See "Essentials of Glycobiology, 4th Edition"
See the updated version of this chapter
Change to text in Chapter 9
In the section “Synthesis of O-GalNAc Glycan Core Structures,” the following text was edited to read: “Cosmc is an ER protein that appears to bind specifically to T synthase and ensures its full activity in the Golgi. Lack of core 1 synthesis can be due to either defective T synthase or the absence of functional Cosmc chaperone. The result is high expression of Tn and sialyl-Tn antigens.”
In the section “Functions of O-GalNAc Glycans,” the following text was edited to read: “Some sugar residues or their modifications can mask underlying antigens or receptors. For example, O-acetyl groups on the sialic acid residue of the sialyl-Tn antigen prevent recognition by anti-sialyl-Tn antibodies. Gut bacteria enzymatically remove this blocking group.”
O-glycosylation is a common covalent modification of serine and threonine residues of mammalian glycoproteins. This chapter describes the structures, biosynthesis, and functions of glycoproteins that are often termed mucins. In mucins, O-glycans are covalently α-linked via an N-acetylgalactosamine (GalNAc) moiety to the -OH of serine or threonine by an O-glycosidic bond, and the structures are named mucin O-glycans or O-GalNAc glycans. The focus of this chapter is on mucins and mucin-like glycoproteins that are heavily O-glycosylated, although glycoproteins that carry only one or a few O-GalNAc glycans are also briefly discussed. There are also several types of nonmucin O-glycans, including α-linked O-fucose, β-linked O-xylose, α-linked O-mannose, β-linked O-GlcNAc (N-acetylglucosamine), α- or β-linked O-galactose, and α- or β-linked O-glucose glycans (discussed in Chapters 12 and 16–18). In this chapter, however, the term O-glycan refers to mucin O-glycans, unless otherwise specified. Mucin glycoproteins are ubiquitous in mucous secretions on cell surfaces and in body fluids.
DISCOVERY AND BACKGROUND
In 1865, E. Eichwald, a Russian physician working in Germany, provided the first chemical evidence that mucins are proteins conjugated to carbohydrate. He also showed that mucins are widely distributed in the animal body. In 1877, F. Hoppe-Seyler discovered that mucins produced by the epithelial cells of mucous membranes and salivary glands contain an acidic carbohydrate, identified many years later to be sialic acid. Mucins are present at many epithelial surfaces of the body, including the gastrointestinal, genitourinary, and respiratory tracts, where they shield the epithelial surfaces against physical and chemical damage and protect against infection by pathogens. Mucin O-glycans begin with an α-linked N-acetylgalactosamine residue linked to serine or threonine. The N-acetylgalactosamine may be extended with sugars including galactose, N-acetylglucosamine, fucose, or sialic acid, but not mannose, glucose, or xylose residues. There are four common O-GalNAc glycan core structures, designated cores 1 through 4 and an additional four designated cores 5 though 8 (Table 9.1). Mucin O-glycans can be branched, and many sugars or groups of sugars on mucin O-glycans are antigenic (Table 9.1). Important modifications of mucin O-glycans include O-acetylation of sialic acid and O-sulfation of galactose and N-acetylglucosamine. Thus, mucin O-glycans are often very heterogeneous, with hundreds of different chains being present in some mucins. Numerous chemical, enzymatic, and spectroscopic methods are applied to analyze the sugar composition and the linkages among sugars of mucin O-glycans.
TABLE 9.1
Structures of O-glycan cores and antigenic epitopes found in mucins
MUCIN GLYCOPROTEINS
Mucins are heavily O-glycosylated glycoproteins found in mucous secretions and as transmembrane glycoproteins of the cell surface with the glycan exposed to the external environment. The mucins in mucous secretions can be large and polymeric (gel-forming mucins) or smaller and monomeric (soluble mucins). Many epithelial cells produce mucin, but gel-forming mucins are produced primarily in the goblet or mucous cells of the tracheobronchial, gastrointestinal, and genitourinary tracts. In goblet cells, mucins are stored intracellularly in mucin granules from which they can be quickly secreted upon external stimuli.
The hallmark of mucins is the presence of repeated peptide stretches called “variable number of tandem repeat” (VNTR) regions that are rich in serine or threonine O-glycan acceptor sites and have an abundance of clustered mucin O-glycans that may comprise 80% of the molecule by weight. The tandem repeats are usually rich in proline residues that appear to facilitate O-GalNAc glycosylation. Mucins may have hundreds of O-GalNAc glycans attached to serine or threonine residues in the VNTR regions. The clustering of O-GalNAc glycans causes mucin glycoproteins to adopt an extended “bottle brush” conformation (Figure 9.1).
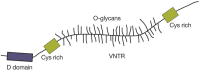
FIGURE 9.1
A simplified model of a large secreted mucin. The VNTR (variable number of tandem repeat) region rich in serine, threonine, and proline is highly O-glycosylated and the peptide assumes an extended “bottle brush” conformation. Hundreds (more...)
About 20 different mucin genes have been cloned, and they are expressed in a tissue-specific fashion. For example, different mucin genes are expressed in different regions of the gastrointestinal tract, suggesting that they serve specific functions. The first mucin gene to be cloned encoded a transmembrane mucin termed MUC1. Mucins that span the plasma membrane are known to be involved in signal transduction, to mediate cell–cell adhesion, or to have an antiadhesive function. Cell-surface mucins contain an extracellular domain with a central VNTR region that carries O-GalNAc glycan chains, a single transmembrane domain, and a small cytoplasmic tail at the carboxyl terminus.
The mucin genes, their transcripts, the resulting mucin proteins, and the attached O-GalNAc glycans all exhibit extreme variability. Different mucins vary in the number and composition of the peptide repeats in their VNTR regions. Within the same mucin, the repeats usually vary in their amino acid sequences. It has not yet been possible to determine exactly which of the many different O-GalNAc glycan structures isolated from a purified mucin preparation are attached to specific serine or threonine residues in the tandem repeats. However, in some less densely glycosylated glycoproteins, specific O-GalNAc glycosylation sites have been identified. The structures of O-glycans at these defined sites are usually heterogeneous and any mucin includes a range of glycoforms. One of the exceptions is the Antarctic fish antifreeze glycoprotein, which carries only Galβ1-3GalNAc- at threonine residues of alanine-alanine-threonine repeat sequences.
Some mucins contain a hydrophobic transmembrane domain that serves to insert the molecule into the cell membrane. Secreted mucins have cysteine-rich regions and cystine knots that are responsible for their polymerization and the formation of extremely large molecules of several million daltons. Mucins may also have protein domains similar to motifs present in other glycoproteins. For example, D domains found in mucins are also found in von Willebrand factor, a glycoprotein important in blood clotting. The D domain may regulate mucin polymerization (Figure 9.1).
Mucin-like glycoproteins such as GlyCAM-1, CD34, and PSGL-1 are less densely O-glycosylated membrane-associated glycoproteins that mediate cell–cell adhesion. Most glycoproteins with O-GalNAc glycans carry several O-glycans, although some, such as interleukin-2 and erythropoietin, carry a single O-GalNAc glycan. The expression of mucin genes is regulated by a large number of cytokines and growth factors, differentiation factors, and bacterial products.
The purification of gel-forming mucins is achieved by density-gradient centrifugation. Purified mucins have distinct viscoelastic properties that contribute to the high viscosity of mucous secretions. They are hydrophilic and contain charges that attract water and salts. Bacteria, viruses, and other microbes are trapped by mucins and sometimes adhere to specific O-GalNAc glycans that serve as receptors (see Tables 34.1 and 34.2). The ciliary action of the mucous membrane epithelium aids in the removal of microbes and small particles.
Mucins hydrate and protect the underlying epithelial cells, but they have also been shown to have roles in fertilization, blastocyst implantation, and the immune response. Cell-surface MUC1 and mucin-like glycoproteins have roles in cell adhesion (see Chapter 31). A number of diseases are associated with abnormal mucin gene expression and abnormal mucin carbohydrate structures and properties. These include cancer, inflammatory bowel disease, lung disease, and cystic fibrosis. The expression of underglycosylated MUC1 is often increased in individuals with cancer (see Chapters 43 and 44).
O-GalNAc GLYCAN STRUCTURES
The simplest mucin O-glycan is a single N-acetylgalactosamine residue linked to serine or threonine. Named the Tn antigen, this glycan is often antigenic. The most common O-GalNAc glycan is Galβ1-3GalNAc-, and it is found in many glycoproteins and mucins (Table 9.1, Figure 9.2). It is termed a core 1 O-GalNAc glycan because it forms the core of many longer, more complex structures. It is antigenic and is also named the T antigen. Both Tn and T antigens may be modified by sialic acid to form sialylated-Tn or -T antigens, respectively.
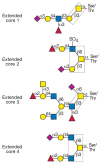
FIGURE 9.2
Complex O-GalNAc glycans with different core structures. Representative examples of complex O-GalNAc glycans with extended core 1, 2, or 4 structures from human respiratory mucins and an O-GalNAc glycan with an extended core 3 structure from human colonic (more...)
Another common core structure contains a branching N-acetylglucosamine attached to core 1 and is termed core 2 (Table 9.1, Figure 9.2). Core 2 O-GalNAc glycans are found in both glycoproteins and mucins from a variety of cells and tissues. Linear core 3 and branched core 4 O-GalNAc glycans (Table 9.1, Figure 9.2) have been found only in secreted mucins of certain mucin-secreting tissues, such as bronchi, colon, and salivary glands. Core structures 5–8 have an extremely restricted occurrence. Mucins with core 5 have been reported in human meconium and intestinal adenocarcinoma tissue, whereas core 6 structures are found in human intestinal mucin and ovarian cyst mucin. Core 8 has been reported in human respiratory mucin. Bovine submaxillary mucin was shown to contain O-GalNAc glycans with relatively short chains, including those containing a core 7 structure (Table 9.1).
All of the core structures can be sialylated. However, only cores 1–4 and core 6 have been shown to occur as extended, complex O-glycans that carry antigens such as the ABO and Lewis blood group determinants, the linear i antigen (Galβ1-4GlcNAcβ1-3Gal-), and the GlcNAcβ1-6 branched I antigens (Table 9.1). Both type 1 (based on the Galβ1-3GlcNAc sequence) and type 2 (based on the Galβ1-4GlcNAc sequence) extensions can be repeated several times in poly-N-acetyllactosamine units, which provide a scaffold for the attachment of additional sugars or functional groups. The terminal structures of O-GalNAc glycans may contain fucose, galactose, N-acetylglucosamine, and sialic acid in α-linkages, N-acetylgalac-tosamine in both α- and β-linkages, and sulfate. Many of these terminal sugar structures are antigenic or represent recognition sites for lectins. In particular, the sialylated and sulfated Lewis antigens are ligands for selectins (see Chapter 31). Poly-N-acetyllactosamine units and terminal structures may also be found on N-glycans and glycolipids (see Chapter 13).
ISOLATION, PURIFICATION, AND ANALYSIS OF MUCIN O-GLYCANS
All O-GalNAc glycans may be released from the peptide by an alkali treatment called β-elimination. The N-acetylgalactosamine residue attached to serine or threonine is reduced to N-acetylgalactosaminitol by borohydride as it is released. Conversion to N-acetylgalactosaminitol prevents the rapid alkali-catalyzed degradation of the released O-GalNAc glycan by a “peeling” reaction. Glycoproteins that contain both N- and O-glycans will retain their N-glycans and specifically lose O-glycans when subjected to β-elimination. However, harsh alkali treatment will also disrupt peptide bonds and degrade the mucin. Unsubstituted N-acetylgalactosamine residues may also be released from serine or threonine by a specific N-acetylgalactosaminidase, and unsubstituted Galβ1-3GalNAc (core 1) can be released with an enzyme termed O-glycanase. For core 1 O-GalNAc glycans substituted with sialic acids, the sialic acid must first be removed by treatment with sialidase or mild acid before O-glycanase treatment will be successful. No enzymes are known to release larger O-glycans from the peptide, in contrast to the availability of several enzymes that release N-glycans from asparagine (see Chapter 8).
Released O-GalNAc glycans may be separated into different species by chromatographic methods such as gel filtration, anion-exchange chromatography, and high-pressure liquid chromatography. Chemical derivatization of purified reduced O-glycans helps in the analysis of sugar composition and the determination of linkages between sugars by gas chromatography and mass spectrometry (see Chapter 47). With improvements in the sensitivity of mass spectrometry, it is now possible to analyze small amounts and identify structures on the basis of their mass in complex mixtures. Structural analysis by nuclear magnetic resonance (NMR) spectroscopy is more precise, but requires more material and highly purified compounds. The NMR spectra of hundreds of O-glycans have been published and are used as references to identify known and new O-glycan structures. Other tools to analyze the structures of O-GalNAc glycans include specific exoglycosidases that remove terminal sugars, antibodies to N-acetylgalactosamine (anti-Tn) and core 1 (anti-T), and lectins such as peanut agglutinin (which binds to core 1) and Helix pomatia agglutinin (which binds to terminal N-acetylgalactosamine) (see Chapter 45). O-GalNAc glycans in a specific cell line or tissue may be predicted based on the presence of active glycosyltransferases required for their synthesis.
BIOSYNTHESIS OF O-GalNAc GLYCANS
Polypeptide-N-Acetylgalactosaminyltransferases
The first step of mucin O-glycosylation is the transfer of N-acetylgalactosamine from UDP-GalNAc to serine or threonine residues, which is catalyzed by a polypeptide-N-acetyl-galactosaminyltransferase (ppGalNAcT). There are at least 21 polypeptide-N-acetylgalactosaminetransferases (ppGalNAcT-1 to -21) that differ in their amino acid sequences and are encoded by different genes. Homologs of ppGalNAcT genes are expressed in all eukaryotic organisms and a high degree of sequence identity exists between mouse and human ppGalNAcTs.
Immunohistochemistry studies have demonstrated that some ppGalNAcTs localize to the cis-Golgi in submaxillary glands (see Chapter 3). However, more recent studies suggest that in human cervical cancer cells, several enzymes of this family are located throughout the Golgi. The subcellular localization of ppGalNAcTs and other glycosyltransferases involved in O-glycosylation has a critical role in determining the range of O-glycans synthesized by a cell. Thus, a ppGalNAcT localized to a late compartment of the Golgi will act just before the secretion of a glycoprotein and is likely to produce a number of “incomplete” shorter O-GalNAc glycans, a factor that will contribute to the glycan heterogeneity of a mucin.
ppGalNAcT expression levels vary considerably between cell types and mammalian tissues. All ppGalNAcTs bind UDP-GalNAc (the donor of N-acetylgalactosamine), but they often differ in the protein substrates to which they transfer N-acetylgalactosamine. Such differences allow ppGalNAcTs to be distinguished. Many ppGalNAcTs do not efficiently transfer N-acetylgalactosamine to serine, but only to threonine in peptide acceptors used in in vitro assays. Nevertheless, the VNTR regions of mucins appear to be fully O-glycosylated. Many ppGalNAcTs appear to have a hierarchical relationship with one another, such that one enzyme cannot attach an N-acetylgalactosamine until an adjacent serine or threonine is glycosylated by a different ppGalNAcT. Thus, coexpression in the same cell of ppGalNAcTs with complementary, partly overlapping acceptor substrate specificities probably ensures efficient O-GalNAc glycosylation.
Although defined amino acid sequons that accept N-acetylgalactosamine have not been identified, certain amino acids are preferred in the substrate. Proline residues near the site of N-acetylgalactosamine addition are usually favorable to mucin O-glycosylation, whereas charged amino acids may interfere with ppGalNAcT activity. It is possible that the role of proline is to expose serine or threonine residues in a β-turn conformation, leading to more efficient O-glycosylation. Databases are available that estimate the likelihood of O-glycosylation at specific sites based on known sequences around O-glycosylation sites of several hundreds of glycoproteins (e.g., OGLYCBASE and NetOGlyc).
The domain structure of ppGalNAcTs is that of a type II membrane protein, typical of all Golgi glycosyltransferases (see Chapter 3). In addition, a distinct lectin-like domain has been discovered at the carboxyl terminus of several ppGalNAcTs. The lectin domain is used for binding to N-acetylgalactosamine residues that have already been added to the glycoprotein. In vitro studies using O-glycosylated peptides as acceptor substrates have shown that further addition of N-acetylgalactosamine is strongly influenced by the presence of existing N-acetylgalactosamine and larger O-glycans in the acceptor substrate, suggesting a molecular mechanism for the hierarchical relationships of ppGalNAcTs.
Purification of ppGalNAcTs has been achieved using mucin peptide and nucleotide sugar derivatives as affinity reagents. The recent elucidation of the crystal structure of ppGalNAcT-1 showed that the catalytic site of the enzyme is spatially separated from the lectin-binding site, and that there is a nucleotide sugar- and Mn++-binding groove containing an aspartate-X-histidine motif. The unique structure of ppGalNAcT-1 suggests that this transferase can accommodate a number of different glycosylated acceptor substrates.
Synthesis of O-GalNAc Glycan Core Structures
The transfer of the first sugar from UDP-GalNAc directly to serine or threonine in a protein initiates the biosynthesis of all O-GalNAc glycans. Subsequently, with the addition of the next sugar, different mucin O-glycan core structures are synthesized (Figure 9.2). In contrast to the initial reactions of N-glycosylation and O-mannosylation, no lipid-linked intermediates are involved in O-GalNAc glycan biosynthesis, and no glycosidases appear to be involved in the processing of O-GalNAc glycans within the Golgi.
The O-glycans of mucins produced in a specific tissue predict the enzyme activities that are present in that tissue. The glycosyltransferases that are involved solely in the assembly of mucin O-GalNAc glycans are listed in Table 9.2. The many other enzymes that may be involved also contribute to the synthesis of N-glycans and glycolipids. The first sugar (N-acetylgalactosamine) added to the protein creates the Tn antigen (GalNAc-Ser/Thr), which is uncommon in normal mucins, but is often found in mucins derived from tumors. This suggests that the extension of O-GalNAc glycans beyond the first sugar is blocked in some cancer cells. Another common cancer-associated structure found in mucins is the sialyl-Tn antigen, which contains a sialic acid residue linked to C-6 of N-acetylgalactosamine (Table 9.1). O-Acetylation of the sialic acid residue masks the recognition of sialyl-Tn by anti-sialyl-Tn antibodies. No other sugars are known to be added to the sialyl-Tn antigen.
TABLE 9.2
Glycosyltransferases specific for mucin O-GalNAc glycans
There are eight O-GalNAc glycan core structures (Table 9.1), most of which may be further substituted by other sugars. N-Acetylgalactosamine is converted to core 1 (Galβ1-3GalNAc-) by a core 1 β1-3 galactosyltransferase termed T synthase or C1GalT-1 (Figure 9.3). This activity is present in most cell types. However, to be exported from the endoplasmic reticulum in vertebrates, T synthase requires a specific molecular chaperone called Cosmc. Cosmc is an ER protein that appears to bind specifically to T synthase and ensures its full activity in the Golgi. Lack of core 1 synthesis can be due to either defective T synthase or the absence of functional Cosmc chaperone. The result is high expression of Tn and sialyl-Tn antigens. Immunoglobulin A nephropathy in humans is associated with low expression of Cosmc, and a mutation in the Cosmc gene gives rise to a condition called the Tn syndrome.
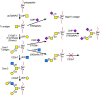
FIGURE 9.3
Biosynthesis of core 1 and 2 O-GalNAc glycans. Shown is the biosynthesis of some extended core 1 and core 2 O-GalNAc glycans. The linkage of N-acetylgalactosamine to serine or threonine to form the Tn antigen, catalyzed by polypeptide-N-acetylgalactosaminyltransferases (more...)
In many serum glycoproteins and mucins, the T antigen is substituted by sialic acid at C-3 of galactose and at C-6 of N-acetylgalactosamine (Figure 9.3). These substitutions add a negative charge to the O-GalNAc glycan. They also prevent other modifications of core 1. The cell surfaces of many leukemia and tumor cells contain large numbers of sialylated core 1 O-GalNAc glycans. On rare occasions, core 1 remains unsubstituted, leaving the T antigen exposed, for example, in cancer and inflammatory bowel disease. In these cases, it is likely that there is an abnormality in either the sialylation of core 1 or further extension and branching of core 1.
Core 2 mucin O-glycans are branched core 1 structures that are produced in many tissues, including the intestinal mucosa. The synthesis of core 2 O-GalNAc glycans is regulated during activation of lymphocytes, cytokine stimulation, and embryonic development. Leukemia and cancer cells and other diseased tissues have abnormal amounts of core 2 O-GalNAc glycans. The synthesis of core 2 O-GalNAc glycans has been correlated with tumor progression. Because of their branched nature, core 2 O-glycans can block the exposure of mucin peptide epitopes. The enzyme responsible for core 2 synthesis is core 2 β1-6 N-acetylglucosaminyltransferase or C2GnT (Figure 9.3). At least three genes encode this subfamily (C2GnT-1 to -3) of a larger family of β1-6 N-acetylglucosaminyltransferases that catalyze the synthesis of GlcNAcβ1-6-linked branches. There are two major types of core 2 β1-6 N-acetylglucosaminyltransferases. The L type (leukocyte type, C2GnT-1 and -3) synthesizes only the core 2 structure, whereas the M type (mucin type, C2GnT-2) is also involved in the synthesis of core 4 and other GlcNAcβ1-6-linked branches. The L enzyme is active in many tissues and cell types, but the M enzyme is found only in mucin-secreting cell types. The expression and activity of both the L and M enzymes are altered in certain tumors.
The synthesis of core 3 O-GalNAc glycans appears to be restricted mostly to mucous epithelia from the gastrointestinal and respiratory tracts and the salivary glands. The enzyme responsible is core 3 β1-3 N-acetylglucosaminetransferase (C3GnT; Figure 9.4). Although in vitro assays of this enzyme suggest that it is relatively inefficient, the enzyme must act efficiently in vivo in colonic goblet or mucous cells because secreted colonic mucins mainly carry core 3 and few core 1, core 2, or core 4 O-GalNAc glycans. The activity of C3GnT is especially low in colonic tumors and virtually absent from tumor cells in culture. The synthesis of core 4 by the M-type β1-6 N-acetylglucosaminetransferase (C2GnT-2; see above) requires the prior synthesis of a core 3 O-GalNAc glycan (Figure 9.4). The glycosyltransferase synthesizing core 5 must exist in colonic tissues and in colonic adenocarcinoma because these tissues produce mucin with core 5. The enzymes synthesizing cores 5 to 8 remain to be characterized.
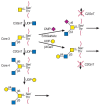
FIGURE 9.4
Biosynthesis of core 3 and core 4 O-GalNAc glycans. N-Acetylgalactosamine is substituted by a β1-3 N-acetylglucosamine residue to form core 3. This reaction is catalyzed by core 3 β1-3 N-acetyl-glucosaminyltransferase (C3GnT), which is (more...)
Synthesis of Complex O-GalNAc Glycans
The elongation of the galactose residue of core 1 and core 2 O-GalNAc glycans is catalyzed by a β1-3 N-acetylglucosaminyltransferase (Table 9.2) that is specific for O-GalNAc glycans. These O-glycans can also be extended by N-acetylglucosaminyltransferases and galactosyltransferases to form repeated GlcNAcβ1-3Galβ1-4 (poly-N-acetyllactosamine) sequences that represent the little i antigen. Less common elongation reactions are the formation of GalNAcβ1-4GlcNAc- (LacdiNAc) and Galβ1-3GlcNAc- sequences. Linear poly-N-acetyllactosamine units can be branched by members of the β1-6 N-acetylglucosaminyltransferase family, resulting in the large I antigen. Some of these branching reactions are common to other O- and N-glycans and glycolipids (see Chapter 13).
The ABO and other glycan-based blood groups as well as sialic acids, fucose, and sulfate are common terminal structures in mucins as in other glycoconjugates. The families of glycosyltransferases that catalyze the addition of these terminal structures are described in more detail in Chapters 13 and 14. In contrast to N-glycans, O-GalNAc glycans do not have NeuAcα2-6Gal linkages, although the NeuAcα2-6GalNAc moiety is common, for example, in the sialyl-Tn antigen and in elongated core 1 and 3 structures. Thus, in most mammalian mucin-producing cells, α2-6 sialyltransferases act on N-acetylgalactosamine, and α2-3 sialyltransferases act on galactose. Some of the sialyltransferases and sulfotransferases prefer O-GalNAc glycans as their substrate (Table 9.2), but many of these enzymes have an overlapping specificity and also act on N-glycan structures as acceptor substrates.
Control of O-GalNAc Glycan Synthesis
The acceptor specificities of glycosyltransferases and sulfotransferases are the main factors determining the structures of O-GalNAc glycans found in mucins, and these specificities restrict the high number of theoretically possible O-glycans to “only” a few hundred. The specificities also direct the pathways that are feasible. For example, the sialylated core 1 structure NeuAcα2-6(Galβ1-3)GalNAc- can be synthesized only by adding a sialic acid residue to core 1, but not by adding a galactose residue to the sialyl-Tn antigen, because the sialic acid prevents the action of core 1 β1-3 galactosyltransferase. The disialylated core 1 structure NeuAcα2-3Galβ1-3(NeuAcα2-6)GalNAc- is synthesized by the addition of the α2-3-linked sialic acid first to core 1, followed by the addition of the α2-6-linked sialic acid to N-acetylgalactosamine. These enzymes can therefore recognize at least two sugar residues and are often (but not always) blocked by the presence of sialic acid. Expression of sialyltransferases is consequently often associated with shorter O-GalNAc glycans. When a core 1 O-GalNAc glycan is sialylated to form NeuAcα2-3Galβ1-3GalNAc-, core 2 cannot be synthesized because the L-type core 2 β1-6 N-acetylglucosaminyltransferase requires an unsubstituted core 1 structure as the acceptor substrate. Detailed specificity studies suggest that the L-type core 2 β1-6 N-acetylglucosaminyltransferase recognizes unsubstituted C-4 and C-6 hydroxyl groups of both the galactose and N-acetylgalactosamine residues of a core 1 O-GalNAc glycan.
Another important control factor is that the relative activities of the glycosyltransferases determine the relative amounts of O-GalNAc glycans in mucins. Because of partially overlapping localization in the cis- and medial-Golgi compartments of breast cancer cells, core 2 β1-6 N-acetylglucosaminyltransferase (C2GnT-1) competes with α2-3 sialyltransferase for the common core 1 substrate (Figure 9.3). Therefore, the relative activities of these two enzymes determine the nature of the O-GalNAc glycans synthesized and the antigenic properties of the cell surface. Depending on which activity predominates, the cell-surface mucin MUC1 may be highly sialylated and its O-glycans relatively small or its O-glycans may be large and more complex due to the presence of extended, branched core 2 O-GalNAc glycans. In MUC1 from breast cancer cells, the presence of these sialylated small O-glycans leads to the exposure of underlying mucin peptide epitopes.
In vitro studies have shown that the activities of transferases are controlled by many factors such as metal ions and membrane components. Only two activities involved in O-glycan biosynthesis have been found to be regulated by the presence of specific binding proteins. The first is β1-4 galactosyltransferase 1 (β4GalT-1), which can bind to α-lactalbumin in mammary gland to change its preferred acceptor substrate from N-acetylglucosamine to glucose, thereby forming lactose. The second enzyme is core 1 β1-3 galactosyltransferase (C1GalT-1), which requires the coexpression of the molecular chaperone Cosmc.
The first step of O-GalNAc glycosylation is clearly regulated by the amino acid sequence of the acceptor substrate. Although individual ppGalNAcTs may prefer certain sites in the acceptor substrate, a combination of enzymes expressed in the same cell compartment ensures efficient mucin O-glycosylation. Several enzymes of the O-glycosylation pathway recognize their substrates in the context of the underlying peptide. The sequence of the peptide moiety of the acceptor substrate may direct the synthesis of cores 1, 2, and 3 as well as the extension of N-acetylglucosamine-terminating core structures by galactose (Figures 9.3 and 9.4).
It appears that glycosyltransferases are arranged in a diffuse “assembly line” within the Golgi compartments. This intracellular localization of enzymes allows them to act on mucin substrates in a specific sequence. Thus, early-acting enzymes synthesize the substrates for the next reactions. Because several enzymes can often use the same substrate, and there is extensive overlap of both localization and specificities, large numbers of different O-glycan structures are synthesized, leading to the heterogeneity seen in mucins. The concentrations of nucleotide sugar and sulfate-donor substrates within the Golgi and the rate of substrate transport throughout the Golgi are additional important control factors (see Chapter 4). The sensitive and complex balance of these various factors appears to be altered upon cell differentiation, cytokine stimulation, and in disease states.
FUNCTIONS OF O-GalNAc GLYCANS
O-GalNAc glycosylation is probably an essential process because all mammalian cell types studied to date express ppGalNAcTs. However, when the ppGalNAcT-1 gene was deleted in mice the animals appeared to be unaffected, possibly due to the fact that another ppGalNAcT replaces the function of ppGalNAcT-1. In the secreted mucins of the respiratory, gastrointestinal, and genitourinary tracts, as well as those of the eyes, the O-GalNAc glycans of mucous glycoproteins are essential for their ability to hydrate and protect the underlying epithelium. Mucins also trap bacteria via specific receptor sites within the O-glycans of the mucin. Some sugar residues or their modifications can mask underlying antigens or receptors. For example, O-acetyl groups on the sialic acid residue of the sialyl-Tn antigen prevent recognition by anti-sialyl-Tn antibodies. Gut bacteria enzymatically remove this blocking group. Bacteria can cleave sulfate with sulfatases or terminal sugars with glycosidases. Because the O-glycans are hydrophilic and usually negatively charged, they promote binding of water and salts and are major contributors to the viscosity and adhesiveness of mucus, which forms a physical barrier between lumen and epithelium. The removal of microbes and particles trapped in mucus is an important physiological process. However, in diseases such as cystic fibrosis, the abnormally high viscosity of the mucus leads to obstruction and life-threatening tissue malfunction.
O-GalNAc glycans, especially in the highly glycosylated mucins, have a significant effect on the conformation of the attached protein. Depending on the size and bulkiness of O-glycans, underlying peptide epitopes can be variably recognized by antibodies. O-glycosylation of mucins provides almost complete protection from protease degradation, and it is possible that the sparse O-glycosylation of some secreted glycoproteins, such as the single O-GalNAc glycan on interleukin-2, has a similar protective role.
Cell lines defective in specific O-glycosylation pathways can be used as models to study the role of O-glycosylation (see Chapter 46). For example, due to a defect in UDP-Glc-4-epimerase that reversibly converts UDP-GlcNAc to UDP-GalNAc, the ldlD cell line does not produce GalNAc-Ser/Thr linkages unless N-acetylgalactosamine is added to the cell medium. The addition of galactose as well as N-acetylgalactosamine results in complete O-GalNAc glycans. This model has been used to demonstrate that O-glycans of cell-surface receptors may regulate receptor stability and expression levels.
O-GalNAc glycans change during lymphocyte activation and are abnormal in leukemic cells where an increase in core 2 and a decrease in core 1 O-glycans are often seen. The ligands for selectin-mediated interactions between endothelial cells and leukocytes are commonly based upon sialyl Lewisx epitopes attached to core 2 O-GalNAc glycans. This type of selectin–glycan interaction is important for the attachment of leukocytes to the capillary endothelium during homing of lymphocytes or the extravasation of leukocytes during the inflammatory response (see Chapter 31). Removal of core 2 O-GalNAc glycans by eliminating the C2GnT-1 gene in mice results in a severe deficiency in the immune system, particularly in the selectin-binding capability of leukocytes (see Chapter 50).
Cancer cells often express sialyl Lewisx epitopes and may thus use the selectin-binding properties of their cell surface as a mechanism to invade tissues. The critical role of O-glycans in this process has been studied in cell lines treated with the O-glycan extension inhibitor benzyl-α-GalNAc (see Chapter 50). This compound acts as a competitive substrate for the synthesis of core 1, core 2, core 3, and core 4 O-glycans in cells and thus causes a reduction in the synthesis of complex O-GalNAc glycans. As a consequence, mucins carry a higher number of unmodified N-acetylgalactosamine residues and shorter O-GalNAc glycans. Inhibitor-treated cancer cells lose the ability to bind to E-selectin and endothelial cells in vitro.
Reproductive tissues produce mucins and O-glycosylated glycoproteins that may have important roles in fertilization. Specific terminal O-glycan structures have been shown to form the ligands for sperm-egg interactions in several species (see Chapter 25).
The changes of O-glycans commonly observed in diseases can be due to the actions of cytokines or growth factors that affect cell growth, differentiation, and cell death and alter the expression of glycosyltransferase genes. Although these glycosylation changes may be considered a “side effect” of a pathological condition, they can also significantly contribute to the ultimate pathology and the course of disease. In cancer, the biosynthesis of O-GalNAc glycans is often abnormal due to either decreased or increased expression and activities of specific glycosyltransferases. An altered cell-surface glycocalyx may then affect the biology and survival of the cancer cell.
FURTHER READING
- Tabak LA. In defense of the oral cavity: Structure, biosynthesis, and function of salivary mucins. Annu Rev Physiol. 1995;57:547–564. [PubMed: 7778877]
- van Klinken BJW, Dekker J, Büller HA, Einerhand AWC. Mucin gene structure and expression: Protection vs. adhesion. Am J Physiol. 1995;269:G613–627. [PubMed: 7491952]
- Hansen JE, Lund O, Nielsen JO, Brunak S. O-GLYCBASE: A revised database of O-glycosylated proteins. Nucleic Acids Res. 1996;24:248–252. [PMC free article: PMC145605] [PubMed: 8594592]
- Brockhausen I. Pathways of O-glycan biosynthesis in cancer cells. Biochim. Biophys. Acta. 1999;1473:67–95. [PubMed: 10580130]
- Fukuda M. Roles of mucin-type O-glycans in cell adhesion. Biochim. Biophys. Acta. 2002;1573:394–405. [PubMed: 12417424]
- Brockhausen I. Sulphotransferases acting on mucin-type oligosaccharides. Biochem Soc Trans. 2003;31:318–325. [PubMed: 12653628]
- Lowe JB, Marth J. A genetic approach to mammalian glycan function. Annu Rev Biochem. 2003;72:643–691. [PubMed: 12676797]
- ten Hagen KG, Fritz TA, Tabak LA. All in the family: The UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferases. Glycobiology. 2003;13:1R–16R. [PubMed: 12634319]
- Hollingsworth MA, Swanson BJ. Mucins in cancer: Protection and control of the cell surface. Nat. Rev. Cancer. 2004;4:45–60. [PubMed: 14681689]
- Robbe C, Capon C, Coddeville B, Michalski JC. Structural diversity and specific distribution of O-glycans in normal human mucins along the intestinal tract. Biochem J. 2004;384:307–316. [PMC free article: PMC1134114] [PubMed: 15361072]
- Wandall HH, Irazoqui F, Tarp MA, Bennett EP, Mandel U, Takeuchi H, Kato K, Irimura T, Suryanarayanan G, Hollingsworth MA, Clausen H. The lectin domains of polypeptide GalNAc-transferases exhibit carbohydrate-binding specificity for GalNAc: Lectin binding to GalNAc-glycopeptide substrates is required for high density GalNAc-O-glycosylation. Glycobiology. 2007;17:374–387. [PubMed: 17215257]
- Hattrup CL, Gendler SJ. Structure and function of the cell surface (tethered) mucins. Annu Rev Physiol. 2008;70:431–457. [PubMed: 17850209]
Publication Details
Author Information and Affiliations
Authors
Inka Brockhausen, Harry Schachter, and Pamela Stanley.Copyright
Publisher
Cold Spring Harbor Laboratory Press, Cold Spring Harbor (NY)
NLM Citation
Brockhausen I, Schachter H, Stanley P. O-GalNAc Glycans. In: Varki A, Cummings RD, Esko JD, et al., editors. Essentials of Glycobiology. 2nd edition. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2009. Chapter 9.