See "Essentials of Glycobiology, 4th Edition"
See the updated version of this chapter
Antiglycan antibodies and lectins are widely used in glycan analysis because their specificities enable them to discriminate among a variety of glycan structures and their multivalency ensures high-affinity binding to the glycans and cell surfaces containing those glycans. This chapter describes the variety of commonly used antibodies and lectins and the types of analyses to which they may be applied.
BACKGROUND
The first evidence that carbohydrates were antigenic arose from the discovery that the human blood group ABO antigens were glycans (see Chapter 13). A key tool in these studies was the use of plant lectins, and by the mid-1940s they had found widespread use in typing blood.
Since this early beginning, hundreds of different plant and animal lectins have been identified and characterized. Because of their abundance in many plant seeds and tissues, lectins from these sources have been analyzed in detail and used for practical research on glycans. Thus, although monoclonal antibodies might be more specific for glycan determinants, plant and animal lectins have useful specificities, are usually less expensive, are better characterized with respect to binding specificity, and are more stable. The availability of the plant lectins and some animal lectins and their exquisite specificity for complex glycans helped to catapult the field of glycobiology into the modern era.
LECTINS MOST COMMONLY USED IN ANALYSIS OF GLYCANS
Many of the lectins currently used as tools in glycobiology come from plants and are commercially available. Most of these lectins were characterized initially by inhibition assays, in which monosaccharides, monosaccharide derivatives, or small oligosaccharides are used to block lectin binding to cells or some other glycan-coated target. Small-sized molecules that compete with binding of a lectin or antibody to a larger-sized ligand are termed haptens. These lectins are grouped by specificity depending on the monosaccharide(s) for which they show the highest affinity and their distinct preference for α- or β-anomers of the sugar. However, lectins within a particular specificity group also may differ in their affinities for different glycans. The common method for grouping lectins according to monosaccharide specificity should thus be used with caution because it does not reflect the complex specific determinants a given lectin may recognize with high affinity. The binding affinity (Kd) of lectins for complex glycans is often in the range of 1 to 10 μM. For complex glycoconjugates with multiple determinants or multivalency, the binding affinity of lectins may approach nanomolar values. In contrast, the affinity of most lectins for monosaccharides is in the millimolar range. The specificity of con-canavalin A (ConA), perhaps the most widely used lectin, demonstrates this point. This lectin (which is an α-mannose/α-glucose-binding lectin) binds to N-glycans and is not known to bind O-glycans on animal cell glycoproteins. However, it binds oligomannose-type N-glycans with much higher affinity than it binds complex-type biantennary N-glycans, and it fails to bind more highly branched complex-type N-glycans (Figure 45.1). Other lectins, such as L-phytohemagglutinin (L-PHA) and E-PHA from Phaseolus vulgaris and lentil lectin (LCA) from Lens culinaris, also recognize specific aspects of N-glycan structures. Many lectins, such as Ulex europaeus agglutinin I (UEA-I), Sambucus nigra agglutinin (SNA), Maackia amurensis leukoagglutinin (MAL), and Griffonia simplicifolia I-B4 agglutinin (GSI-B4), bind terminal structures with specific determinants (Figures 45.2–45.4) (see also Chapters 28 and 29).

FIGURE 45.1
Examples of N-glycans recognized by concanavalin A (ConA) from Canavalia ensiformis and Galanthus nivalis agglutinin (GNA). The determinants required for binding are indicated in the boxed areas. Hapten sugars that can competitively inhibit binding of (more...)
FIGURE 45.2
Examples of types of N-glycans recognized by L-PHA, E-PHA, and DSA. The determinants required for binding are indicated in the boxed areas. Hapten sugars that can competitively inhibit binding of the lectin to the indicated glycans are shown on the right (more...)
FIGURE 45.4
Examples of types of glycan determinants bound with high affinity by different plant lectins. The determinants required for binding are indicated in the boxed areas. Hapten sugars that can competitively inhibit binding of the lectin to the indicated glycans (more...)
Some of the animal lectins that are widely used in glycobiology include Helix pomatia agglutinin (HPA) from the snail, Limulus polyphemus agglutinin (LPA) from the hemolymph of the horseshoe crab, Limax flavus agglutinin (LFA) from the garden slug, and Anguilla anguilla agglutinin (AAA) from the freshwater eel.
GENERATION OF MONOCLONAL ANTIBODIES TO GLYCAN ANTIGENS
There are several approaches for generating antibodies to glycan antigens.
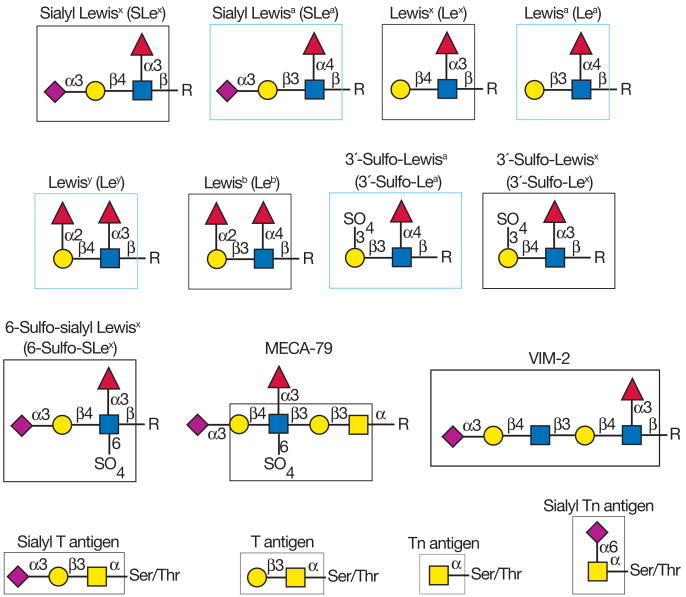
- Whole cells have been used to immunize mice to generate specific monoclonal antibodies to various glycoprotein and glycolipid antigens, including the Tn antigen (GalNAcα1-Ser/Thr) and the stage-specific embryonic antigen-1 (SSEA-1), now known as the Lewisx (Lex) antigen (Figure 45.5). In this approach, hybridomas are screened for monoclonal antibodies that recognize the immunizing cell but not other types of cells. A large number of antibodies to glycan determinants were generated by immunizing mice with different types of cancer cells.
- Glycan-protein conjugates (glycans coupled to carrier proteins such as bovine serum albumin [BSA] or keyhole limpet hemocyanin [KLH]) have been used to generate monoclonal antibodies to specific structures. A common variation of this approach is to immunize mice directly with a preparation of glycoproteins (such as a membrane fraction) or even a purified glycoprotein, glycolipid, or glycosaminoglycan. For example, antibodies to the plant glycoprotein horseradish peroxidase detect the presence of the unusual modifications Xylβ1-2Man-R and Fucα1-3GlcNAc-R in the core regions of complex-type N-glycans. Immunization with glycan conjugates has also been used to generate polyclonal antisera in rabbits and chickens. Using such antisera, it is possible to purify antibodies to specific glycan determinants by affinity chromatography on immobilized glycans. Knockout mice lacking specific glycoconjugates are also useful for generating antibodies to common structures. For example, antibodies to the common glycolipid sulfatide (3-O-sulfate-Galβ1-ceramide) were generated by taking mice that lacked the cerebroside sulfotransferase and immunizing them with sulfatide. If desired, it is possible to produce recombinant single-chain antibodies to glycan determinants after cloning the VH and VL domains of antibodies from hybridomas expressing specific monoclonal antibodies.
- A novel approach for obtaining monoclonal antibodies to specific glycan antigens has been to use mice that were infected with specific parasites or bacteria and then preparing hybridomas from the splenocytes of the infected animals. This approach has been used to generate many specific monoclonal antibodies to pathogen-specific glycan antigens.
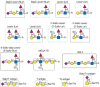
FIGURE 45.5
Examples of different glycan antigens recognized by specific monoclonal antibodies. The antigens have the structures shown within the boxed area and are named as indicated. Usually, the antigen shown in the box can be linked to almost any glycan and antibodies (more...)
Screening for appropriate antibodies to the desired antigen usually involves ELISA-type assays with immobilized target glycans. These targets may be specific glycolipids, which can be directly adsorbed to plastic wells; neoglycoproteins, such as BSA derivatives with covalently bound glycans; glycoproteins with known glycan structures; glycosaminoglycans, such as keratan sulfate, chondroitin sulfate, dermatan sulfate, and heparin; or biotinylated glycans captured on streptavidin-coated plates. However, in many cases, it is very difficult to define the specific epitopes recognized by these antibodies, because a wide variety of related compounds are not readily available for comparison. Thus, it is always advisable to be cautious in interpreting results based on monoclonal antibodies to glycan antigens, unless the antibody specificities have been well defined. In the future it will be important to define the specificities of each monoclonal antibody precisely using glycan microarrays and related approaches, where binding to a large number of glycans can be compared (see Chapter 27). Antiglycan antibodies are widely used in glycobiology and some of the more common antigens they recognize are shown in Figures 45.5 and 45.6. Most of the murine monoclonal antibodies to these antigens are of the IgM variety, but some are IgGs. The higher valency of IgM antibodies can affect the binding specificity. Some antibodies to glycan antigens are commercially available, whereas others are often obtained directly from individual laboratories.
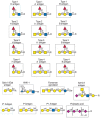
FIGURE 45.6
Additional examples of different glycan antigens recognized by specific monoclonal antibodies. The antigens have the structures shown within the boxed area and are named as indicated. Usually, the antigen shown in the box can be linked to almost any glycan (more...)
Most of the antibodies against the antigens shown in Figures 45.5 and 45.6 recognize terminal glycan determinants, although subterminal sequences may also be required for binding. In addition, the context of expression (glycoprotein vs. glycolipid) or the class of glycan (N- vs. O-linked) carrying the antigen may play a significant role in determining specificity. For example, antibodies to the 6-sulfo-SLex antigen require the fucose, sialic acid, and GlcNAc-6-O-sulfate residues. In contrast, the MECA-79 antibody recognizes extended core-1 O-glycans that contain internal GlcNAc-6-O-sulfate residues; it does not require either the fucose or sialic acid for recognition, but it does require the core-1 O-glycan. There are also many monoclonal antibodies that recognize different glycolipids and glycosaminoglycans.
USES OF ANTIBODIES AND LECTINS IN GLYCAN IDENTIFICATION
Lectins and antibodies are useful reagents for aiding in glycan identification. Some of the important uses are illustrated in Figure 45.7. They include agglutination of cells and blood typing, cell separation and analysis, bacterial typing, identification and selection of mutated cells with altered glycosylation, toxic conjugates for tumor cell killing, cytochemical characterization/staining of cells and tissues, inducing mitogenesis of cells, acting as growth inhibitors, mapping neuronal pathways, purification and characterization of glyco-conjugates, assays of glycosyltransferases and glycosidases, and defining glycosylation status of target glycoconjugates and cells.
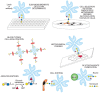
FIGURE 45.7
Examples of different uses of plant and animal lectins and antibodies in glycobiology. Many plant and animal lectins are multivalent as shown, and antibodies are always multivalent. They can be used to detect glycan structures in all of the formats shown. (more...)
Antibodies and lectins each have distinct advantages. For example, plant and animal lectins are less expensive than antibodies, a major consideration in affinity chromatography approaches where milligram amounts of the glycan-binding protein might be needed for purification. However, antibodies may be needed to bind specifically to certain determinants. For example, no lectin specific for the SLea antigen has been found. Conversely, no monoclonal antibodies have been identified that bind general determinants, such as α2-6-linked sialic acid and α1-2-linked fucose, whereas lectins have this specificity (e.g., SNA and Aleuria aurantia lectin [AAL], respectively). Of course antibodies that are specific to some restricted determinants, such as Lex and SLea antigens, still cannot distinguish their presentation on O-glycans, N-glycans, or glycolipids. In addition, although antibodies cannot distinguish N-glycan structural motifs, such features are well recognized by some plant lectins. For example, the plant lectin ConA does not bind mucin-type O-glycans in animal cells, but binds only to some specific classes of N-glycans (Figure 45.1). Additionally, E-PHA binds “bisected” complex-type N-glycans (Figure 45.2) and does not bind any known glycolipid or O-glycan. In all studies, care should always be taken to use lectins and antibodies at appropriate concentrations where their specificity can be exploited. Thus, it can be seen that antibodies and lectins each offer distinct advantages in defining glycan structures.
The glycan determinants bound with highest affinity by each of these probes have been identified by a combination of approaches, including affinity chromatography, glycan synthesis, and binding to specific glycoconjugates and cells. A good example of this is the phytohemagglutinin L-PHA, which is often used by immunologists as a mitogen to stimulate quiescent T cells to divide. L-PHA originates from the red kidney bean Phaseolus vulgaris, which also contains isolectins to L-PHA, notably E-PHA. L-PHA binds to certain branched, complex-type N-glycans containing the pentasaccharide sequence Galβ1-4GlcNAcβ1-2(Galβ1-4GlcNAcβ1-6)Manα1-R (the so-called “2,6-branch”), as shown in the boxed portion of the glycan in Figure 45.2. Curiously, the only monosaccharide that effectively inhibits either L-PHA or E-PHA is N-acetylgalactosamine, although this monosaccharide is not part of the determinants in N-glycans recognized by these lectins (Fig. 45.2). This finding arose from a combination of studies. First, L-PHA does not bind to any known glycolipid or O-glycan. Interestingly, L-PHA does not bind to human erythrocytes; thus, it is not a hemagglutinating lectin. It can, however, agglutinate white blood cells, and thus it is called a leukoagglutinin. Affinity chromatography approaches using immobilized L-PHA showed that it binds well to highly branched complex-type N-glycans containing the 2,6-branched structure, but it does not bind to alternate branched N-glycans lacking the 2,6-branch. In addition, L-PHA is highly inhibited by synthetic glycans containing the pentasaccharide sequence Galβ1-4GlcNAcβ1-2(Galβ1-4GlcNAcβ1-6)Man. Finally, L-PHA binds to the murine thymic leukemia cell line BW5147, but not to a mutated derivative cell line PHAR2.1. This mutant lacks the β1-6 N-acetylglucosaminyltransferase that creates the 2,6-branched complex-type N-glycan containing the pentasaccharide sequence. Thus, it has become clear that differential binding to erythrocytes versus leukocytes results because 2,6-branched N-glycans are not expressed on glycoproteins in human erythrocytes. Indeed, the binding of L-PHA has been used by glycobiologists to identify these types of branched N-glycans in cells. Interestingly, such L-PHA-binding glycoproteins are increased in many tumor cells (see Chapter 44), whereas they are dramatically decreased in mice genetically null for the branching β1-6 N-acetylglucosaminyltransferase (GNT-V).
Another good example of lectin specificity is G. simplicifolia I-B4 agglutinin (GSI-B4). This isolectin (from the seeds of a shrub native to West and Central Africa) agglutinates human blood group B and AB erythrocytes, poorly agglutinates A erythrocytes, and does not agglutinate H(O) erythrocytes. These seeds also contain other isolectins, such as GSI-A4, which binds terminal α-linked N-acetylgalactosamine residues. Glycoconjugates with terminal α-linked galactose, but not β-linked galactose, inhibited agglutination by GSI-B4. Affinity chromatography of glycoproteins from murine tumor cells on immobilized GSI-B4 led to purification of a subset of glycoproteins strongly enriched in terminal α-linked galactose determinants. The lectin has also been used to expression clone the cDNA encoding the α1-3-galactosyltransferase that synthesizes the terminal sequence Galα1-3Galβ1-4GlcNAc-R, as discussed below. Thus, GSI-B4 shows a strong preference for glycoconjugates containing the terminal sequence Galα1-3Galβ1-R.
Using a variety of lectins and antibodies to probe glycoproteins or cells, it is possible to deduce many aspects of glycan structure. This approach is especially sensitive in regard to defining whether two samples differ in glycosylation. For example, such approaches have been adapted to study differential glycosylation of prion glycoproteins using a panel of biotinylated lectins in ELISA-type formats.
USES OF ANTIBODIES AND LECTINS IN GLYCAN PURIFICATION
There are several approaches to using antibodies and lectins in glycan purification, including affinity chromatography or affinity binding and immunoprecipitation or lectin-induced precipitation. The proteins may be covalently coupled to a carrier such as Sepharose or biotinylated and captured on streptavidin-Sepharose. In addition, antibodies may be noncovalently captured on protein A (or G)-Sepharose. These bound antibodies and lectins can then be used in affinity chromatography or affinity capture to isolate glycoconjugates expressing specific glycan determinants. ConA-Sepharose is commonly used to isolate glycoproteins as it has little binding to nonglycosylated proteins. However, it does not bind all glycoproteins because it recognizes specific N-glycan structures. Using free glycans, ConA-Sepharose has been used to isolate oligomannose-, hybrid-, and complex-type biantennary N-glycans.
When combined in a serial format, multiple lectins can be used in affinity chromatography to isolate most of the major glycan structures present in animal cells, with glycans being separated as classes that share common determinants. An example of serial lectin affinity chromatography is shown in Figure 45.8. A mixture of cell glycans, containing the structures shown, may be applied to columns of immobilized ConA, LCA, and MAL. On the basis of their binding to these lectins, the glycans are separated into bound and unbound fractions, which are serially analyzed as shown. In this example, all glycans are bound by at least one lectin. The binding, or lack of binding, allows structural features of the glycans to be predicted. For example, the biantennary glycans bind weakly to ConA and are eluted with 10 mM α-methylglucoside, whereas the oligomannose- and hybrid-type N-glycans bind strongly and elution requires 100 mM α-methylmannoside. The biantennary N-glycans are fractionated into glycans that bind LCA (which are predicted to have a core α1-6-fucose residue) and those that are unbound (which are predicted to lack this fucose linkage). The unbound biantennary N-glycans from LCA are then bound by MAL and they are predicted to contain terminal α2-3-sialic acid linked to Galβ1-4GlcNAc-R residues. When serial lectin affinity chromatography is coupled with ion-exchange chromatography and HPLC, it is possible to obtain highly purified glycans with predicted structures, which can then be confirmed by mass spectrometry of native and permethylated derivatives (see Chapter 47).
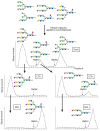
FIGURE 45.8
An example of the use of different immobilized plant lectins in serial lectin affinity chromatography of complex mixtures of glycopeptides. In this example, a mixture of glycopeptides is applied to a column of immobilized ConA, and the bound glycans are (more...)
It should also be noted that mixed-bed lectin chromatography, where a number of different immobilized lectins are combined in a single column or tube, is useful for generally isolating all types of glycoconjugates simultaneously from nonglycosylated material. For example, this can be used to separate glycopeptides from peptides. Thus, the ability of glycans to be recognized by lectins dependent on specific structural features in the glycans is a powerful tool for glycan identification and isolation. In such approaches, the glycans may be tagged at the reducing end by fluorophores and radioisotopes or may be obtained by metabolic radiolabeling from cells or tissues grown in the presence of radiolabeled sugar precursors, such as [2-3H]mannose or [6-3H]glucosamine. Interestingly, glycan fractionation shown on immobilized lectins in Figure 45.8 is currently not possible with antibodies because no antibodies are known that can distinguish such core structural features in glycans.
When intact glycoproteins are analyzed for their interactions with plant lectins, the interpretation of data may be complicated by the multivalency of the glycoprotein and the density of the immobilized lectin. For example, glycoproteins containing multiple high mannose-type N-glycans bind so tightly to immobilized ConA that it is difficult to elute the bound glycoprotein, even with extremely high concentrations of hapten and under harsh conditions. Lower densities of ConA conjugation reduce its avidity for the glycoproteins and promote hapten dissociation of bound ligands with lower concentrations of sugars. When used in combination, multiple lectins, such as ConA, AAL, LCA, and RCA (Ricinus communis agglutinin), can be used to isolate most glycoproteins containing N-and O-glycans from animal cells. This is a potentially powerful approach for glycoproteomics, or the identification of glycoproteins and their glycosylation status.
Lectins can also be used in western blotting (lectin blotting) approaches to characterize protein and lipid glycosylation, in which biotinylated lectins are applied to material transferred to nitrocellulose or other supports after electrophoresis or chromatography. In such cases, the bound lectins are visualized by binding streptavidin-alkaline phosphatase and conversion of luminescent substrates. In these approaches, the concentrations of lectins used must be low enough both to reduce false-positive binding and to allow the binding to be inhibited by appropriate haptens to confirm lectin-sugar binding, rather than nonspecific lectin-protein association. These types of lectin blotting studies have been especially useful in characterizing alterations in glycosylation following genetic manipulation of glycosylation pathways.
USES OF ANTIBODIES AND LECTINS IN CHARACTERIZING CELL-SURFACE GLYCOCONJUGATES
The major approaches for using antibodies and lectins to characterize cell-surface glycoconjugates are histochemistry for lectins and immunohistochemistry for antibodies, flow cytometry and cell sorting, and cell agglutination. In histochemistry and immunohistochemistry, tissues are prepared and fixed as usual for histological staining, and then incubated with appropriate biotinylated or peroxidase-labeled lectins or antibodies. The bound lectins or antibodies are visualized after the binding of secondary reagents, such as streptavidin-peroxidase or labeled secondary antibody. These approaches often yield information that is difficult to obtain by any other approach. For example, they can reveal the spatial orientation of different glycans, their relative abundance, and whether they are intracellular and/or extracellular. Three important controls in such studies are (1) use of lectins or antibodies at limiting concentrations to avoid nonspecific binding; (2) confirmation of the specificity of binding by appropriate inhibition by haptens or by destruction of the predicted target glycans with glycosidases; and (3) use of multiple lectins or antibodies to provide further confirmation of the conclusions.
Lectins and antibodies to glycans have also been widely used in flow cytometry and cell sorting. In such studies, cells are incubated with low, nonagglutinating levels of lectins or antibodies that are biotinylated and conjugated to fluorescently labeled streptavidin or directly fluorescently labeled. Cells with bound lectins can then be identified by their fluorescence in the flow cytometer, and the degree of fluorescence can be correlated with the number of binding sites. Again, appropriate controls include the three mentioned above. A key consideration in flow cytometry is to avoid concentrations of lectins that cause agglutination of cells, which must be ruled out by direct microscopic visualization. Lectins and antibodies can also be used to identify specific membrane localization of different glycans, using confocal microscopy and electron microscopy.
Lectins and antibodies are also especially useful for characterizing cell-surface glycans when limited numbers of cells are available. For example, studies on glycosylation of embryonic stem cells have been greatly aided by using panels of specific lectins to identify unique glycan determinants and changes in their expression during cellular differentiation. A recent variation of this approach is to use a microarray of immobilized lectins, which are probed with fluorescently labeled extracts of cells. Such assays can reveal minor differences in protein glycosylation between different samples and give insight into the glycan structures that are present.
Finally, one of the oldest uses of lectins is in the agglutination and precipitation of glycoconjugates, cells, and membrane vesicle preparations. The easiest assay for many soluble lectins that are multivalent is agglutination of target cells, such as erythrocytes, leukocytes, or even bacteria or fungi. Agglutination can often be easily observed without a microscope, but it is also measurable in instruments such as aggregometers. In these assays, a lectin solution is serially diluted and the reciprocal of the final dilution that gives measurable cell agglutination is taken to define the activity. Bacterial agglutination by plant and animal lectins is often used to explore the surface glycocalyx and changes in glycocalyx upon culture conditions and to define phenotypes of different serotypes. Lectin precipitation and aggregation can be used to define the glycan composition and overall architecture of polysaccharides, as has been done for bacterial, algal, plant, and animal polysaccharides. Some lectins also have antiviral activity, as a result of aggregation of the viruses and blocking of viral adhesion and infection. These antiviral activities are being explored as new treatments for human diseases.
USES OF ANTIBODIES AND LECTINS FOR GENERATING ANIMAL CELL GLYCOSYLATION MUTANTS
An important use of lectins and antibodies has been in the selection of cell lines that express altered cell-surface glycans. A good example of this approach involves studies using the Chinese hamster ovary cell line (CHO) as a model system. The common lectins that have been used are ConA, WGA (wheat germ agglutinin), L-PHA, LCA, PSA (Pisum sativum agglutinin), E-PHA, ricin, modeccin, and abrin. The latter three lectins are heterodimeric, disulfide-bonded proteins that are classified as type II ribosome-inactivating proteins; they contain an A subunit that encodes an enzyme called RNA-N-glycosidase, which inactivates the 28S ribosome, and a B subunit that is a galactose-binding lectin. Many plant lectins such as ConA, WGA, or LCA (which lack an enzymatic or toxic A subunit) are toxic to animal cells via poorly understood mechanisms, whereas other lectins, such as soybean agglutinin (SBA) and peanut agglutinin (PNA), are not toxic to animal cells in culture. One mechanism of toxicity of plant lectins is the induction of apoptosis, perhaps by blocking receptor or transport functions in cells. Noncytotoxic lectins and antibodies to specific glycans can be rendered toxic by conjugating them with ricin A subunit or other toxic proteins. Using these agents, both loss-of-function (e.g., loss of a glycosyl-transferase or glycosidase) and gain-of-function (e.g., activation of a latent transferase gene) mutants have been obtained. More details about glycosylation mutants of cultured cells are presented in Chapter 46.
USES OF ANTIBODIES AND LECTINS FOR CLONING GLYCOSYLTRANSFERASE GENES BY EXPRESSION
The specificity of lectins and antibodies to particular glycans has made them especially useful in cloning genes encoding glycosyltransferases or other proteins required for proper glycosylation, such as nucleotide sugar transporters. To illustrate the approaches that are usually taken, several examples will be discussed. CHO cells and the African green monkey kidney cell line COS lack glycans with terminal α-galactose residues. Consequently, the cells do not bind the plant lectin GSI-B4 (Figure 45.3). When the cells are transfected with a cDNA library prepared from cells that do express terminal α-galactose residues (such as murine teratocarcinoma cells F9), a few of the transfected cells will have taken up a plasmid encoding the cognate α1-3 galactosyltransferase and consequently express terminal α-galactose residues. Those cells will bind to GSI-B4 and can be identified by binding to plates coated with the lectin. Isolation of the transfected plasmids from the bound cells and repeated recloning and reexpression by this technique (called expression cloning) led to the identification of a specific gene encoding the murine α1-3 galactosyltransferase.
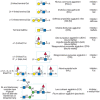
FIGURE 45.3
Examples of types of glycan determinants bound with high affinity by different plant and animal lectins. The determinants required for binding are indicated in the boxed areas. Hapten sugars that can competitively inhibit binding of the lectin to the (more...)
Related approaches using antibodies and lectins to other glycan structures and wild-type CHO cells and CHO mutants led to the identification of the genes encoding many other glycosyltransferases, including some involved in extending glycosphingolipids and nucleotide sugar transporters, such as the transporter for CMP-NeuAc. Such approaches based on lectin selection are also useful with yeast. For example, the gene encoding a yeast N-acetyl-glucosaminyltransferase (GlcNAcT) was identified by expression cloning in yeast using a GlcNAcT-deficient yeast. The mannan chains of the yeast Kluyveromyces lactis normally contain some terminal N-acetylglucosamine residues and are bound by the plant lectin GSL-II, which binds terminal N-acetylglucosamine residues (Figure 45.4). A mutant lacking the GlcNAcT and lacking terminal N-acetylglucosamine residues on mannoproteins was identified. Transformation of yeast with DNA containing the gene encoding the GlcNAcT led to production of some yeast that were bound by the fluorescently labeled GSL-II. This strategy was used to identify the gene encoding the GlcNAcT. The transporters for UDP-Gal in Leishmania parasites and in the plant Arabidopsis were also identified by expression cloning in Lec8 CHO cells; these cells have a mutation in their endogenous UDP-Gal transporter, and consequently lack galactose-containing glycans on their surface. For the UDP-Gal transporter from Arabidopsis, Lec8 cells were co-transfected with a cDNA library encoding the putative Arabidopsis UDP-Gal transporter, along with a glucuronosyltransferase that can lead to synthesis of the unsulfated version of the HNK epitope (Figure 45.6), GlcAβ1-3Galβ-R that is bound by a specific antibody. This identification strategy led to the expression cloning of several UDP-Gal transporters from Arabidopsis.
CHO and COS cell lines have also been useful in characterizing the activities of novel glycosyltransferase genes that were identified by other approaches. For example, candidate fucosyltransferase genes encoding α1–2 or α1–3 fucosyltransferases have been expressed in CHO and COS cells, which lack these enzymes. Expression of these enzymes then leads to expression on the cell surface of antigens recognized by specific antibodies and lectins, such as the H-antigen (for the α1–2 FucT) or Lex and SLex (for the α1–3 FucT).
USES OF ANTIBODIES AND LECTINS IN ASSAYING GLYCOSYLTRANSFERASES AND GLYCOSIDASES
Lectins and antibodies to glycan antigens have been very useful in assaying specific glyco-syltransferases and glycosidases in a variety of formats (Figure 45.7). Immobilized lectins have been used to isolate products of glycosyltransferases assays, such as chitin polysaccharides on WGA or glycosylated peptides on mixed-bed lectin columns. Assays for several specific glycosyltransferases have relied on lectin and antibody binding to the products. For example, α1-3 fucosyltransferases that synthesize the Lex and SLex antigens have been assayed based on capture of the product on immobilized antibodies to these antigens or binding of antibody to the immobilized fucosylated product in an ELISA-type format. Likewise, α2-3 sialyltransferases and α2-6 sialyltransferases have been assayed using immobilized acceptors in ELISA-type format and in BIAcore formats (see Chapter 27), and their products have been measured with MAL (which binds to the α2-3-sialylated product) or SNA (which binds to the α2-6-sialylated product). Similarly, α1-3 galactosyltransferases have been assayed using immobilized acceptors in an ELISA-type format, and the product has been measured with GSI-B4 or Viscum album agglutinin, which binds to the α1-3 galactosylated product. The glycoprotein-specific β1-4 N-acetylgalactosaminyltransferase has been assayed using glycoprotein acceptors in solution, capture by a specific monoclonal antibody in a microtiter plate, and measurement of product by ELISA-type assay with a Wisteria floribunda lectin (WFA) that binds to terminal β1-4-linked GalNAc residues generated by the enzyme. These are just some of the examples of ELISA-type, relatively high-throughput, and nonradioactive assays that have been developed for various glycosyltransferases using plant lectins and antibodies to detect the specific products. Conversely, glycosidases can be assayed by measuring the gain in binding of lectins. For example, the lectin PNA, which binds to nonsialylated Galβ1-3GalNAcα1-Ser/Thr in O-glycans, can be used to measure bacterial sialidases by agglutination of treated erythrocytes. It is easy to envision how lectins and antibodies can be used to probe the products of other specific glycosidases and glycosyltransferases, given the specificities of the lectins described here.
FURTHER READING
- Hakomori S. Tumor-associated carbohydrate antigens. Annu Rev Immunol. 1984;2:103–126. [PubMed: 6085749]
- Merkle RK, Cummings RD. Lectin affinity chromatography of glycopeptides. Methods Enzymol. 1987;138:232–259. [PubMed: 3600324]
- Osawa T, Tsuji T. Fractionation and structural assessment of oligosaccharides and glycopeptides by use of immobilized lectins. Annu Rev Biochem. 1987;56:21–42. [PubMed: 3304133]
- Osawa T. The separation of immunocyte subpopulations by use of various lectins. Adv Exp Med Biol. 1988;228:83–104. [PubMed: 3051929]
- Esko JD. Animal cell mutants defective in heparan sulfate polymerization. Adv Exp Med Biol. 1992;313:97–106. [PubMed: 1442273]
- Kobata A, Endo T. Immobilized lectin columns: Useful tools for the fractionation and structural analysis of oligosaccharides. J Chromatogr. 1992;597:111–122. [PubMed: 1517308]
- Cummings RD. Use of lectins in analysis of glycoconjugates. Methods Enzymol. 1994;230:66–86. [PubMed: 8139516]
- Lis H, Sharon N. Lectins: Carbohydrate-specific proteins that mediate cellular recognition. Chem Rev. 1998;98:637–674. [PubMed: 11848911]
- Bush CA, Martin-Pastor M, Imberty A. Structure and conformation of complex carbohydrates of glycoproteins, glycolipids, and bacterial polysaccharides. Annu Rev Biophys Biomol Struct. 1999;28:269–293. [PubMed: 10410803]
- Morgan WT, Watkins WM. Unravelling the biochemical basis of blood group ABO and Lewis antigenic specificity. Glycoconj J. 2000;17:501–530. [PubMed: 11421345]
- Goldstein IJ. Lectin structure-activity: The story is never over. J Agric Food Chem. 2002;50:6583–6585. [PubMed: 12381155]
- Paschinger K, Fabini G, Schuster D, Rendic D, Wilson IB. Definition of immunogenic carbohydrate epitopes. Acta Biochim Pol. 2005;52:629–632. [PubMed: 16175237]
- Akama TO, Fukuda MN. N-Glycan structure analysis using lectins and an α-mannosidase activity assay. Methods Enzymol. 2006;416:304–314. [PubMed: 17113875]
- Lehmann F, Tiralongo E, Tiralongo J. Sialic acid-specific lectins: Occurrence, specificity and function. Cell Mol Life Sci. 2006;63:1331–1354. [PMC free article: PMC7079783] [PubMed: 16596337]
- Maeda Y, Ashida H, Kinoshita T. CHO glycosylation mutants: GPI anchor. Methods Enzymol. 2006;416:182–205. [PubMed: 17113867]
- Patnaik SK, Stanley P. Lectin-resistant CHO glycosylation mutants. Methods Enzymol. 2006;416:159–182. [PubMed: 17113866]
- Varki NM, Varki A. Diversity in cell surface sialic acid presentations: Implications for biology and disease. Lab Invest. 2007;87:851–857. [PMC free article: PMC7100186] [PubMed: 17632542]
Publication Details
Author Information and Affiliations
Authors
Richard D Cummings and Marilynn E Etzler.Copyright
Publisher
Cold Spring Harbor Laboratory Press, Cold Spring Harbor (NY)
NLM Citation
Cummings RD, Etzler ME. Antibodies and Lectins in Glycan Analysis. In: Varki A, Cummings RD, Esko JD, et al., editors. Essentials of Glycobiology. 2nd edition. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2009. Chapter 45.