Except where otherwise noted, this work is distributed under the terms of a Creative Commons Attribution-NonCommercial- NoDerivatives 4.0 International licence (CC BY-NC-ND), a copy of which is available at http://creativecommons.org/licenses/by-nc-nd/4.0/
3.1. Literature Selection
A total of 1,160 citations were reviewed and eight articles were selected for inclusion in this review. There were two systematic reviews18,19 and six RCTs that investigated the use of incretin agents in patients who were inadequately controlled with an insulin-containing treatment regimen. Neither systematic review performed a meta-analysis; therefore, the individual clinical trials are summarized in this review.
3.2. Study Characteristics
The characteristics of the included RCTs are summarized in Table 3. The six RCTs included five placebo-controlled trials investigating the use of four DPP-4 inhibitors (sitagliptin, saxagliptin, alogliptin, and vildagliptin)20–23 and one GLP-1 analogue (exenatide).24 There was one RCT that compared sitagliptin against an increased dosage of insulin.25 The studies were similar in duration, ranging from 24 to 30 weeks. All of the placebo-controlled trials were double-blind and the active comparison of sitagliptin versus an increase in insulin dose was open-label. Sample sizes ranged from 259 to 641 in the placebo-controlled trials. The active comparison had the smallest sample size with 140 patients.
Table 3
Summary of Trial and Patient Characteristics.
The trial by Buse et al. (2011)24 was limited to participants who had been receiving a basal insulin. All of the other studies included a heterogeneous population of patients using basal, biphasic, and basal/bolus insulin regimens. For trials reporting the total insulin dosage at baseline (i.e., regardless of treatment regimen), the dosage ranged from 35 to 82 units per day (Table 4).
Table 4
Insulin Usage at Baseline.
3.3. Addition of an Incretin versus Placebo
Five RCTs20–24 were identified that compared the addition of a DPP-4 inhibitor to an unchanged insulin regimen20–23 or a GLP-1 analogue to an insulin regimen adjusted using a standardized algorithm.24 The results for changes in A1C, changes in body weight, and hypoglycemia are summarized in Figure 2, and detailed results for each end point are summarized in Table 5. Changes from baseline in A1C with the DPP-4 inhibitor groups ranged from −0.5% to −0.7%. All of the DPP-4 inhibitors demonstrated a statistically significant improvement in A1C relative to placebo (range −0.3% to −0.6%). The GLP-1 analogue exenatide also demonstrated a significant improvement compared with placebo (−0.7%). All participants in the exenatide trial received “optimized insulin glargine,” which consisted of a standardized titration algorithm based on the Treat-To-Target trial26 in addition to the randomized treatment; therefore, both the exenatide and placebo groups demonstrated large improvements from baseline (−1.7% and −1.0%, respectively).
Table 5
Detailed Results for Hemoglobin A1C, Body Weight, and Hypoglycemia.
The addition of a DPP-4 inhibitor did not result in significant changes in body weight relative to placebo, and the GLP-analogue resulted in statistically significant weight loss (−2.7 kg). Trials involving saxagliptin, vildagliptin, alogliptin, and exenatide demonstrated no significant increase in risk of overall hypoglycemia relative to placebo; however, a single trial involving sitagliptin showed a significantly increased risk of hypoglycemia compared with placebo (odds ratio [95% CI] = 2.16 [1.30 to 3.59]). Events of severe hypoglycemia were rare in the included trials, making it difficult to conduct meaningful comparisons.
Total adverse events, serious adverse events, and withdrawals due to adverse events are summarized in Table 6. A larger proportion of sitagliptin-treated patients reported at least one adverse event compared with placebo (52% versus 43%).21 With the exception of hypoglycemia (15% versus 8%), there were no specific categories of adverse events responsible for the increase in the sitagliptin group.27 Exenatide was associated with a greater proportion of patients with at least one adverse event (79% versus 70%), largely due to an increase in gastrointestinal adverse events (i.e., nausea, vomiting, diarrhea, and constipation).24 Sitagliptin was the only treatment associated with a larger proportion of patients that reported at least one serious adverse event relative to placebo (6.2% versus 3.5%). The serious adverse events reported in this trial represented a wide range of categories, with few events occurring in a single category. Two DPP-4 inhibitors, vildagliptin (50 mg twice daily) and sitagliptin (100 mg once a day), were associated with more withdrawals due to adverse events than placebo (6% versus 0.7% and 3% versus 1%, respectively). Exenatide (10 mcg twice daily) was also associated with more withdrawals due to adverse events than placebo (9% versus 1%); however, the reasons for withdrawal were not reported for this trial.
Table 6
Summary of Adverse Events.
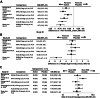
Figure 1
Results for Hemoglobin A1C (%) (A), Body Weight (kg) (B), Hypoglycemia (C).
3.4. Addition of DPP-4 Inhibitor versus Intensified Insulin
Only a single RCT was identified that compared the addition of an incretin against an intensified insulin regimen for patients who were inadequately controlled on their existing insulin therapy. Hong et al., 2012 compared the addition of sitagliptin (100 mg once daily) against an increase in insulin dosage in 140 Korean patients.25 Patients allocated to the increased insulin dosage group were instructed to increase their daily dosage of insulin as follows:
- ≥ 10% at randomization
- ≥ 10% at the 12-week follow-up, if their A1C was not within the target level (i.e., ≤ 7.0%)
- an additional 2 U every week, based on the self-monitoring of their blood glucose.
Eligible patients were required to have an A1C of between 7.5% and 11.0%, and to have received insulin injections for at least three months at a dose of at least 10 U/day for a minimum of four weeks. Forty-eight per cent of participants were using insulin glargine only, 20% were using insulin glargine and a rapid-acting insulin, and 31% were insulin NPH and regular insulin.
Compared with an increased insulin dosage, the addition of sitagliptin (100 mg once daily) was associated with statistically significant improvements in A1C (MD [95% CI] = −0.42% (−0.91 to −0.11]) and body weight (MD [95% CI] = −1.7 kg [−2.5 to −0.5]) (Table 7). The mean dosage of insulin at baseline was numerically greater in the sitagliptin group (39.6 ± 19.1) compared with the intensified insulin group (35.4 ± 16.3). After 24 weeks, the mean insulin dosage in the intensified insulin group had increased by 10.1 U per day and had decreased in the sitagliptin group by 2.5 U per day (P < 0.05). There was also a lower rate of overall and severe hypoglycemia with sitagliptin compared with the increased insulin dosage (both P < 0.01).
Table 7
Summary of Results for Intensified Insulin Versus Addition of an Incretin.
- RESULTS - Combination Use of Insulin and Incretins in Type 2 DiabetesRESULTS - Combination Use of Insulin and Incretins in Type 2 Diabetes
Your browsing activity is empty.
Activity recording is turned off.
See more...
