“The attempt and not the deed, Confounds us.”
Macbeth, William Shakespeare.
Functions of hormones and their regulation
The word hormone is derived from the Greek hormao meaning ‘I excite or arouse’. Hormones communicate this effect by their unique chemical structures recognized by specific receptors on their target cells, by their patterns of secretion and their concentrations in the general or localized circulation. The major hormones discussed in this book are listed in Box 1.1.
Box 1.1
Classification by structure of the major human hormones*.
Their functions can be broadly grouped into several categories: reproduction and sexual differentiation; development and growth; maintenance of the internal environment; and regulation of metabolism and nutrient supply. A single hormone may affect more than one of these functions and each function may be controlled by several hormones. For example, thyroid hormone is essential in development as well as many aspects of homeostasis and metabolism, whilst glucocorticoids, such as cortisol, are important both in growth and nutrient supply and are also modulators of immune function. The roles several hormones play in one function is exemplified by the control of blood glucose which involves the pancreatic peptide insulin and its counter regulatory hormone, glucagon, as well as cortisol, growth hormone and epinephrine. Hormones act in concert and thus, an abnormality in a controlled variable, such as blood glucose concentration may result from defects in the control of any one of several hormones.
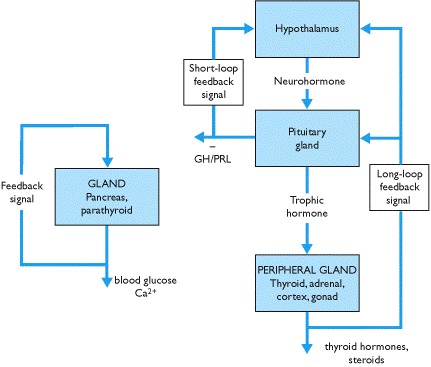
The secretion of hormones is subject to negative feedback control, and there are several ways by which this is achieved (Box 1.2). Feedback loops may involve the hypothalamo-pituitary axis that detects changes in the concentration of hormones secreted by peripheral endocrine glands or a single gland may both sense and respond to changes in a controlled variable. The integration of feedback loops involving several hormones may be complex. Disturbances in feedback loops are clinically important and their significance in diagnosis is pivotal.
Chemical signalling - endocrine, paracrine, autocrine and intracrine mechanisms
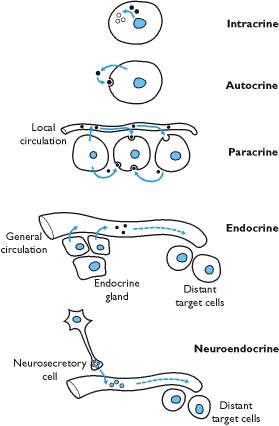
A chemical released by a specialized group of cells into the circulation and acting on a distant target tissue defines the ‘classical’ endocrine and neuroendocrine signalling mechanism. A paracrine mechanism is defined as chemical communication between neighboring cells within a tissue or organ (Box 1.3). Autocrine signals are those in which a chemical acts on the same cell whilst an intracrine signal is generated by a chemical acting within the same cell.
Box 1.3
Different mechanisms of cell signaling.
Chemical classification of hormones and their synthesis
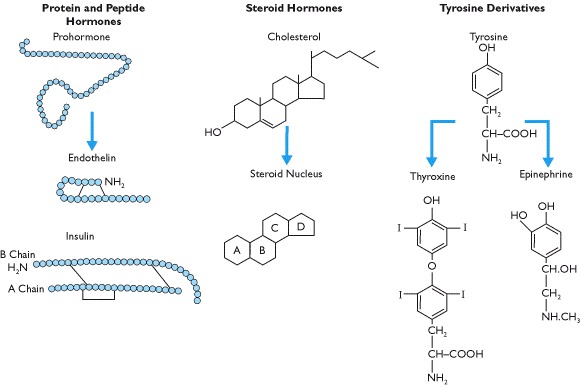
Hormones are derived from amino acids, from cholesterol (Box 1.4) or from phospholipids (Box 8.8). By far the most numerous are the protein or peptide hormones, ranging in size from just three to over 200 amino acids. Some hormones, such as insulin, are made up of two sub-units joined by disulfide bonds between two cysteine molecules whilst the glycoprotein hormones of the anterior pituitary gland are not only made up of two protein sub-units but also have complex sugar moieties attached.
Box 1.4
Chemical structures of the three major classes of human hormones. Other hormones include those derived from tryptophan (serotonin and melatonin, Boxes 7.33 and 8.12) and those derived from fatty acids (eicosanoids, Box 8.8).
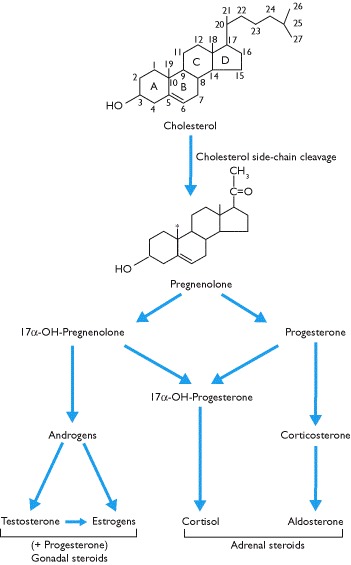
The steroid hormones, which include vitamin D and those secreted by the adrenal cortex and gonads, are derived from cholesterol. All adrenal and gonadal steroids have the same basic ring structure (Box 1.4) and despite superficial 2D structural similarity, the side chains and spatial orientation generate specificity.
The third group of hormones are those derived either from tyrosine or from tryptophan. A single tyrosine molecule yields the catecholamines, epinephrine and norepinephrine, the latter being both a neurotransmitter and a hormone. In the endo-crine system, these hormones are secreted by the adrenal medulla and are rapidly broken down once released into the circulation. The thyroid hormones are formed by the conjugation of two tyrosine molecules and resemble steroid hormones in binding to serum proteins and in the mechanism of action. Tryptophan is the precursor of serotonin (5-hydroxytryptamine) and melatonin synthesis. Finally, hormones derived from lipids and phospholipids include the major classes of eicosanoids including prostaglandins, prostacyclins, thromboxanes and leukotrienes (Box 8.8).
Hormone synthesis
Most protein and peptide hormones require the transcription of a single gene though the α and β subunits of the glycoprotein hormones (TSH, LH and FSH) are derived from different genes. The initial RNA undergoes modifications such that the introns are excised from the molecule and there are modifications to the 3′ and 5′ ends of the messenger (m) RNA. The mature mRNA, containing only the exons, is then used as the template for the assembly of amino acids, through transfer (t) RNA, on the rough endoplasmic reticulum (Box 1.5).
Box 1.5
Synthesis of protein and peptide hormones.  Transcription of the DNA sequence into RNA
Transcription of the DNA sequence into RNA  Excision of sequences (introns) from the initial DNA transcript and modifications of the 3′ and 5′ terminals
Excision of sequences (introns) from the initial DNA transcript and modifications of the 3′ and 5′ terminals
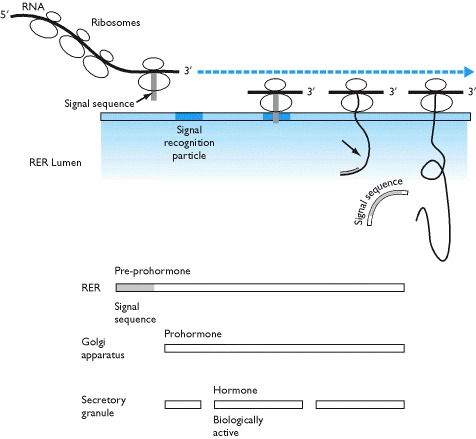
Since protein and peptide hormones are stored in, and secreted from, secretory granules it is necessary for their synthesis and packaging to take place within membrane-bound structures of the cell. For this reason, the first amino acids that are translated from the mRNA template form a signal sequence. This signal sequence finds a docking protein, the signal recognition particle, on the rough endoplasmic reticulum so that as protein synthesis continues the assembled amino acids move into the membranes of the rough endoplasmic reticulum. The signal sequence is rapidly cleaved from the growing protein pre-prohormone and eventually a large pro-hormone is left within the membrane-bound rough endoplasmic reticulum (Box 1.6).
Inside the endoplasmic reticulum, the protein moves into the Golgi apparatus by fission and fusion of protein containing vesicles in which the large pro-hormone is cleaved by peptidases into the biologically active hormone and one or more fragments of the original molecule. Fragments are frequently co-secreted with the active hormone. Secretory granules are formed from budding of the Golgi apparatus and hormones and their associated fragments are stored in these prior to their release.
Thus, protein and peptide hormone synthesis requires transcription of gene, post-transcriptional modification by exision of the introns, translation of the mRNA and post-translational modifications of the original amino acid sequence. As a result, more than one pro-hormone may be derived from a single gene (e.g. calcitonin and calcitonin-gene related peptide, Box 5.38). Furthermore, post-translational processing of a pro-hormone may result in the formation of different biologically active peptide fragments (e.g. pro-opiomelanocortin). These processes are typically tissue-specific.
In contrast, the synthesis of steroid hormones (Box 1.7) that occurs in the mitochondria and rough endoplasmic reticulum does not require immediate gene expression. It requires the presence of specific enzymes that convert cholesterol into the appropriate steroid (Box 4.5). Different enzymes are expressed in different steroid secreting cells and their expression is controlled by trophic hormones and/or other factors. Cholesterol for steroid synthesis and the amino acid, tyrosine, for thyroid hormone synthesis are ubiquitous, but synthesis of thyroid hormones (Box 1.4) requires both specific enzymes (containing selenium) and iodine, both of which are trace elements (Box 3.5).
The amine hormones such as the catecho-lamines, melatonin and serotonin are formed by side-chain modifications of either a single tyrosine or tryptophan molecule while the eicosanoid family of hormones are formed from lipids (Box 8.8).
Transport of hormones in the circulation and their half-lives
Steroid and thyroid hormones are less soluble in aqueous solution than protein and peptide hormones and over 90% circulate in blood as complexes bound to specific plasma globulins or albumin. Bound and free hormones are in equilibrium (Box 8.28). More recently, binding proteins for several protein and peptide hormones (e.g. CRH, GH) as well as growth factors (e.g. IGF) have also been identified.
It is generally accepted that it is the unbound or free hormone that is biologically active and that hormone binding delays metabolism and provides a circulating reservoir of hormones. More recently, it has been suggested that the specific binding globulins are not just passive transporters but may interact with membrane receptors and that hormone binding to the globulins initiates a signal transduction pathway.
Most binding proteins are synthesized in the liver and alterations in the serum concentrations of these proteins alter total serum concentrations of a hormone but may have much less effect on the concentrations of free hormone. As a result, situations may arise in which assays of total hormone concentrations do not reflect changes in free hormone concentrations. Measurement of biologically relevant free hormone concentrations (Box 3.30), however, is generally more difficult than measuring total hormone concentrations.
The rates of metabolism of hormones in the circulation vary but generally speaking the half life (t½) of catecholamines from the adrenal medulla is in the order of seconds, minutes for protein and peptide hormones and hours for steroid and thyroid hormones.
Hormone receptors - cell surface
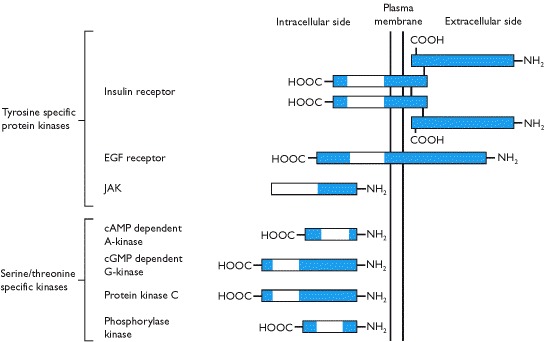
Proteins and peptides are water soluble and, hence, do not diffuse across hydrophobic lipid cell membranes. Thus, parts of their receptors lie extracellularly (where hormone-receptor interactions occur) and they couple with intracellular signal transducing molecules by traversing the cell membrane. The majority of classical protein and peptide hormone receptors are the G-protein linked receptors (Box 1.8) and these may either have a relatively short extracellular amino terminal domain (e.g. epinephrine, GnRH) or a much longer extracellular domain (e.g. TSH, LH, PTH). Extracellular hormone-receptor interactions induce dissociation of the associated intracellular trimeric G protein (Box 1.10). This may either open ion channels in the membrane or activate a membrane bound enzyme that stimulates (or inhibits) the production of a second messenger such as cyclic AMP or diacylglycerol and inositol trisphosphate (Box 1.10). These second messengers then activate serine/threonine kinases (Box 1.9) or phosphatases.
Activation of these protein kinases may have three consequences. It can lead to alterations in specific cytosolic enzyme activity, activation of nuclear transcription factors (Boxes 1.10 and 1.13) or initiation of a cascade of subsequent phosphorylations on the serine or threonine residues of protein kinases that can also regulate transcription (Box 1.10).
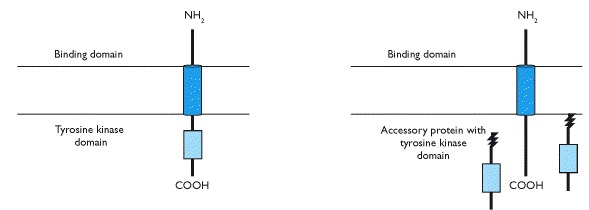
The second most common type of cell surface receptors is that used in the signalling of insulin, growth hormone, prolactin, most growth factors and cytokines. This type is a transmembrane receptor with either inherent protein tyrosine kinase activity on the intracellular domain (e.g. insulin and growth factor receptors) or associated intracellular molecules (Box 1.9) that have this activity (e.g. receptors for growth hormone, prolactin and cytokines). Binding of the hormone or growth factor to the extracellular domain results in receptor dimerization with an adjacent receptor initiating either autophosphosphorylation (Box 1.10) or phosphorylation of an associated enzyme. Subsequently, there are similar signal transduction events to those described above that involve both cytoplasmic and nuclear events.
Signal transduction pathways for cell surface receptors
These are complex processes and unfortunately dogged by terminology that is confusing and not always logical - a legacy from the periodic discovery of intracellular factors that were subsequently assembled into a relatively complete sequence of signal tranduction processes. It is not in the scope of this book to pursue all the molecular events but a broad outline is pertinent to understanding hormone action and genetic mutations that can cause endocrine disorders.
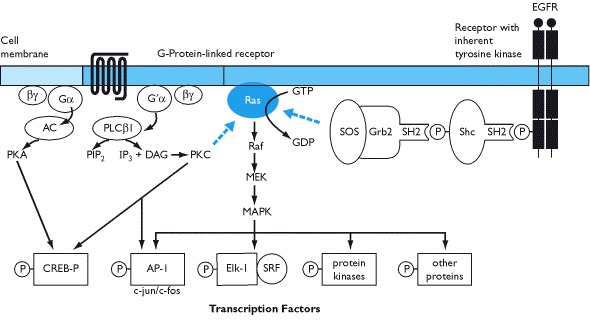
Receptors that have inherent tyrosine kinase activity bind molecules that have a specific SH2 domain (src homology domain). In turn, another accessory protein may be activated such as SOS (son-of-sevenless). This can activate a monomeric G-protein known as Ras that essentially acts as a signal transduction switch. Its activation can lead to phosphorylation of Raf, MEK and eventually to mitogen activated protein kinase (MAPK) which can initiate transcription (termed the MEK-MAPK pathway).
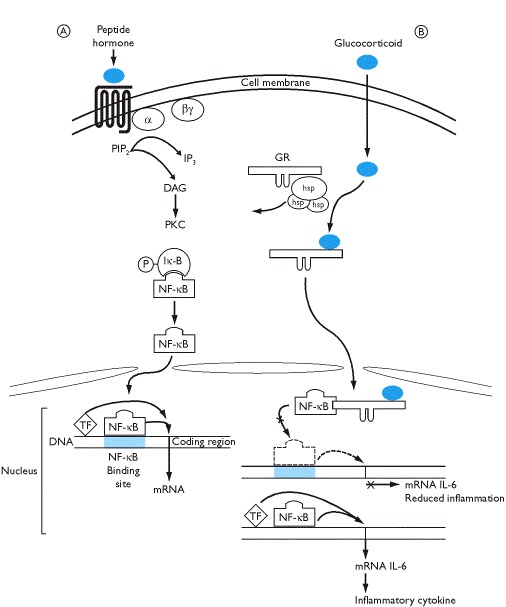
Receptors for GH, prolactin, erythropoietin, insulin and a variety of cytokines and growth factors do not have inherent protein kinase activity but are associated with a protein that has tyrosine kinase activity. One of these proteins, known as JAK (just another kinase) may activate downstream effectors that include the STAT proteins - the JAK-STAT pathway. Binding of insulin to its receptor induces phosphorylation of insulin receptor substrate proteins (IRS) which activates further signal transduction pathways including activation of nuclear transcription factor κB (NF-κB). In essence, there is a cascade of protein phosphorylations that ultimately end in the nucleus to induce transcription.
The transcription factor targets for kinases that are activated by protein and peptide hormones include c-jun and c-fos which make up the heterodimeric AP-1 complex, the serum response factor (often targeted by the MAP kinase dependent pathway), and nuclear CREB-P (cAMP response element binding protein) which is phosphorylated by protein kinase A and enhances transcriptional activity of closely positioned promoters (Box 1.10).
Hormone receptors - intracellular
Steroid and thyroid hormones are lipophilic and readily diffuse across cell membranes. Their receptors are typically intracellular and are classified according to their cellular location, their dimerization and the sequences of DNA to which they bind. There is a large family of steroid receptors, all of which are transcription factors. They bind to DNA and with other transcription factors initiate RNA synthesis. Whilst receptors for the major steroid hormones have been identified (Box 1.11) other structurally similar molecules have been identified though their ligands have not. These have been termed orphan receptors.
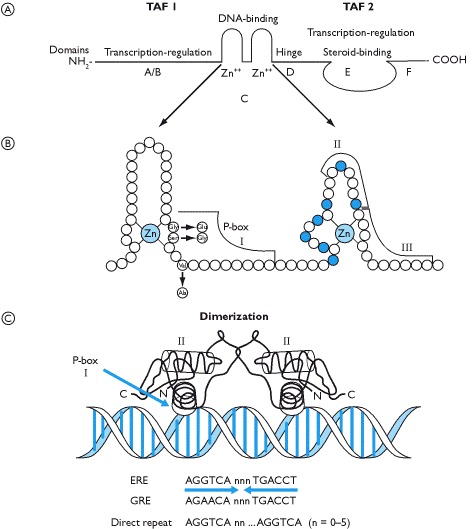
The characteristic single polypeptide chain is structurally and functionally divided into six domains. At the amino terminus are the A/B domains that are variable both in sequence and length. The C domain, also called the DNA binding domain (DBD), is a highly conserved sequence across all steroid receptors and is characterized by possessing two zinc fingers which readily slot into the helix of the DNA molecule. The D domain is thought to represent a hinge region in the molecule whilst E represents the ligand binding domain and F a variable region in the carboxyl terminus. This end of the molecule is also the region where the heat shock proteins (hsps) are bound and where dimerization occurs.
Receptors that exist predominantly in the cytoplasm are classified as Type 1 receptors and these include the glucocorticoid, mineralocorticoid, androgen and progesterone receptors. They are bound to heat shock proteins (e.g. hsp 90, hsp70 and hsp 56). Upon steroid binding the hsp complex is released and the receptor forms a dimer with another identical receptor (Boxes 3.9 and 5.7). The homodimer translocates to the nucleus where it binds to a specific base sequence on the DNA. The estrogen receptor is also associated with hsps and whilst this receptor shuttles between the nucleus and cytoplasm, most are confined to the nuclear compartment. Type 2 receptors are typically located in the nucleus and may be bound to DNA. They characteristically form heterodimers (e.g. thyroid hormone receptor and retinoid X receptor) or may initiate transcription as monomers upon ligand binding.
The specific amino acid sequence of the zinc fingers in the DNA binding domain is important for determining the bases in the DNA helix to which the receptor binds and, thus, the specificity of the transcriptional activity of the receptor. This is determined through what is called the recognition helix that lies at the end of the first zinc finger and part of the amino acid sequence between the two zinc fingers. Amino acids in the second zinc finger make specific contacts with the phosphate backbone of the DNA.
Type 1 receptors recognize a base sequence AGAACA whilst Type 2 receptors and the estrogen receptors recognize a base sequence AGGTCA. These are known as hormone response elements on the DNA and can be further defined as a glucocorticoid response element (GRE) or estrogen response element (ERE), respectively (Box 1.12). These, however, are half-site specificities of hormone receptors; the other half-site forms an inverted palindrome, as recognized by Type 1 and the estrogen receptors, or by a direct repeat of bases with variable number of bases between the half-site specificity. These are generally recognized by thyroid hormones, vitamin D, and retinoid receptors. The other way in which steroid hormones can alter transcription is not via interaction with a GRE or ERE on the DNA but by binding to and activating/repressing other transcription factors that recognize a particular site on DNA (Box 1.13).
Many steroids and thyroid hormones can stimulate rapid responses in target cells that are clearly non-genomic and may be explained by interaction with cell surface receptors. Such receptors may initiate the opening of ion channels or activate classical second messenger systems. The difficulty of isolating such receptors has hampered their investigation but it is clear that steroids exert membrane effects.
Hormones and gene transcription
The receptors of all classes of hormones may regulate gene transcription either by activating transcription factors or by acting as transcription factors in their own right. Transcription is not, however, a simple process of a factor or receptor binding to DNA and activating RNA polymerase at an initiation site. It also requires a complex of enzymes, referred to as the holo-enzyme complex, before transcription is initiated down-stream of the factors and promoters. Thus, for example, a steroid receptor may have transcriptional activity but only when promoters (or repressors) bind with a particular part of the molecule. Sometimes these factors may act independently of ligand binding, others require ligand binding before they can be activated.
Hormone receptor regulation
Receptor regulation is an important part of endocrine function and this occurs through up or down-regulation of the number of receptors and by desensitization of the receptors. This occurs by increasing or decreasing receptor synthesis, by internalization of membrane receptors after ligand binding, or by uncoupling of the receptor from its signal transduction pathway (desensitization). The latter usually involves phosphorylation of the receptor. Some hormones may regulate their own receptors (homologous regulation) such as GnRH on the pituitary gonadotrophs whilst other receptors are regulated by other hormones (heterologous regulation) e.g. estrogen regulating oxytocin receptors.
Interaction between hormones and their receptors depends on the number of receptors, the concentration of circulating hormone and the affinity of the hormone for the receptor. The latter is defined as the concentration of a hormone at which half the total number of receptors is occupied (Box 1.14) and the higher the affinity the lower the concentration of hormone required. Generally speaking the affinity of hormone receptors does not change and thus the biological response depends on the number of receptors and the concentration of hormone.
Usually less than 5% of hormone receptors are occupied at any one time and maximum biological responses are achieved when only a fraction of the total number of receptors are occupied. Thus, it might be questioned why a small reduction in receptor number or a change in hormone concentration should make much difference to the overall biological response. This is governed by the law of mass action. If receptor numbers are reduced then the chances of a hormone binding to a receptor are decreased. Thus, a higher concentration of hormone is required to achieve a similar receptor occupancy. A similar argument may be applied when hormone concentrations are reduced. Together these two parameters are important in determining the target cell's response to a hormone despite low occupancy of receptors (Box 1.14).
Neuroendocrine interactions
All endocrine glands are innervated by autonomic nerves and these may either directly control their endocrine function and/or regulate blood flow (and hence function) within the gland. Hormones, in turn, may affect central nervous system functions such as mood, anxiety and behavior.
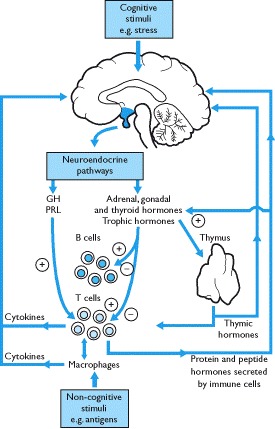
Neurosecretory cells (Box 1.3) may directly convert a neural signal into a hormonal signal. In other words they act as transducers converting electrical energy into chemical energy. Thus, activation of neurosecretory cells leads to secretion of a hormone into the circulation. These neurosecretory cells include: those that secrete hypothalamic releasing and inhibiting hormones controlling TSH, ACTH, LH and FSH release from the anterior pituitary gland; the hypothalamic neurons the axon terminals of which secrete oxytocin and vasopressin from the posterior pituitary gland; the chromaffin cells of the adrenal medulla (embryologically modified neurons) that secrete epinephrine and norepinephrine into the general circulation. The significance of these neurosecretory cells is that they allow the endocrine system to integrate and respond to changes in the external environment. Thus, for example, the CRH-ACTH-cortisol axis can be activated by stress generated from external cues as is oxytocin secretion by a suckling baby. The recent discovery that the nervous system itself can synthesize neurosteroids has added new dimensions to the concept of neuroendocrine integration. With regard to endocrine function per se, the significance of these discoveries remains to be elucidated.
Hormones and the immune system
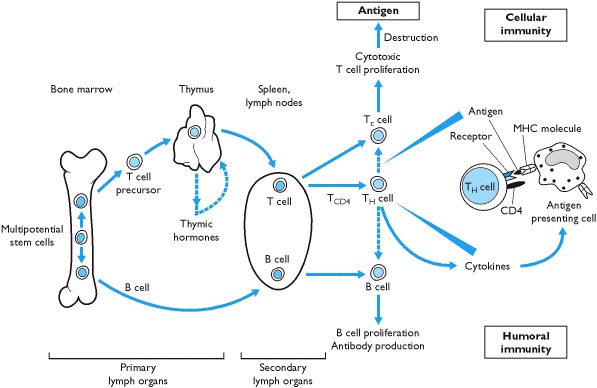
Since the discovery that surgical ablation of the pituitary gland caused atrophy of the thymus gland, experimental evidence from animal studies has indicated that there is a complex network of interactions between these two systems. The thymus gland, which is essential for orchestrating immune responses, has two regions - one in which T cell precursors from bone marrow mature and the other that secretes thymic hormones (Box 1.15). The physiological role of thymic hormones is not clear but they are postulated to promote T cell maturation (Box 1.15).
The immune system can be considered a sensory system as it responds to stimuli such as bacteria, viruses, tumors and other antigens. When stimulated, cell-mediated or humoral immune responses are activated and this information is sent to the hypothalamus (via the circumventricular organs) by cytokines and peptide hormones secreted from cells of the immune system. In addition neural and non-neural cells in the brain also synthesize cytokines. The neuroendocrine system responds to these signals which, in many ways, may be considered a stress response.
Both cytokines and thymic hormones may influence the release of hypothalamic neurohormones and, hence, pituitary secretions; overall, immune activation is stimulatory to the release of pituitary hormones (Box 1.16). There is also evidence that cytokines act directly on endocrine glands such as the pituitary, thyroid, pancreas, adrenals and gonads and alter their secretions. Pituitary hormones and the secretions of their peripheral target endocrine glands may modulate immune function. Thus, for example, ACTH/glucocorticoids are immunosuppressants as are progesterone and testosterone whilst the action of estrogens is both stimulatory and inhibitory. GH and prolactin may also potentiate immune responses through a variety of effects and can stimulate growth and activity of the thymus gland (Box 1.16).
Box 1.16
Overview of interactions between the endocrine and immune systems and feedback effects of immunomodulatory peptides and hormones on the hypothalamo-pituitary axis and on peripheral endocrine glands.
Thymic hormones and immunomodulators may alter endocrine function and hormones may modulate immune responses. Cross-talk between the systems also occurs because immune cells release peptides that are identical to those produced by the hypothalamus and pituitary gland. They are released in response to stimulation from both antigens and hypothalamic hormones. For example, immune cells release ACTH that can stimulate the release of glucocorticoids and thus, to some extent over-ride the negative feedback effects of stress-induced increases in cortisol secretion on the hypothalamo-pituitary axis. Furthermore, interleukin-1, released by macrophages, stimulates the secretion of hypothalamic CRH release. Thus, high cortisol secretion rates are maintained. It should be noted, however, that recent studies on knock-out mice suggest that many hormones are not essential immunomodulators but act as stress-modulating hormones in most cells, including those of the immune system.
Autoimmunity
The primary role of the immune system is defensive and it is required to distinguish normal self- components from those of foreign invaders or pathogens. Tolerization is a complex process and loss of tolerance may lead to inappropriate activation of the immune system causing tissue destruction and autoimmune disease. Theoretically all tissues in the body should be equally frequent targets for autoimmune destruction but endocrine tissues appear more susceptible.
The factors that predispose to the development of autommunity are not completely understood but there is clearly a genetic association and the most clearly established is that of the genotype of the major histocompatability complex (MHC). This is a set of linked genes coding for glycoproteins through which monocytes/dendritic cells and B lymphocytes present antigens to receptor molecules on T cells (Box 1.15). In humans, the MHC is referred to as human leukocyte antigen (HLA) and the HLA region, located on the short arm of chromosome 6, contains at least 50 genes extending over 4 million base pairs.
Whilst genetic linkages in the HLA complex and autoimmune disease have been established, how they contribute to the pathogenesis of autoimmun-ity remains unknown. The strongest linkage has been with certain HLA-DQ β-chains (specifically the presence of aspartic acid at position 57). Since this is involved in the peptide binding cleft of the molecule it has been thought that it involves an error in antigen presentation. However, the exact mechanism remains uncertain.
The same can be said of the role of T and B cells in the pathogenesis of autoimmunity. T cells maturing in the thymus gland can certainly be deleted to prevent autoimmune disease (if they react too strongly to the MHC complex) and the same may be true of B cells. Whatever the mechanism of autoimmunity, there is no simple explanation of the sequence of events. Human autoimmune diseases only become clinically evident after considerable tissue damage or disruption has occurred and this makes it difficult to establish the course of pathogenesis and particularly its initiation. There are clearly familial traits of inheritance that can predispose to autoimmune disease, but the fact that only 30–50% of identical twins develop the same autoimmune disease suggests that other factors are involved, including those of the environment.
Hormones, growth promotion and malignancy
Many hormones and growth factors promote growth in fetal and post-natal life and, thus, it has been suggested that they may also promote tumorigenesis. Whilst it is known that many growth factors such as the insulin-like growth factors induce proliferation in both normal and malignant cells, their precise role in the development of malignancy is unknown. There is, however, substantial evidence that human cancers do not result from a single genetic event but from stepwise genetic changes that result in the activation of proto-oncogenes and the inactivation of so-called antioncogenes or tumor suppressor genes.
Proto-oncogenes are cellular (c) genes that are thought to have been captured and recombined into transforming (viral) oncogenes (v) by certain retroviruses. Such genes code for growth factors or their receptors that are homologous or identical to the proteins coded by the normal cellular proto-oncogenes. For example c-erb B-1 codes for the EGF receptor whilst its transformed oncogene v-erb B-1 codes for a truncated form of the EGF receptor. Similarly c-src which codes for the IGF-1 receptor, can be tranformed into v-src that codes for a modified protein kinase. These receptors may be constitutively active, not requiring the presence of a ligand. Similarly, genes for the growth factors, e.g. c-sis and c-int that code for platelet derived growth factor beta chain and basic fibroblast factor, respectively, can be transformed and inappropriately expressed in tumor cells. The importance of growth factors and their receptors in the phenotype of many malignancies makes them potential therapeutic targets.
Sex steroids are well known to cause or promote tumor growth in target tissues such as breast, endometrium and prostate. Thyroid, testicular and ovarian tumors, occurring in glands controlled by the trophic hormones TSH, LH and FSH may also be putatively included in the list of endocrine-dependent cancers. Two final points need to be noted with regard to growth factors, hormones and cancer. First, transformed cells may produce protein and peptide hormones providing an ectopic source of a hormone that is not under the regulatory feedback control of normally functioning endocrine glands. Second, tumors or their treatment may cause long-term endocrine complications.
Genes, mutations and endocrine function
It has been said that the practice of clinical genetics has always been facilitated by the fact that those carrying mutations always present to clinicians. Clearly this is not always true as some mutations in crucially important genes will be uniformly fatal in utero and never present clinically. The genetic study of such, albeit rare, patients presenting clinically has provided an enormous amount of knowledge in endocrinology. When such mutations are in the germ line, such conditions present as familial diseases. Novel somatic mutations may present with expression only within specific tissues. Clinical examples of the effects of mutations are given in the text where appropriate.
Animal models (predominantly rodent) have also provided an enormous insight into endocrine diseases. Some of these mouse and rat strains were the result of naturally occurring mutations. More recently genes have been experimentally inserted (transgenic) or deleted (knockout) in mice and these animal models have provided considerable scientific knowledge. Not all the insights gained from such approaches can be directly extrapolated to the human and it has to be borne in mind that redundancy within endocrine and biochemical systems means that the phenotypic results of such experiments are not always as straightforward as expected.
As the human genome project reaches its target, it is to be hoped that the knowledge gained may be used to further knowledge of the causes of the polygenic disorders so common in endocrinology such as obesity, diabetes mellitus and autoimmune diseases.
Clinical evaluation of endocrine disorders
It is important to emphasize the oxymoron that patients are bioassays of their circulating hormone concentrations. This is seen repeatedly in the clinical cases that form the springboards for the scientific knowledge throughout this book. Generally, in endocrinology patients come to attention for only two reasons - because of excess hormone action or through its lack. Each has a number of possible causes.
Excessive amounts of a hormone may be secreted by tumors. The normal feedback loop may be reset so that the amount of hormone secreted is abnormal only in the context of the concentration of the variable that it controls. In some cases, the relevant hormone may, in fact, be absent but its receptor may be constitutively activated. On the other hand, there may be a non-physiological stimulator of the receptor such as an antibody. The post-receptor signal transduction pathway may contain an abnormal protein that signals continued receptor occupancy. Finally, excess hormone may be ingested accidentally, deliberately or, indeed, therapeutically.
A lack of hormone effect may result from a lack of the hormone however caused (e.g. genetic deletion, damage to the endocrine gland, lack of a synthetic enzyme) or from the production of a biologically inactive hormone. The hormone receptor (or the down-stream signalling pathways) may be structurally abnormal and inactive leading to hormone resistance.
The pace of progress in endocrinology has always been dictated by the development of assays to measure the hormones. By and large, clinical endocrinology is limited to the measurement of hormone concentrations in venous serum or body fluids such as urine or saliva. Interpretation of the results of these assays should always take into account three factors - the clinical features of the patient, the concentration of the variable regulated by the hormone, and the concentration of other hormones in the feedback loop. For example, a serum insulin concentration can only be interpreted in light of the simultaneous glucose concentration and correct interpretation of thyroid, adrenal or gonadal hormone concentrations requires in many cases the results of the appropriate pituitary hormone concentrations. Tests of the feedback loops may be used; in general, in situations in which hormone concentrations are expected to be high, suppression tests are used and in those in which low concentrations are expected stimulation tests are used.
The practice of clinical endocrinology, like all branches of medicine, has been greatly facilitated by the technological progress in imaging techniques, for example MR and CT scanning. It has also been aided by the exquisite specificity of the biochemistry of individual tissues. Thus, radioactive iodine has been used for over 50 years to image the thyroid gland whilst recent techniques allow the use of radiolabeled agents such as metaiodobenzylguanidine (MIBG) or radio-labeled somatostatin analogs to visualize biologically active endocrine tissues. Examples of these and other techniques are given in appropriate clinical settings.
Publication Details
Copyright
Publisher
BIOS Scientific Publishers, Oxford
NLM Citation
Nussey S, Whitehead S. Endocrinology: An Integrated Approach. Oxford: BIOS Scientific Publishers; 2001. Chapter 1, Principles of endocrinology.
 Protein kinase C (PKC), activated by
Protein kinase C (PKC), activated by  Two-dimensional
Two-dimensional