, Anderson T J, Brown H K. et al. 14 years of follow-up from the Edinburgh randomized trial of breast-cancer screening. Lancet. 1999;353:1903–1908. [PubMed: 10371567]
- 10.
- Moss S M, Coleman D A, Chamberlain T J. et al. 16-year mortality from breast cancer in the UK Trial of Early Detection of Breast Cancer. Lancet. 1999;353:1909–1914. [PubMed: 10371568]
- 11.
- American College of Radiology (ACR): Standards for the performance of diagnostic mammography and problem-solving breast evaluation [Adopted by the ACR Council 1994]. In: ACR digest of official actions. Reston, VA: ACR; 1994.
- 12.
- FDA/CDRH. Writing the mammography report. Mammography Matters 1998;5:10 .
- 13.
- Sickles E A. Management of probably benign breast lesions. Radiol Clin North Am. 1995;33:1123–1130. [PubMed: 7480660]
- 14.
- Jackson V P, Dines K A, Bassett L W. et al. Diagnostic importance of radiographic density of noncalcified breast masses: analysis of 91 lesions. AJR Am J Roentgenol. 1991;157:25–28. [PubMed: 1646563]
- 15.
- Egan R L, McSweeney M B, Sewell C W. Intramammary calcifications without an associated mass in benign and malignant diseases. Radiology. 1980;137:1–7. [PubMed: 7422830]
- 16.
- Stomper P C, Connelly J L, Meyer J E, Harris J R. Clinically occult ductal carcinoma in situ detected with mammography: analysis of 100 cases with radiologic-pathologic correlation. Radiology. 1989;172:235–241. [PubMed: 2544922]
- 17.
- Sickles E A. Mammographic features of 300 consecutive nonpalpable breast cancers. AJR Am J Roentgenol. 1986;146:661–663. [PubMed: 3485337]
- 18.
- Mendelson E B. Evaluation of the postoperative breast. Radiol Clin North Am. 1992;30:107–138. [PubMed: 1732922]
- 19.
- Stomper P C, Margolin F R. Ductal carcinoma in situ: the mammographer’s perspective. AJR Am J Roentgenol. 1994;162:585–591. [PubMed: 8109501]
- 20.
- Kalisher L, Chu A M, Peyster R G. Clinicopathological correlations of xeroradiography in determining involvement of metastatic axillary nodes in female breast cancer. Radiology. 1976;121:333–335. [PubMed: 981609]
- 21.
- Sickles E A, Filly R A, Callen P W. Breast cancer detection with ultrasonography and mammography: comparison using state-of-the-art equipment. AJR Am J Roentgenol. 1983;140:843–845. [PubMed: 6601422]
- 22.
- Kopans D B, Meyer J E, Lindfords K K. Whole-breast US imaging: 4 year follow-up. Radiology. 1985;157:505–507. [PubMed: 3901112]
- 23.
- Bassett L W, Kimme-Smith C, Sutherland L K. et al. Automated and hand-held breast US: effect on patient management. Radiology. 1987;165:103–108. [PubMed: 3306779]
- 24.
- Stavros A T, Thickman D, Rapp C L. et al. Solid breast nodules: use of sonography to distinguish between benign and malignant lesions. Radiology. 1995;196:123–134. [PubMed: 7784555]
- 25.
- Fornage B D, Coan J D, David C L. Ultrasound-guided needle biopsy of the breast and other interventional procedures. Radiol Clin North Am. 1992;30:167–185. [PubMed: 1732925]
- 26.
- Jackson V P. The current role of ultrasonography in breast imaging. Radiol Clin North Am. 1995;33:1161–1170. [PubMed: 7480663]
- 27.
- Bassett L W, Liu T -H, Giuliano A E, Gold R H. The prevalence of carcinoma in palpable vs. impalpable mammographically detected lesions. AJR Am J Roentgenol. 1991;157:21–24. [PubMed: 1646562]
- 28.
- Cyrlak, D Induced costs of low-cost screening mammography. Radiology. 1988;168:661–663. [PubMed: 3406395]
- 29.
- Azevado E, Svane G, Aver G. Stereotactic fine needle biopsy in 2594 mammographically-detected nonpalpable lesions. Lancet. 1989;1:1033–1036. [PubMed: 2565996]
- 30.
- Jackson V P, Bassett L W. Stereotactic fine-needle aspiration biopsy for nonpalpable breast lesions. AJR Am J Roentgenol. 1990;154:1196–1197. [PubMed: 2110727]
- 31.
- Parker S H, Lovin J D, Jobe W E. et al. Nonpalpable breast lesions: stereotactic automated large-core biopsies. Radiology. 1991;180:403–407. [PubMed: 1648757]
- 32.
- Parker S H, Jobe W E, Dennis M A. et al. US-guided automated large core breast biopsy. Radiology. 1993;187:507–511. [PubMed: 8475299]
- 33.
- Bassett L W, Winchester D P, Caplan R B. et al. Stereotactic breast CNB: report of the Joint Task Force of the ACR, ACS, COAP. CA Cancer J Clin. 1997;47:171–190. [PubMed: 9152175]
- 34.
- Jackman, RJ, Nowels K W, Shepard M J, etal Stereotaxic large-core needle biopsy of 450 nonpalpable breast lesions with surgical correlation in lesions with cancer or atypical hyperplasia. Radiology. 1994;193:91–95. [PubMed: 8090927]
- 35.
- Shtern F. Digital mammography and related technologies: a perspective from the National Cancer Institute. Radiology. 1992;183:629–630. [PubMed: 1584908]
- 36.
- Gorczyca D P, Sinha S, Ahn C Y. et al. Silicone breast implants in vivo: MR imaging. Radiology. 1992;185:407–410. [PubMed: 1410346]
- 37.
- El Yousef S J, O’Connell D M, Duchesneau R H. et al. Benign and malignant breast disease: magnetic resonance and radiofrequency pulse sequences. AJR Am J Roentgenol. 1985;145:1–8. [PubMed: 2988320]
- 38.
- Harms S E, Flamig D P, Evans W P. et al. MR imaging of the breast: current status and future potential. AJR Am J Roentgenol. 1994;163:1039–1047. [PubMed: 7976873]
- 39.
- Heywang S H, Wolf A, Pruss E. et al. MR imaging of the breast with Gd-DTPA: use and limitations. Radiology. 1989;171:95–103. [PubMed: 2648479]
- 40.
- Heywang-Kobrunner S H, Schlegel A. et al. Contrast-enhanced MRI of the breast after limited surgery and radiation therapy. J Comput Assist Tomogr. 1993;17:891–900. [PubMed: 8227574]
- 41.
- Orel S G, Schnall M D, LiVolsi V A, Troupin R H. Suspicious breast lesions: MR imaging with radiographic-pathologic correlation. Radiology. 1994;190:485–493. [PubMed: 8284404]
- 42.
- Khalkhali I, Mena I, Jouanne E. et al. Prone scintimammography in patients with suspicion of carcinoma of the breast. J Am Coll Surg. 1994;178:491–497. [PubMed: 8167887]
- 43.
- Tolmos J, Cutrone J A, Wang B. et al. Scintimammographic analysis of nonpalpable breast lesions previously identified by conventional mammography. J Natl Cancer Inst. 1998;90:846–849. [PubMed: 9625173]
- 44.
- Prats E, Carril J, Herranz R. et al. A Spanish multicenter scintigraphic study of the breast using Tc99m MIBI. Report of results. Revista Espanola de Medicina Nuclear. 1998;17:338–350. [PubMed: 9812008]
- 45.
- Adler L P, Crowe J P, Al-Kaisi N K, Sunshine J L. Evaluation of breast masses and axillary lymph nodes with (F-18) 2-deoxy-2-fluoro-D-glucose PET. Radiology. 1993;187:743–750. [PubMed: 8497624]
- 46.
- Winchester D J, Sener S F, Winchester D P. et al. Sentinel lymphadenectomy for breast cancer: experience with 180 consecutive patients: efficacy of filtered technetium 99m sulphur colloid with overnight migration time. J Am Coll Surg. 1999;188:597–603. [PubMed: 10359352]
- Imaging the Breast - Holland-Frei Cancer MedicineImaging the Breast - Holland-Frei Cancer Medicine
- ni79c08.s1 NCI_CGAP_Pr12 Homo sapiens cDNA clone IMAGE:983054, mRNA sequenceni79c08.s1 NCI_CGAP_Pr12 Homo sapiens cDNA clone IMAGE:983054, mRNA sequencegi|2287962|gnl|dbEST|1187576|gb|AA5 .1|Nucleotide
- CLXN [Nipponia nippon]CLXN [Nipponia nippon]Gene ID:104014048Gene
- PREX1 [Falco peregrinus]PREX1 [Falco peregrinus]Gene ID:101916586Gene
Your browsing activity is empty.
Activity recording is turned off.
See more...

