NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Maher AR, Miake-Lye IM, Beroes JM, et al. Treatment of Metastatic Non-Small Cell Lung Cancer: A Systematic Review of Comparative Effectiveness and Cost-Effectiveness [Internet]. Washington (DC): Department of Veterans Affairs (US); 2012 Oct.

Treatment of Metastatic Non-Small Cell Lung Cancer: A Systematic Review of Comparative Effectiveness and Cost-Effectiveness [Internet].
Show detailsLITERATURE FLOW
From the search for systematic reviews and cost-effectiveness analyses, we received 736 titles. We narrowed the scope to systematic reviews published after 2010 during the title screen, and identified 88 potential citations for inclusion. Full articles were retrieved and screened for 55 articles. Of these, 22 were cost-effectiveness analyses relevant to Key Question #4. The remaining 33 included articles were systematic reviews of first line, second line, or maintenance therapies for advanced, stage III, or stage IV NSCLC. These were then distributed among the relevant Key Questions: 22 were relevant to Key Quest #1, 6 were relevant to Key Question #2, and 3 were relevant to Key Question #3. One additional systematic review was identified by our peer reviewers relevant to Key Question #3.
From the search for trials, we received 820 titles, and after a title screen, there were 158 citations identified for potential inclusion. From these citations, 120 articles were identified for full article screening. Of the 60 meeting the final inclusion criteria, there were 43 articles relevant to Key Question #1, 14 relevant to Key Question #2, and three relevant to Key Question #3. An additional seven trials, plus one update on a trial already included in the review, were identified by our peer reviewers and distributed as follows: four were relevant to Key Question #1, two were relevant to Key Question #2, and one trial plus the update were relevant to Key Question #3.
Figure 2 details the review process and the number of references related to each of the key questions.
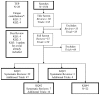
Figure 2
Literature Flow. * Most TEP recommendations were already included in either a systematic review or the newly identified trials in from our search. Only the six noted above were not included from another source.
KEY QUESTION #1. For patients with metastatic non-small cell lung cancer (NSCLC) what is the comparative effectiveness of the different recommended (e.g. NCCN guidelines) first line chemotherapy regimens?
We identified 22 systematic reviews or meta-analyses published since 2010 that addressed NSCLC first-line therapy. 2, 5–25 Most of these reviews were narrowly targeted at specific questions. The most comprehensive of these reviews was published by the Cancer Care Ontario Program in Evidence-Based Care (CCO) in 2010 by Goffin and colleagues.2 This review scored a “yes” on 9 out of 11 of the AMSTAR criteria,26 searched multiple databases through 2007, and included 10 systematic reviews and 46 randomized trials. The CCO first searched for relevant systematic reviews, and then added new trials that were not included in those reviews. In our review we follow the same format: we summarize the literature included in the CCO review and then discuss additional trials, systematic reviews, and meta-analyses not included in this main review. The CCO review had 13 key questions, which we incorporate into this report as key sub-questions:
- Does doublet chemotherapy consisting of a platinum agent plus a new agent improve outcomes compared with doublets using older agents?
- Does doublet chemotherapy consisting of a platinum agent plus a new agent improve outcomes compared with a new single agent alone or to a platinum agent alone?
- Which doublet chemotherapy regimen consisting of a platinum agent plus a new agent is most effective in improving clinical outcomes?
- Does doublet chemotherapy consisting of a platinum agent plus a new agent improve outcomes compared with nonplatinum combination chemotherapy including a new agent?
- Are new doublets containing cisplatin more effective than doublets containing carboplatin?
- Does triplet chemotherapy consisting of a platinum agent plus a new agent improve clinical outcomes compared with doublet chemotherapy consisting of a platinum agent plus a new agent?
- Does the addition of targeted therapy to doublet chemotherapy consisting of a platinum agent plus a new agent improve outcomes compared with doublet chemotherapy consisting of a platinum agent and a new agent?
- Is a single new agent superior to best supportive case?
- Is a single new agent superior to single-agent or doublet therapy including older agents?
- Which single new agent is most effective?
- What is the optimal administration, duration, and timing of chemotherapy for advanced nsclc?
- Is a doublet regimen better than a single agent for the elderly population? This was modified by our tep from the original question in the cco review: which single or doublet regimen including a new agent is superior for the elderly population?
- Toxicity of first-line systemic chemotherapy regimens
The overall conclusion of the review by the CCO was that “data continue to support the use of a platinum agent plus a new agent as the reference standard.”2 They also concluded that the combination of a platinum agent and a new agent had “a slight advantage over pairs of new agents, although at the cost of greater toxicity.” Cisplatin combinations were judged to have a “slight” advantage over carboplatin combinations, although carboplatin combinations had a more favorable toxicity profile. Finally, the CCO concluded that “as differences between regimens are small, toxicities and patient preference should help guide treatment choice.” Data on the new targeted therapies were just emerging at the time of the CCO review. A subsequent addendum limited to pemetrexed concluded that the data “are sufficiently compelling to recommend that pemetrexed should not be used in the treatment of squamous cell carcinomas for first line treatment.”
Key Sub-question 1.1. Does doublet chemotherapy consisting of a platinum agent plus a new agent improve outcomes compared with doublets using older agents?
The CCO review included one meta-analysis by Baggstrom and colleagues,27 which itself consisted of six RCTs, and five additional RCTs.28–32 The meta-analysis of six trials of doublet regimens found results favoring chemotherapy regimens containing platinum and a new agent, with a 1-year survival rate risk difference of 6% (95% CI: 2–10%).27 The five additional trials comparing old to new doublet therapies, only one found superior survival with a newer agent, that being docetaxel plus cisplatin over vindesine plus cisplatin.29
New Literature Since 2007 Relevant to this Sub-question
Our update search identified seven new RCTs relevant to this sub-question.33–39 Details of these studies, plus those from the CCO review, are in Table F1. Among these seven new studies, none reported significant benefit in outcomes when comparing the newer, to older, doublets. One study actually found worse outcomes with the newer agent, pemetrexed, versus the older agent, etoposide.36 Overall survival was 8.1 months with pemetrexed, versus 10.6 months with etoposide (HR=1.56, 95% CI: 1.27–1.92; log-rank P<.01) and the objective response rate was 31% vs. 52% (P<.001).
Table F1
New Doublets vs. Older Doublets (adapted from Goffin).
Summary of Key Sub-question 1.1
New trials continue to support the conclusion by the CCO that any differences in survival between platinum-based doublets are modest (GRADE=High).
Key Sub-question 1.2. Does doublet chemotherapy consisting of a platinum agent plus a new agent improve outcomes compared with a new single agent alone or to a platinum agent alone?
The review by the CCO included a meta-analysis by Hotta, which itself included eight trials of 2,374 patients.40 In these trials, a doublet of platinum plus a new agent versus a new agent alone found an improvement in survival for platinum-based doublets (HR=0.87; 95% CI: 0.80–0.94; p<0.001) and a higher response rate (OR=2.32, 95% CI: 1.68–3.20) compared with new single agent therapy.
New Literature Since 2007 Relevant to this Sub-question
We identified one new published trial relevant to this Key Sub-question. Reynolds and colleagues randomized 170 patients with performance status of two to either gemcitabine or gemcitabine plus carboplatin.41 The median survival was 6.7 (4.9–10.0) months in the gemcitabine plus carboplatin group and 5.1 (3.9–6.3) months for gemcitabine. This difference was not statistically significant (p=0.14). Overall survival at one year was 31.3 percent in the doublet therapy group and 21.2 percent in the gemcitabine alone therapy group (statistical testing not performed). There was a statistically significant higher rate of confirmed response in the gemcitabine plus carboplatin (21.1%) group compared to gemcitabine alone (6.3%, p=.01). In addition, patients’ tumors were evaluated for RRM1 and ERCC1 protein levels and found that these were significantly and inversely correlated with disease response.
During peer review, we were directed to a recently presented abstract of a second relevant study42. Patients with advanced NSCLC and a performance status of 2 were randomized to receive pemetrexed or carboplatin plus pemetrexed. During the trial, eligibility was later restriced to only patients with nonsquamous histology. Overall survival among 205 patients studied was 9.1 months versus 5.6 months, favoring doublet therapy (p=0.001). We await full publication of these results, which provide additional support favoring doublet therapy, even in patients with a poor performance status.
Summary of Key Sub-question 1.2
This result continues to support the conclusions by the CCO that doublet chemotherapy including a platinum agent has a higher survival rate and a higher response rate than a single agent (GRADE=High).
Key Sub-question 1.3. Which doublet chemotherapy regimen consisting of a platinum agent plus a new agent is most effective in improving clinical outcomes?
The review by the CCO identified two meta-analyses43, 44 and nine studies,32, 45–52 three of which were included in one of the two meta-analyses.45, 46, 50 The first meta-analysis, by Le Chevalier et al, examined the efficacy of gemcitabine plus platinum combinations versus any other platinum-based regimen.43 Out of six trials, there was a trend toward superior survival with gemcitabine-based regimens and improved progression-free survival (HR= 0.89; 95% CI: 0.82–0.96). The second meta-analysis, by Douillard et al., compared seven trials of docetaxel-containing regimens with vinca-alkaloid regimens.44 Docetaxel led to greater overall survival (HR=0.89; 95% CI: 0.82–0.96; P=0.004). In the nine additional studies, median survival ranged from 6.4–14.0 months, without consistent differences between arms.
New Literature Since 2007 Relevant to this Sub-question
Our search identified nine additional studies53–61 and two subsequent meta-analyses62, 63 relevant to this Key Sub-question. These new results did not alter the conclusions reached by the CCO. The combinations studied included gemcitabine, paclitaxel, paclitaxel poliglumex (CT-2013 PPX), S-1, pemetrexed, vinorelbine, and docetaxel. Details of these studies, as well as the studies contained in the CCO review, are in Table F2. No particular combination was shown to be significantly superior in outcomes. Median survival ranged from 7.0 to 15.2 months. Of note, the study comparing paclitaxel plus carboplatin vs. gemcitabine plus oxaliptin had to be terminated after adverse events exceeded the safety threshold set by the data safety and monitoring board.60
Table F2
Comparison of Doublets of New Agents in Combination with Platinum Analogues (adapted from Goffin).
We also identified one clinical trial comparing doublet chemotherapy regimens, but neither included a platinum agent. This study compared paclitaxel and gemcitabine to paclitaxel and vinorelbine in 39+ patients with stage IIIB or IV NSCLC. There was no difference in median overall survival (11.1 months vs. 8.6 months, p=0.14); the group treated with vinorelbine had more grade 3/4 toxicities.25
Summary of Key Sub-question 1.3
New trials continue to support the conclusion by the CCO that any differences in outcomes between doublet therapies with platinum-based agents are modest (GRADE=High).
Key Sub-question 1.4. Does doublet chemotherapy consisting of a platinum agent plus a new agent improve outcomes compared with nonplatinum combination chemotherapy including a new agent?
The review by the CCO identified two meta-analyses and four additional relevant RCTs.65–70 The first meta-analysis, by Pujol et al., analyzed 11 RCTs comparing platinum-based new doublets or a combination of new nonplatinum agents, in 4602 patients.67 There was a 2.9% absolute reduction in the risk of death at 1-year with the platinum-based regimens (OR=0.88; 95% CI: 0.78–0.99, P=0.044). The other meta-analysis, by D’Addario et al, examined 14 trials and found no survival benefit with platinum-based agents over nonplatinum chemotherapy regimens (OR, 1.11; 95% CI: 0.096–1.28; P=0.17).65 In the four additional studies, median survival ranged from 7.6 to 13.8 months.66, 68–70 One of these studies, Tan et al, found improvement in the median survival in those patients receiving gemcitabine-vinorelbine (11.5 months) compared to those receiving vinorelbine-carboplatin (8.6 months, P=0.01).69
New Literature Since 2007 Relevant to this Sub-question
The additional three trials we identified did not find a significant difference between agents and did not alter the conclusions reached by the CCO.59, 71, 72 Details of these three and the four RCTs identified by the CCO are presented in Table F3. In one of these trials, platinum-based regimens of gemcitabine or paclitaxel with carboplatin led to a median survival of 7.9 (7.1–9.2, P=0.693) and 8.7 (7.7–9.9) months, compared to gemcitabine with paclitaxel which led to 8.5 (7.6–10.0) months.59 In another trial, the combination of gemcitabine with paclitaxel led to a median survival of 9.97 (8.74–12.0) months compared to 10.49 months with gemcitabine and carboplatin (9.04–11.94).72 Though the final study appeared to favor gemcitabine and epirubicin with a median survival of 21.5 months versus 13.2 months for gemcitabine-cisplatin, the confidence intervals were wide (9.4–33.6 and 10.4–16.0) and the number of patients small (80 and 85).71
Table F3
New Platinum-Based Doublets vs. New Nonplatinum-Based Doublets (adapted from Goffin).
Summary of Key Sub-question 1.4
New trials continue to support the conclusion by the CCO that doublet chemotherapy including a platinum agent probably has a slight advantage over nonplatinum doublets (GRADE=moderate).
Key Sub-question 1.5. Are new doublets containing cisplatin more effective than doublets containing carboplatin?
The review by the CCO identified three relevant meta-analyses.73–75 In the meta-analysis by Jiang et al., they found a higher overall response rate with cisplatin-based regimens when added to new drugs (RR 0.87;0.78–0.97, P=0.01) and when added to the same drug (RR 0.79; 0.70–0.89; P=0.0001) without a corresponding improvement in 1-year survival (RR 0.98; 0.90–1.07; P=0.66- new agent) (RR, 0.91;0.82–1.01; P=0.07).75 The meta-analysis by Hotta et al. found superior survival with platinum-based combinations containing cisplatin over those containing carboplatin plus the same agent (HR= 1.106; 95% CI: 1.005–1.218; P=0.039).74 It also found a greater objective response rate when using cisplatin over carboplatin (OR= 1.38; 95% CI: 1.14–1.67; P=0.001). In the meta-analysis by Ardizzoni et al, there was a greater mortality risk in those treated with carboplatin compared to those treated with cisplatin in patients with nonsquamous histology (HR=1.12; 95% CI: 1.01–1.23).73
Literature Relevant to this Sub-question Not Included in the CCO Review
We found one additional trial.64 It compared vinorelbine plus cisplatin and paclitaxel plus carboplatin finding no significant difference between the two regimens in median survival 8.1 (6.7–9.6) months for the cisplatin containing regimen compared to 8.6 (7.2–10.7) for the carboplatin containing regimen. The overall response rates were 28 and 25%, respectively. However, the difference in second agents in the doublet precludes reaching a conclusion about differences between carboplatin and cisplatin.
Summary of Key Sub-question 1.5
One new trial does not alter the conclusion by the CCO that cisplatin combinations may have a slight advantage over carboplatin combinations in terms of survival and response rate. However, carboplatin generally has a milder toxicity than cisplatin (GRADE=moderate).
Key Sub-question 1.6. Does triplet chemotherapy consisting of a platinum agent plus a new agent improve clinical outcomes compared with doublet chemotherapy consisting of a platinum agent plus a new agent?
The review by the CCO identified updated guidelines published by the ACCP in 2007 which contained a meta-analysis of 28 trials and 12 additional RCTs where the addition of a third chemotherapeutic agent failed to show superiority over conventional doublets.76 Though they found that response rates did improve, this was at the cost of substantially increased toxicity with the triplets, leading to a recommendation of the two-drug combination. In addition to the trials analyzed for the ACCP guidelines, the CCO found six additional trials investigating triplet regimens, none of which found a difference in median or 1-year survival with toxicity generally more frequent with the triplet regimens.77–82
New Literature Since 2007 Relevant to this Sub-question
We identified a systematic review not in the review by the CCO that was relevant to this question.24 Azim and colleagues identified six trials including 1,932 patients who received either third generation triplet therapy or standard doublet therapy. Pooled analyses showed that triplet therapy resulted in a statistically significant increase in response rate (pooled OR=1.33, 95% CI: 1.50–2.23), however overall survival was improved by less than six weeks (42.8 vs. 37.4 weeks) and this was not statistically significant. Patients receiving triplet therapy had statistically significantly more grade III/IV toxicities than patients receiving doublet therapy. The authors concluded that triplet therapy “is associated with higher tumor response rate at the expense of increased toxicity.”24
We found four new trials,83, 84 one of which was included in the review by Azim and colleagues.84 The first trial randomized 433 patients into 4 arms of treatment- gemcitabine-cisplatin, gemcitabine-vinorelbine, gemcitabine-ifosfamide-cisplatin or gemcitabine-ifosfamide-vinorelbine. 83 They found no benefit with the addition of ifosfamide to a gemcitabine based doublet. Overall survival was 10.4 (9.4–12.2) months for the doublet and 10.3 (9.2–11.8) months for the triplet and the response rate was 29% (23–35) for the doublet and 26% (21–33) for the triplet. The second trial had 433 patients randomly assigned to gemcitabine-vinorelbine, gemcitabine-paclitaxel, gemcitabine-vinorelbine-cisplatin, gemcitabine-paclitaxel-cisplatin.84 They found an increase in the response rate with triplets over doublets, 48% (42–54) vs. 35% (32–38). However, there was no benefit in overall survival with triplets having a median OS of 10.7 months and doublets having an overall survival of 10.5 months (P=0.379).
We also found one trial that compared a platinum-based triplet (paclitaxel, carboplatin, gemcitabine) versus a nonplatinum doublet (gemcitabine + vinorelbine). There were no statistically significant differences in overall survival between groups (10.7 vs. 10.3 months), and certain toxicities were greater in the triplet therapy group.85 Another “triplet vs. doublet” study compared a nonplatinum triplet (vinorelbine, gemcitabine, docetaxel) to carboplatin plus paclitaxel and showed no statistically significantly difference in overall survival (13.6 vs. 14.1 months), with toxicity greater in the platinum doublet treated patients.86
Summary of Key Sub-question 1.6
New trials continue to support the conclusion by the CCO that triplet cytotoxic therapy might have some slight advantages in terms of response rate but at an increased risk of toxicity (GRADE=high).
Key Sub-question 1.7. Does the addition of targeted therapy to doublet chemotherapy consisting of a platinum agent plus a new agent improve outcomes compared with doublet chemotherapy consisting of a platinum agent and a new agent?
The review by the CCO identified eight trials of adding targeted therapy to conventional chemotherapy. They found that aprinocarsen did not improve outcomes in patients with advanced disease. Four trials of the tyrosine kinase inhibitors (TKI) failed to show an improvement in survival or response. However erlotinib was shown to significantly increase survival when a particular subgroup of 116 patients, who had never smoked, received the drug. When combined with paclitaxel and carboplatin, this subgroup had a median survival of 22.5 months compared with 10.1 months for placebo patients (P=0.01). In another subgroup study of only 18 patients, the Tarceva Lung Cancer Investigation trial, erlotinib led to significantly longer progression-free survival compared to placebo (7.9 months versus 5.4 months, P=0.02). Two of the trials looked at addition of bevacizumab. The first added bevacizumab to paclitaxel and carboplatin in a population restricted to those with Eastern Cooperative Oncology Group (ECOG) performance scores of 0–1, no brain metastases, nonsquamous cell histology, no hemoptysis and no history of bleeding diathesis or coagulopathy. In these 878 patients median survival increased by 2 months in the bevacizumab group (12.3 versus 10.3 months, HR= 0.79, 95% CI: 0.67–0.92; P=0.003) in addition to an improvement in response rate (35 vs. 15%, P<0.001). The second trial added bevacizumab to gemcitabine and cisplatin and found some improvement in response and a very modest improvement in progression-free survival, but no difference in overall survival. There were two trials adding cetuximab. The first added cetuximab to cisplatin and vinorelbine in 1125 patients whose tumors were immunohistochemically positive for epidermal growth factor receptor (EGFR) expression. There was improved survival with cetuximab (HR=0.871; 95% CI: 0.762–0.996; median 11.3 vs. 10.1 months). Response rates were also improved with cetuximab (36 vs. 29%, P=0.01). The other trial, which added cetuximab to carboplatin and taxane, did not show improvement in survival, or progression-free survival.
New Literature Since 2007 Relevant to this Sub-question
Our update search identified an additional 10 trials relevant to this sub-question.87–96 Details of these trials, in addition to the eight contained in the review by the CCO, are presented in Table F4. Two of the new trials were of the agent PF-3512676. The first of these two trials, of 839 patients, did not find an improvement in OS or PFS and, in fact was halted based on efficacy, futility, and increased toxicity.92 The other study of this agent had similar findings in 828 patients and was therefore also halted.90 There was one study of endostar which enrolled 126 patients and found improvement in the overall response rate of 39.3% in the treatment group versus 23.0% in the control group (p=0.078).89 However, there was no significant difference in the overall survival (17.6 vs. 15.8 months, p=0.696). There were two reports of bevacizumab. One of these had 1043 patients and was included in the review by the CCO,95 and the other was the Asian subset of that population, consisting of 105 patients.93 In the overall sample, as noted above, an improvement in progression-free survival was found. However this did not translate into an improvement in overall survival, which was about 13 months in each group. 95 In the study of the Asian subset, however, bevacizumab at a dose of 7.5 mg/kg improved the overall survival in Asian patients compared to placebo (HR=0.46, 95% CI: 0.22–0.97).93 The other agents failed to show positive results. One trial of sorafenib found no clinical benefit and, in fact, was halted after an interim analysis found higher mortality in patients with squamous cell histology taking sorafenib than those in the placebo group.96 Another study that was halted examined cediranib.88 That trial was halted to review imbalances in assigned causes of death. A trial of cetuximab found a slight improvement in overall survival but this was not statistically significant, 9.69 months with cetuximab vs. 8.38 months with standard chemotherapy alone, (HR= 0.890, 95% CI: 0.754–1.051; p=.169).91 There were two trials of bexarotene with chemotherapy.87, 94 The first looked at 612 patients and found little difference in overall survival (8.5 vs. 9.2 months) or overall response (19.3 vs. 23.5%, p=.24).87 However, when they examined a subpopulation of those with hypertriglyceridemia, they found that those with hypertriglyceridemia had significantly longer median survival than control patients (12.4 vs. 9.2 months; log-rank, P=.014). The other trial was of 623 patients and found the same, no significant difference (OS: 8.7 vs. 9.9 months, P=.3, ORR: 16.7 vs. 24.4%, P=.0224) except in those with hypertriglyceridemia (OS 12.3 months).94 As both of these results were post-hoc subgroup analyses, their results should be interpreted with caution.
Table F4
Targeted Agents (adapted from Goffin).
Key Sub-question 1.7.1. Does targeted monotherapy improve outcomes in selected patient populations?
We also identified seven publications (from six trials) that assessed the use of targeted monotherapy compared to conventional chemotherapy, primarily in the population of patients with the EGFR gene mutation. We consider this to be related but distinct to the above discussion regarding the addition of targeted therapy to conventional chemotherapy, and therefore have created a new key sub-question for this topic. These studies, which compared gefitinib to carboplatin-paclitaxel in a general population and then performed a subgroup analysis on patients with the EGFR mutation,107, 108 gefitinib vs. chemotherapy in patients selected for EGFR gene mutations,109, 110 gefitinib vs. chemotherapy in a general population and then performed a subgroup analysis on patients with EGFR mutations,111 and erlotinib vs. chemotherapy in patients selected for EGFR gene mutations,112, 113 consistently show large differences in progression-free survival favoring targeted monotherapy, such as10.1 months vs. 5.4 months(Table F5). Overall survival also tended to favor groups treated with targeted monotherapy as opposed to chemotherapy treatment, but this has not reached statistical significance, and two trials favored chemotherapy over targeted monotherapy, however neither of these contradictory findings reached statistically significance either.111, 113 One of these trials was consistent with the support for targeted monotherapy compared to chemotherapy within a subgroup analysis of patients with EGFR mutations.111 Other than rash and aminotransferase elevation, erlotinib and gefitinib were also in general better tolerated than cytotoxic chemotherapy.
Table F5
Targeted Monotherapy.
One additional trial is relevant to this topic. Gridelli and colleagues compared a strategy of using erlotinib as first-line therapy in unselected patients with advanced NSCLC, followed by cisplatin and gemcitabine at the first sign of progression, versus the opposite strategy of initial therapy of cisplatin and gemcitabine followed by erlotinib given at the first sign of progression.114 Among nearly 800 patients, the erlotinib first strategy was significantly worse in terms of overall survival.
As part of an update search, we identified two recently published meta-analyses of tyrosine kinase inhibitors.13, 22 One meta-analysis was of monotherapy with either erlotinib or gefitinib compared to cytotoxic therapy in patients with advanced NSCLC and EGFR mutations.13 It included six trials, all of which are included in Table F5. The pooled analysis showed a statistically significant benefit for progression-free survival (HR=0.37; 95% CI: 0.27–0.52), and a nonstatistically significant benefit in overall survival (HR=0.94; 95% CI: 0.77–1.15). The second meta-analysis was restricted to gefitinib, and included studies of it as monotherapy, combined with systemic chemotherapy, and then given after systemic chemotherapy (only one trial of this).22 The monotherapy portion included four trials, three of which are included in Table F5, and also found improvement in progression-free survival for patients with EGFR mutations.
Summary of Key Sub-question 1.7
New trials of a number of novel targeted agents have so far failed to find results equivalent to the increases in progression-free survival seen with erlotinib (mostly in patients who have never smoked) and bevacizumab (in an Asian population subgroup analysis) in the CCO review (GRADE=moderate).
Erlotinib or gefitinib monotherapy is in general superior in terms of beneficial outcomes and adverse events than cytotoxic chemotherapy in patients with EGFR mutations (GRADE=high).
Key Sub-question 1.8. Is a single new agent superior to best supportive care?
We did not update this key sub-question as our scope was the comparative effectiveness between drugs.
Key Sub-question 1.9. Is a single new agent superior to single-agent or doublet therapy including older agents?
The CCO review focused on cytotoxic “new agents” such as vinorelbine and irinotecan and not targeted therapies. As an earlier conclusion had already established doublet therapy as the treatment of choice, we did not update this key sub-question.
Key Sub-question 1.10. Which single new agent is most effective?
The CCO review focused on the single agent cytotoxic agents gemcitabine, vinorelbine, paclitaxel, and docetaxel. Since an earlier conclusion had established doublet therapy as the treatment of choice, we did not update this key sub-question.
Key Sub-question 1.11. What is the optimal administration, duration, and timing of chemotherapy for advanced nsclc?
We did not update this key sub-question as it did not assess comparative effectiveness of agents and therefore did not fit the scope of key question #1 for this current review.
Key Sub-question 1.12. Is a Doublet Regimen Better than a Single Agent for the Elderly Population?
In the systematic review by the CCO, six trials were identified as being relevant to the treatment of elderly patients with NSCLC.2 These trials compared a variety of different regimens, including singlet vs. singlet, singlet vs. doublet, and singlet vs. best supportive care. The CCO original key question was refined by our TEP to focus solely on singlet vs. doublet therapy in elderly patients. Four trials in the CCO review compared singlet versus doublet therapy. Two of the trials reported modestly improved overall survival in the doublet-treated patients, and the other two reported no differences. The CCO review concluded that using a single new agent was superior to best supportive care in patients > 70 years of age; doublet therapy could be considered in those that could tolerate combination therapy or platinum agents; and that no trial that had been dedicated to assessing platinum agents in the elderly population has been completed. For patients > 70 years, survival was similar to that in younger patients, even though the elderly experienced more frequent leucopenia. There was a lack of data regarding the use of chemotherapy in patients >80 years of age and, therefore, chemotherapy should be used cautiously in that population.
New Literature Since 2007 Relevant to this Sub-question
We identified four new trials that compared single agents to doublet regimens in the elderly population.108, 113, 115, 116 The first two trials were focused on elderly patients.108, 113 The second two trials examined the elderly as a subpopulation.115, 116
The first trial focused specifically on the elderly population, comparing treatment with docetaxel to treatment with both docetaxel and gemcitabine.116 Three hundred and fifty patients were randomized, with the median age of 74 years. ITT analysis showed median survival of 5.5 months in the group receiving the doublet chemotherapy, compared to 5.1 months in those receiving a single agent (P=.65). The median time-to-progression was longer in those receiving the doublet regimen (4.8 months) compared to those receiving a single agent (2.9 months; P=.004).
The second trial compared individuals aged 70–89 receiving either the doublet regimen of carboplatin and paclitaxel or therapy with a single agent of vinorelbine or gemcitabine.115 The 451 patients were randomly assigned to the two groups. Median overall survival was 10.3 months for the doublet chemotherapy compared to 6.2 months for therapy with a single agent (HR 0.64, 95% CI 0.52–0.78; p<0.0001). One year survival was also greater in the doublet group with 44.5% (95% CI 37.9–50.9) alive at one year compared to 25.5% in the single agent group (95% CI 19.9–31.3). There were greater toxic effects in the doublet chemotherapy group but this was still considered a superior regimen to single agent therapy.
The first subgroup trial assessed elderly patients within a group of adults treated for NSCLC compared erlotinib to standard chemotherapy of cisplatin or carboplatin plus docetaxel or gemcitabine.113 The study was halted after interim analysis revealed that the study had met its primary endpoint. In the final analysis, the median progression-free survival for those treated with erlotinib was 9.7 months (95% CI 8.4–12.3) compared to 5.2 months for those treated with the doublet regimen (95% CI 4.5–5.8) (HR 0.37, 95% CI 0.25–0.54; p<0.0001). A multivariable analysis of progression-free survival found that age was not a significant factor.
Another subgroup study assessed differences in progression-free survival in patients treated with gefitinib compared to carboplatin plus paclitaxel.108 They found that progression-free survival was longer in the gefitinib group but that this was affected by age. For those patients younger than 65, the hazard ratio was 0.81; 95% CI, 0.70 – 0.95, and for those patients 65 years or older, the hazard ratio was 0.58; 95% CI, 0.45–0.76; P<0.001. There was a statistically significant interaction between age and treatment effect (p=0.03).
We add here one additional trial, which compared singlet vs. doublet therapy, although the mean age of patients was “elderly” (mean age = 63), and not all clinical experts would judge this study as relevant to the elderly population.117 This study compared docetaxel alone to docetaxel plus gemcitabine in 312 patients with stage IIIB or IV NSCLC. There was a slight, statistically significant advantage for overall survival in the doublet-treated group (9.4 months vs. 8.3 months). There was a greater proportion of patients in the doublet-treated group with grade 2–4 anemia, plus two treatment-related deaths.
Summary of Key Sub-question 1.12
With the exception of studies of gefitinib and erlotinib monotherapy (in patients with EGFR mutations), doublet chemotherapy probably has a slight benefit in terms of survival compared to singlet therapy, but causes more toxicity (GRADE=moderate). Also, there now has been one trial of platinum therapy in the elderly taken to completion that found a near-doubling of the proportion of patients alive at one year in the doublet therapy group compared to monotherapy.
Key Sub-question 1.13. Toxicity of first-line systemic chemotherapy regimens
The CCO reported a summary of Grade 3 or 4 chemotherapy toxicities from 7 large trials of various agents. They cautioned that “comparisons between trials should be avoided and numbers are provided only to give a general sense of the toxicity of the regimens,” which we now repeat. To the table from the CCO we have added the Grade 3 or 4 toxicities from additional large trials of first line treatment, see Table F6.
Table F6
Summary of Grade 3 or 4 Chemotherapy Toxicity (% Patients) from Large, Selected Trials in NSCLC (adapted from Goffin).
KEY QUESTION 2. For patients with metastatic NSCLC what is the comparative effectiveness of the different recommended (e.g. NCCN guidelines) second line chemotherapy regimens?
“Second-line therapy,” by definition in this review, means active therapy. It has already been established that docetaxel monotherapy resulted in improved overall survival when compared to best supportive care. For example, more than a decade ago Shepherd and colleagues randomized 104 patients to either docetaxel at 75mg/m2 or 100mg/m2 or best supportive care (BSC).123 The median overall survival was longer in the docetaxel groups with 7.0 months compared to the 4.6 months in the BSC group. The difference was more significant when comparing the docetaxel 75mg/m2 to the BSC with the median survival of 7.5 months compared to the 4.6 months of BSC (P=.010) One-year survival was also increased in the docetaxel 75mg/m2 group with 37% versus 11% in the BSC group (p=.003).
There was no single systematic review of second-line therapy that was both comprehensive and high quality, thus we found no equivalent to the review by the CCO. Therefore our synthesis of the second-line therapy first discusses the existing systematic reviews, and then the trials.
Existing Systematic Reviews
There were seven systematic reviews, details of which can be found in Table S1.
Table S1
Second Line Systematic Reviews Published Since 2010.
The first three reviews were done by Qi and colleagues.124–126 The first of these consisted of five RCTs comparing pemetrexed doublets versus pemetrexed alone.124 The two other trials by Qi and colleagues included drugs that do not have FDA approval (enzastaurin and matuximab).125,126 Futhermore, the authors pooled data across studies that included a very heterogeneous group of drugs, including cytotoxic therapies and targeted therapies. We did not judge this pooling to be clinically sensible and hence concluded this meta-analysis was not relevant to clinical practice. Similarly, the second review by Qi and colleagues assesses the efficacy of vandetanib in a pooled analysis of heterogeneous comparators, and we likewise did not judge this as clinically meaningful.125
The third review consisted of eight RCTs comparing docetaxel- based doublets with docetaxel as a single agent.126 These trials resulted in 2,126 patients for analysis. Meta-analysis showed no difference in overall survival (HR=0.93; 95% CI: 0.80–1.07, P=0.308) but did show a difference in overall response rate (OR=1.42, 95% CI: 1.13–1.80, P=0.03). In addition, 1-year survival was not significantly improved (OR=1.09, 95% CI: 0.92–1.28, P=0.328).
The next review was by Yang and colleagues and included four RCTs.127 Two of these compared bevacizumab plus carboplatin and paclitaxel to carboplatin plus paclitaxel alone, one compared bevacizumab with chemotherapy to bevacizumab with erlotinib or chemotherapy alone, and the last compared cisplatin plus gemcitabine with either placebo or bevacizumab. Only one of these trials studied treatment exclusively as second-line therapy,128 one trial included both untreated and recurrent nonsquamous cell disease,102 and the other two studied bevacizumab as part of first line treatment.101, 129 These four studies combined treated 2101 patients. Meta-analysis found that tumor response rate might not be increased with low-dose bevacizumab (7.5mg/kg) (RR= 1.19, p=0.68) but was with high-dose treatment (15mg/kg) (RR=1.69, p=0.002). The one year survival rate was not increased with either low (RR=1.02, p=0.82) or high doses (RR=1.09, p=0.07). The 2 year survival rate was increased by the higher dose regimen but at a cost of greater toxicity. In the one study of bevacizumab as part of second line treatment,128 the addition of bevacizumab to second line treatment with docetaxel or pemetrexed resulted in improvements in progression-free survival and overall survival, with hazard ratios of 0.66 to 0.78, although these improvements were not statistically significant. In this study, adding erlotinib to the bevacizumab therapy did not result in additional improvement. No study demonstrated that continuing bevacizumab beyond first line chemotherapy, either alone or with another agent, offers superior overall survival to stopping bevacizumab at the time of discontinuation of first line cytotoxic therapy.
The fifth systematic review by Mountzios and colleages included four trials of erlotinib versus placebo, erlotinib and gemcitabine-cisplatin, erlotinib and carboplatin-paclitaxel, and erlotinib and bevacizumab.20 This review covered the use of erlotinib as first line, second line, and maintenance therapy. Regarding second-line therapy, assessment of overall survival found a modest absolute gain of 2 months (overall survival of 6.7 months vs. 4.7 months, HR = 0.70, p < 0.001) in the BR.21 trial.130 This led to FDA (11/2004) and EMA (10/2005) approval of erlotinib for the treatment of chemotherapy-resistant patients.
The final two systematic reviews were by Di Maio and colleagues. The first was an individual patient data meta-analysis of docetaxel administered weekly or once every three weeks as second-line therapy in patients with NSCLC.131 A total of 865 patients were included, 81 percent were performance status 0 or 1, 91 percent had received platinum therapy. There was no difference in median survival (27.4 weeks vs. 26.1 weeks), and fewer episodes of severe and febrile neutropenia in patients receiving weekly docetaxel. The second systematic review included the data from six trials comparing single agent and doublet chemotherapy.132 Overall survival was not significantly different with the median of 37.3 weeks with doublets and 34.7 weeks with single agent (P=.32). The response rate was 15.1% with the doublets and 7.3% with a single agent (P=.0004). There were more toxic effects from the doublet, compared to single agent therapy.
The conclusions from the seven systematic reviews can be summarized as:
- doublet second line cytotoxic therapy might offer slight benefits in progression-free survival and response rate, not overall survival, but at a cost of increased toxicity;
- erlotinib produces modest increases in overall survival; and
- in one phase II study, the addition of bevacizumab to second line treatment resulted in improvements in survival that were not statistically significant.
Clinical Trials Published Since 2006
We identified 20 trials, six of which were also included in the systematic reviews above.133–138 Table S2 shows the overlap between trials and systematic reviews.
Table S2
Second Line Trials Appearing in a Systematic Review.
Trials Included in Existing Systematic Reviews
Two of the trials, by Gebbia and colleagues133 and Takeda and colleagues,134 were included in the review by Di Maio and colleagues discussed previously. 132 One trial by Herbst and colleagues136 was in the review by Yang and colleagues, 127 also discussed previously. The remaining three trials were only included in reviews by Qi and colleagues, which were not discussed in detail in the prior section. We therefore summarize these three trials here.
The first trial135 was included in Qi and colleagues (2012).126 In this RCT, patients who had been pretreated with front-line platinum-free regimens were randomized to receive either docetaxel-carboplatin or docetaxel alone. There was no significant difference in overall survival between the two groups with 10.27 (95% CI: 7.07–13.47) months achieved in the combination arm and 7.70 (95% CI: 3.39–12.01) months in the docetaxel alone arm (p=0.550). The response rate was similar 10.4% for the combination versus 7.7% (p=0.764). Likewise, the one-year survival rate was similar 43.8% versus 40.3%.
The next trial137 was included in the systematic reviews by Qi and colleagues (2011)125 and Qi and colleagues (2012).124 In this study, 534 patients were randomized to receive vandetanib added to pemetrexed or pemetrexed plus placebo. There was no significant difference in overall survival. Median overall survival was 10.5 months for vandetanib and 9.2 months for placebo (HR=0.86; 97.54% CI, 0.65–1.13; p=.219). There were statistically significant improvements in overall response rate with 19% in the vandetanib group and 8% in the placebo group (p<.001). Vandetanib is not currently approved for use in the United States.
The last of the trials138 was found in Qi and colleagues (2011).125 In this trial 1,240 patients were randomized to receive vandetanib or erlotinib. There was no significant difference in the superiority analysis as the median overall survival was 6.9 months for vandetanib and 7.8 months with erlotinib (HR=1.01; p=.830). Likewise, the overall response rate was 12% in both arms (two-sided P=.98). A preplanned non-inferiority analysis supported non-inferiority, defined as whether vandetanib “retained at least 50%” of the efficacy of erlotinib.
The remaining 15 trials were not found in any of the systematic reviews.123, 139–153 Table S3 has the details of these trials. We discuss these here in the order in which they emerged into clinical practice: docetaxel, followed by pemetrexed, then tyrosine kinase inhibitors (TKI), and finally newer agents.
Table S3
Trials Not Included in Existing Systematic Reviews.
Docetaxel
We identified one new trial of docetaxel. Fossella and coleagues 373 patients were randomized to receive either docetaxel 100mg/m2, docetaxel 75mg/m2 versus a regimen of either vinorelbine or ifosfamide.151 Overall survival was not significantly different between the three groups, 5.5 months for docetaxel 100mg/m2, 5.7 months for docetaxel 75mg/m2 and 5.6 months for vinorelbine/ifosfamide. However, the 1-year survival was significantly greater with docetaxel 75mg 32% (95% CI: 23–40) than with vinorelbine/ifosfamide 19% (12–26). The overall response rates for both of the docetaxel arms were significantly higher than that of the vinorelbine/ifosfamide arm with 10.8% for docetaxel 100mg/m2, 6.7% for docetaxel 75mg/m2 and 0.8% with vinorelbine/ifosfamide (p=.001 and p=.036, respectively).
Pemetrexed
Cullen et al, randomized 588 patients to either standard or high-dose pemetrexed.145 The goal was to accrue 600 patients, however accrual was terminated after an interim analysis indicated a low probability of improved survival and numerically greater toxicity in the higher dose arm. Of the patients randomized, there was no statistically significant difference between the two groups in median survival reaching 6.7 months with the lower dose and 6.9 months with the higher dose (HR=1.0132, 95% CI: 0.837–1.226). Likewise there was no significant difference in overall response with 7.1% in the lower dose and 4.3% in the higher dose (P=0.1616).
TKI versus Cytotoxic Therapy
There were six trials comparing TKI to cytotoxic therapy.139–144, 152 Ciuleanu et al, included 424 patients who were randomized to receive either erlotonib or chemotherapy.139 About two-thirds of patients were positive for EGFR mutations. Median overall survival was 5.3 months (4.0–6.0) in the erlotinib group and 5.5 months (4.4–7.1) with chemotherapy (HR=0.96, 95% CI: 0.78–1.19; log-rank p=0.73). The response rate was also similar between the 2 groups with a RR of 7.9% (4.6–12.5) in the erlotinib group and 6.3% (3.5–10.4) in the chemotherapy group. The one-year survival was 26% (19–32) in the erlotinib group versus 24% (18–30) in the chemotherapy group.
Maruyama and colleagues randomized 489 patients to receive either gefitinib or docetaxel.140 Patients were enrolled irrespective of EGFR receptor mutation status. There was no significant difference in overall survival, which was 11.5 months (95% CI: 9.8–14.0) in the gefitinib arm and 14.0 months (95% CI: 11.7–16.5)in the docetaxel arm (P=.330). However, for ORR gefitinib was statistically superior to docetaxel (22.5% v 12.8%, odds ratio, 2.14; 95% CI: 1.21–3.78; P=.009).
Sekine and colleagues assessed quality of life outcomes in the preceding study reported by Maruyama and colleagues.142 This studied evaluated the quality of life differences between these two regimens and found gefitinib to have statistically significant benefits over docetaxel. The Functional Assessment of Cancer Therapy-Lung analysis was 23% for gefitinib versus 14% for docetaxel (P=0.023). The Trial Outcome Index was 21% vs 9% (P=0.002). There was no significant differences between treatments in Lung Cancer Subscale improvement rates (23% vs 20%, P=0.562). This study is the quality of life assessment outcome of a trial by Maruyama and colleagues, discussed above.140
Kim and colleagues randomized 1466 patients to either gefitinib or docetaxel.141 Patients were enrolled irrespective of EGFR mutation status. Median overall survival was 7.6 months in the gefitinib group and 8.0 months in the docetaxel group. One-year survival was 32% for gefitinib and 34% for docetaxel.
Lee and colleagues randomized 161 patients to either gefitinib or docetaxel.143 Patients were enrolled irrespective of EGFR mutation status. The overall survival was 14.1 months for gefitinib and 12.2 months for docetaxel (HR=0.870, 95% CI: 0.613–1.236, 2-sided p=0.4370). The objective response rate was statistically superior in the gefitinib group (28.1%) compared to the docetaxel group (7.6%) (P=0.0007).
Lastly, during peer review an abstract presented at the 2012 ASCO Annual Meeting was identified.152 This study randomized 221 patients to erlotinib or docetaxel; all patients had wild type EGFR status. At a median follow-up of 20 months, progression-free survival favored treatment with docetaxel. Overall survival was not yet analyzed. We await full publication of these results, which will provide more complete analyses.
TKI As an Addition to Cytotoxic Therapy
This study of pemetrexed was added during peer review.153 The study has been presented at the the 2011 ASCO Annual Meeting, but full publication is still pending. This was a phase II study in which 165 patients were randomized to receive either pemetrexed or pemetrexed plus erlotinib. The median progression-free survival was 2.9 months versus 3.2 months favoring combination therapy (HR=0.63, 95% CI 0.44–0.90). Overall survival also favored combination therapy (11.8 months vs. 7.8 months, HR=0.68, 95% CI 0.47–0.98). We await full publication of these results, which will provide more complete analyses, and confirmation in a phase III study.
TKI Plus Bevacizumab versus TKI Alone
Herbst and colleagues randomized 636 patients to either bevacizumab plus erlotinib or erlotinib alone.144 Approximately 90 percent of patients had wild type EGFR receptor status. The overall survival did not differ between the two groups with the median overall survival of 9.3 months (IQR 4.1–21.6) in the bevacizumab arm compared to 9.2 months (IQR 3.8–20.2) in the erlotinib alone arm (HR=0.97, 95% CI: 0.80–1.18, p=0.7583). Objective response rate was much higher in the bevacizumab group (13% vs 6%).
Various Other Agents
There were seven trials of various other agents or doses.123, 146–151, 153 Ready and colleagues randomized 106 patients to either the proapoptotic agent AT-101 or placebo in combination with docetaxel.146 There was no significant difference in the median overall survival which was 7.8 months in the AT-101 arm and 5.9 months in the placebo arm (HR=0.82, 95% CI: 0.5–1.3, p=0.21). According to an independent review, the response rate and median PFS were not different between the arms, with the PFS for the AT-101 group of 7.5 weeks and 7.1 weeks for placebo (HR=1.04, p=0.57).
Ramlau and colleagues randomized 829 patients to either oral topotecan or IV docetaxel.147 The median survival was higher with docetaxel at 30.7 weeks (95% CI: 28–34) compared to 27.9 weeks (95% CI: 24–31) with topotecan (p=.0568). Overall response rate was 5% (95% CI: 3–7%) for both docetaxel and topotecan. One-year survival was 29% (24–33%) for docetaxel and 25% (21–29%) for topotecan.
Paz-Ares and colleagues randomized 849 patients to either paclitaxel poliglumex or docetaxel.148 The median survival was not different, at 6.9 months in each arm (HR 1.09, p=0.257). The 1-year survival was also similar with 25% in the paclitaxel poliglumex arm and 29% in the docetaxel arm (P=0.006).
Hermes and colleagues randomized 220 patients to either irinotecan plus carboplatin or etoposide plus carboplatin.39, 154 Overall survival was better in the irinotecan group with a median survival of 8.5 months compared with 7.1 months in the etoposide group (etoposide relative to irinotecan HR=1.41; 95% CI: 1.06–1.87). The one-year survival rate was 34% for irinotecan and 24% for etoposide.
Krzakowski and colleagues randomized 551 patients to either vinflunine or docetaxel.149 The median overall survival was 6.7 months (95% CI: 5.9–7.9) in the vinflunine group and 7.2 months (6.0–8.5) in the docetaxel group (HR=0.973, 95% CI: 0.805–1.176). The overall response rate was 4.4% for vinflunine and 5.5% for docetaxel treatment.
Finally, Schiller and colleagues randomized 150 patients to receive either pemetrexed alone, pemetrexed plus matuzumab 800mg/week, or pemetrexed plus matuzumab 1600mg/3 weeks.150 There was a trend for improved OS in patients receiving matuzumab weekly compared to the every 3 weeks group with the former of 12.4 months and the latter 5.9 months. This was also greater than the 7.9 months of the pemetrexed group. Pooling the matuzumab arms gave an ORR of 11% compared to 5% for pemetrexed alone. Of note, all responses, except for one patient in the pemetrexed group, occurred in patients whose tumors expressed EGFR.
Summary of Second-line therapy Trials Not in Existing Systematic Reviews
The summary of these trials not included in existing systematic reviews is:
- Considering data from first line and maintenance therapy studies in addition to second line studies, there are sufficient data to support the conclusion that histology type influences the effectiveness of potential treatments. Pemetrexed is more effective in nonsquamous NSCLC, while docetaxel is more effective in squamous NSCLC (GRADE=moderate).
- Tyrosine kinase inhibitors, when used as second-line therapy in patients unselected for EGFR mutation status, produce overall survival similar to docetaxel (GRADE=strong).
- There is insufficient data to support effectiveness of other drugs, or drugs in combinations, in second-line therapy (GRADE=moderate).
- The above second line studies are typically undertaken after evidence of disease progression, and should be distinguished from mainenance therapy, which is undertaken when a patient has at least stable disease during treatment (typically four cycles).
Toxicity of Second Line or Maintenance Agents
Analogous to the presentation in first line threatment, we present here a summary of Grade 3 or 4 toxicities from selected trials of second line agents, some of which are also used in maintenance therapy. As noted before, comparisons across trials should be avoided, and “numbers are provided only to give a general sense of the toxicity of the regimens.”2
TABLE S4
Summary of Grade 3 or 4 Chemotherapy Toxicity (% Patients) from Large, Selected Trials of Maintenance or Second-line therapy.
KEY QUESTION 3. For patients with metastatic NSCLC what is the benefit of maintenance therapy following first line chemotherapy regimens compared with no maintenance therapy?
We identified three systematic reviews of maintenance therapy published since 2010.3, 20, 156 Details of these systematic reviews are in Table M1. The most comprehensive and best quality review was completed by Zhang and colleague.3 It met 10 of the 11 AMSTAR criteria and identified eight trials with a total of 3,736 patients with nonprogressing NSCLC. Trials were of either a continuous or switch strategy and were compared to either placebo or observation. Of the nine trials, three employed a continuous maintenance strategy157–159 while six utilized a switch strategy.155, 159, 160, 161–164
Table M1
Maintenance Systematic Reviews Published Since 2010.
In the trials examining continuous maintenance therapy, there was a trend towards benefits in the overall survival, however this did not quite reach statistical significance (HR=0.88; 95% CI: 0.74–1.04; p=.15). There was no evidence of heterogeneity between the studies (p=.66, I2=0%). The first of these trials accrued 215 patients and continued patients on gemcitabine after an initial therapy of gemcitabine and cisplatin. Overall survival was 10.2 months in the maintenance group compared to 8.1 months in the best supportive care group (HR=0.79; 95% CI: 0.57–1.11).157 Another trial of 255 patients on gemcitabine following gemcitabine and cisplatin found overall survival to be 8 months in the maintenance group versus 9.3 months in the best supportive care group (HR=0.97; 95% CI: 0.72–1.30).158 The last of these trials accrued 309 patients, treating them with gemcitabine after gemcitabine and cisplatin and favored continued maintenance, though specific data were not reported.159
The six trials examining switch maintenance therapy found a clinically and statistically significant 15% improvement in OS when compared to the placebo or observation groups (HR=0.85; 95% CI: 0.79–0.92; p<.001). There was no evidence of heterogeneity (p=.77, I2=0%). There were two trials of cytotoxic agents. The first trial accrued 309 patients and treated patients with docetaxel after gemcitabine and carboplatin. The overall survival of the maintenance therapy group was 12.3 months compared to 9.7 months in the best supportive care group (HR=0.81, 95% CI: 0.63–1.03).165 The other trial included 663 patients treated with pemetrexed after platinum-doublet chemotherapy versus placebo and found overall survival to be 13.4 and 10.6 months (HR=0.79, 95% CI: 0.65–0.95).164 There were four trials of molecular-targeted agents. Two trials were combined to give 768 patients treated with bevacizumab and either erlotinib or placebo after bevacizumab and platinum-doublet chemotherapy. The erlotinib group had an overall survival of 15.9 months versus 13.9 in the placebo group (HR=0.90, 95% CI: 0.74–1.09; p=0.2686).161, 162 A trial of 889 patients receiving erlotinib or placebo after platinum-doublet chemotherapy found the overall survival to be 12 in the erlotinib group and 11 in the placebo group.155 Another study of erlotinib included 310 patients and compared it to observation following gemcitabine and cisplatin. The overall survival hazard ratio was 0.91 (95% CI: 0.80–1.04) in favor of the maintenance therapy.159 The last of these studies included 173 patients treated with gefitinib or placebo after platinum-doublet chemotherapy and found an overall survival of 10.9 months in the treatment arm versus 9.4 months (HR=0.87, 95% CI: 0.80–0.95).163
Overall, these nine trials comprised 3,736 patients. When comparing continuous and switch maintenance therapies, the interaction test found no statistically significant difference in overall survival (HR=0.88 vs. 0.85; interaction p=0.78). This result was interpreted as meaning that there is no evidence to conclude superiority for either the continuous or the switch approach. Within the trials of switch maintenance, a subgroup analysis revealed that there was no statistically significant difference in overall survival between the cytotoxic and tyrosine kinase inhibitor agents. Again, the conclusion is that there is no evidence to support that one approach is superior to the other.
The other two systematic reviews were more targeted. The first review identified 5 trial including 3,634 patients.156 Three of these trials were of erlotinib versus either placebo or gemcitabine. Two of the trials were of pemetrexed versus placebo. Two of the trials were included in the review by Zhang.155, 166 Both regimens led to significant improvement in overall survival compared with placebo or observation (erlotinib HR=0.90, 95% CI: 0.83–0.98; pemetrexed HR=0.79; 95% CI: 0.65–0.95) and relative hazards ratio showed no significant difference between the two agents (HR=0.88; 95% CI: 0.71–1.08, p=0.22). These results are compatible with the conclusions of the review by Zhang and colleagues. However, pemetrexed was superior to erlotinib in the outcome of progression free survival. The other systematic review included the SATURN and ATLAS trials which studied erlotinib versus placebo and erlotinib and bevacizumab versus bevacizumab alone.20 There were 2 trials including 1768 patients. In the SATURN trial, patients in the erlotinib arm had a better response rate (12% vs 5%), PFS (12 vs. 11.1 weeks; HR=0.71; 95% CI: 0.62,0.82; p<0.0001) and better overall survival (12 vs. 11 months, HR=0.81, 95% CI: 0.70, 0.95; p=0.0088). The ATLAS trial found an improvement in progression free survival of 4.76 versus 3.75 months (HR=0.722; 95% CI: 0.592, 0.881; p<0.0012).
Finally, we identified four additional trials.167–171 Details of these trials are presented in Table M2. The first was a quality of life assessment of the study by Ciuleanu, included in the systematic review by Zhang.168 This trial treated 663 patients with pemetrexed or placebo after platinum-based therapy. The primary outcome was time to worsening of symptoms and this was longer in the pemetrexed group for pain (HR=0.76, 95% CI: 0.59–0.99; p=0.041) and hemoptysis (HR=0.58, 95% CI: 0.34–0.97; p=0.038). The PARAMOUNT trial of pemetrexed versus placebo treated 539 patients who had undergone therapy with pemetrexed and cisplatin.167 Within the 359 patients treated with pemetrexed, there was a significant reduction in the risk of disease progression compared to the placebo group (HR=0.62, 95% CI: 0.49–0.79; p<0.0001). In addition, the median progression free survival in the pemetrexed group was 4.1 months (95% CI: 3.2–4.6) versus 2.8 months (95% CI: 2.6–3.1) in the placebo group. The results of the PARAMOUNT trial were recently updated in abstract form at the 2012 ASCO Annual Meeting.171 Continuation maintenance therapy with pemetrexed resulted in a 22 percent reduction in the risk of death at a median follow-up of 12.5 months. The INFORM trial by Zhang and colleagues randomized Chinese patients with advanced NCSLC who had received four cycles of platinum-based doublet therapy to gefitinib pr placebo.170 Progression-free survival was approximately doubled from 2.6 to 4.8 months; there was no difference in overall survival. Adverse events, primarily rash, diarrhea, and liver function abnormalities, were much more common in patients taking gefitinib. Lastly, there was a trial of thalidomide versus placebo in 722 patients who had been treated with gemcitabine and carboplatin.169 Thalidomide did not improve overall survival as the median duration in the placebo group was 8.9 months compared to 8.5 months in the thalidomide arm (HR=1.13, 95% CI: 0.97–1.32; p=.12). Unfortunately, the risk of thrombotic stroke was increased by 74% in the thalidomide group (HR=1.74, 95% CI: 1.20–2.52; p=.003). In addition survival was significantly worse in those patients who had nonsquamous histology. In summary, two of these trials reported benefits in additional outcomes for maintanence therapy with pemetrexed compared to placebo, while two other trials did not support any beneficial clinical effect for maintenance treatment with paclitaxel or thalidomide.
Table M2
Trials Not Included in Existing Systematic Reviews.
Summary of Key Question 3: Maintenance Therapy
- Maintenance therapy improves overall survival (GRADE=High).
- Maintenance therapy with gefitinib significantly prolonged preogression-free survival compared with placebo in patients from east Asia with advanced NSCLC who achieved disease control after first-line chemotherapy (GRADE=high).
- There is insufficient evidence to reach conclusions regarding whether a continuous or a switch strategy is superior (GRADE=Very low). However, two drugs have been approved for switch therapy.
- Differences in survival in placebo-controlled trials of erlotinib or cytotoxic agents are sufficiently small that head-to-head comparisons will be required before strong conclusions can be reached about comparative effectiveness.
KEY QUESTION 4. What is the relative cost and cost-effectiveness of the different approaches in Key Questions 1–3?
Our literature review identified one systematic review of cost-effectiveness analyses and 21 cost-effectiveness analyses published since 2010. Of the latter, eight analyses concerned first-line therapy, seven analyses concerned second-line therapy, six analyses concerned maintenance therapy, and one concerned third line therapy. A single publication could deal with more than one kind of therapy. Eleven analyses assessed erlotinib, nine analyses assessed pemetrexed, five analyses assessed bevacizumab, five analyses assessed docetaxel, and a number of other agents were assessed less frequently (analyses could assess more than one agent). Four studies assessed cost-effectiveness from a US payor perspective, while the remainder used non-US payor perspectives. Almost all studies used life expectancy or quality-adjusted life years (QALYs) as the measure of outcome. Details about the 22 analyses are presented in Table CE1.
Table CE1
Cost-Effectiveness Analyses Published Since 2010.
The systematic review by Bongers and colleagues was published in 2012 and searched PubMed, EMBASE, and the Health Economic Evaluations database from 2001 through October 2010. The authors identified 6 prior systematic reviews, six cost-effectiveness analyses, and 6 cost-utility analyses. Four assessments of first-line therapy were included, as were 6 assessments of second-line therapy. The authors noted numerous methodological weaknesses in the included studies. Their overall conclusions were:
- Due to the small number of studies, heterogeneity between studies and lack of a clear and consistent definition of best supportive care in each study, strong conclusions cannot be drawn
- The estimates of key parameters, model assumptions, and calculations in modeling studies were often poorly reported.
- However, there was reasonable consensus between studies that gemcitabine-cisplatin is a cost-effective option for first-line treatment of non-small cell lung cancer, although pemetrexed-cisplatin appears more effective for non-squamous-cell carcinoma
- In second line treatment, docetaxel appears to be cost-effective compared with best supportive care, while erlotinib may be a cost-effective alternative compared to docetaxel.
Our review identified 22 cost-effectiveness analyses published since 2010, of which four overlapped with the systematic review by Bongers and colleagues. We obtained additional information from our TEP to focus our assessment on the following key sub-questions:
First-line Therapy
- Which platinum-doublet is the most cost-effective therapy in squamous and in non-squamous histology types?
- In non-squamous NSCLC, is the carboplatin-paclitaxel-bevacizumab option more cost-effective than platinum-pemetrexed?
Second-line Therapy
- Which of the three approved agents (docetaxel, pemetrexed for non-squamous histology, or erlotinib) is more cost-effective?
Maintenance Therapy
- Which approach is more cost-effective: switch maintenance with pemetrexed, continuation maintenance therapy with pemetrexed or erlotinib?
Key Sub-question 4.1. Which platinum-doublet is the most cost-effective therapy in squamous and in non-squamous histology types?
The review by Bongers and colleagues concluded that there was a “reasonable consensus” that gemcitabine + cisplatin was more cost-effective than other doublet options assessed.172 In the three studies used to reach this conclusion, the comparison options were paclitaxel-cisplatin, gemcitabine-paclitaxel, docetaxel-cisplatinum, vinorelbine-cisplatin, and pemetrexed-cisplatin. The payer perspectives were the Dutch health insurance system, the UK health care system, and a US payer. The study assessing pemetrexed (discussed in more detail, below) concluded that pemetrexed-cisplatin is more cost-effective than cisplatin-gemcitabine for patients with non-squamous disease.
Our search identified eight cost-effectiveness analyses of first-line therapy, but two dealt with agents that were not of interest (rh endostatin173 and cetuximab174) and five dealt with bevacizumab (discussed below). Of the remaining two, one assessed the cost-effectiveness of cisplatin + etoposide compared to carboplatin + paclitaxel, using data from a single Thai hospital,175 and was judged by our TEP to be not of interest for this review. Thus, there are no new cost-effectiveness analyses since the review by Bongers and colleagues that are relevant to this key sub-question. However, Key Question 1 for this review, about first-line therapy, identified one high quality systematic review by the CCO that concluded that “a combination of a platinum agent plus a new agent continues to be the standard of care” and “as differences between regimens are small, toxicity and patient preference should help guide regiment choice.”2 However, the CCO does note the meta-analysis by Le Chevalier that reported a trend toward improved survival with gemcitabine combinations. Eight clinical trials published since that review was completed in aggregate reach the same conclusion – no particular combination has been shown to be consistently superior in terms of effectiveness outcomes. In the absence of clinically important differences in effectiveness, then differences in the costs of the agents, and possibly the cost of treating different treatment toxicities, will drive any differences in cost-effectiveness. An addendum to the CCO review, restricted to pemetrexed, concluded that “data are compelling to recommend that pemetrexed should not be used in the treatment of squamous cell carcinomas for first line treatment” and “on the other hand, these data are not sufficiently compelling to recommend that pemetrexed be used preferentially over all other new agents in doublet therapy to treat adenocarcinoma in first line settings.”
Key Sub-question 4.2. In non-squamous NSCLC, is the carboplatin-paclitaxel-bevacizumab option more cost-effective than platinum-pemetrexed?
This combination was not assessed in the review by Bongers and colleagues, but the cost-effectiveness of bevacizumab was the subject of five recent cost-effectiveness analyses, although none were of exactly this comparison. Four of these analyses were supported by the maker of bevacizumab.176, 177, 178, 179 All three concluded that bevacizumab was a cost-effective approach to care in the following situations: bevacizumab + cisplatin + gemcitabine compared to pemetrexed + cisplatin in non-squamous cell histology, using an Italian payer perspective;178 bevacizumab + cisplatin + gemcitabine compared to cisplatin + pemetrexed in non-squamous cell histology, using a Korean and Taiwanese health payer perspective;176 the same combination using the payer perspective of Italy and Germany;179 and an analysis that combined together the results of two trials, which compared the addition of bevacizumab to either cisplatin + gemcitabine or carboplatin + paclitaxel, and used a societal perspective including time off from work in France, Germany, Italy and Spain.177
The one non-industry funded analysis used a US payer perspective and compared the addition of bevacizumab to carboplatin + paclitaxel versus carboplatin + paclitaxel alone.180 Data about outcomes came from the ECOG 4599 trial. Costs came from Medicare data. The discount rate for future costs and QALYs was 3% per year. The main result was the incremental cost-effectiveness ratio (ICER). Numerous sensitivity analyses were performed. The principal result was that the ICER was $560,000 per QALY gained, or $309,000 per life-year gained, for the addition of bevacizumab to the carboplatin + paclitaxel treatment. The results were most sensitive to the time for survival without progression while on treatment and the number of bevacizumab cycles. In order to reach a value of $100,000 per QALY gained, the addition of bevacizumab would have to result in a mean overall survival advantage of 1.3 years (while holding other model parameters constant). Alternatively, in order to reach the same $100,000/QALY threshold, the acquisition cost of bevacizumab would have to drop to $885, all other model parameters being held constant. The overall conclusion of the authors was that “bevacizumab does not appear to be cost-effective when added to chemotherapy in patients with advanced NSCLC, based on approximate cost-effectiveness thresholds that have been identified in the United States.”
Key Sub-question 4.3. Which of the three approved agents (docetaxel, pemetrexed for non-squamous histology, or erlotinib) is more cost-effective?
The review by Bongers and colleagues concluded that “docetaxel appears to be cost-effective compared to best supportive care, while erlotinib may be a cost-effective alternative compared with docetaxel.”172 These conclusions were based on the results of 6 cost-effectiveness analyses, 3 of them published in 2010 and this also included in our assessment.181–183 The other 3 analyses assessed the cost-effectiveness of docetaxel, in two of these docetaxel was compared to best supportive care and in one compared to pemetrexed and erlotinib. The two cost-effectiveness analyses comparing docetaxel with best supportive care both concluded that second line treatment with docetaxel had an incremental cost-effectiveness ratio well under conventional US thresholds for considering a treatment to be cost-effective: about $32,000 in one study and about $20,000 in the other. The third study assessed second line treatment with docetaxel, pemetrexed, and erlotinib. That analysis assumed that survival with all 3 treatments was equal, but that there was a very slight advantage in QALYs for erlotinib due to fewer adverse events. That, plus the oral administration of erlotinib, led to the finding that erlotinib second line treatment is more cost-effective than pemetrexed or docetaxel. It is unclear whether or not EGFR mutation status or cell histology was considered in this analysis.
Our search identified 7 cost-effectiveness analyses of second line treatment published since 2010. Of these, two analyses assessed erlotinib compared to docetaxel,181, 184 two analyses assessed the gefitinib compared to docetaxel using data from the INTEREST trial,185, 186 two analyses assessed pemetrexed versus docetaxel,183, 187 and one analysis assessed erlotinib compared to placebo using data from the BR.21 trial.182
The assessment of erlotinib compared to placebo used data from the BR.21 study and the actual resource use of the patients enrolled in the trial.182 Resources were converted into Canadian health care costs. Discounting was not used because median survival was only 1 year. The principal finding was the incremental cost-effectiveness ratio of adding erlotinib was $94,000 (Canadian) for life-year gained. In sensitivity analyses, the main driver was the magnitude of the survival benefit. Particular subgroups had more favorable ICERs, corresponding to the increased effectiveness of erlotinib in these subgroups: never smoked = $39,000; EGFR protein expression positive = $64,000; EGFR gene copy number high = $33,000. The authors concluded that “erlotinib treatment is…marginally cost-effective. The use of molecular predictors of benefit for targeted agents may help identify more or less cost-effective subgroups for treatment.” The publication states that the analysis was supported by the University of Toronto.
The two analyses comparing erlotinib to docetaxel used different data sources and reached somewhat different conclusions. The first study was supported by the maker of erlotinib and used data from the BR.21 study to estimate the benefits of erlotinib and data from the TAX317 study to estimate the benefits of docetaxel, and concluded that erlotinib had a small reduction in UK National Health Services costs relative to docetaxel.181 The second study was supported by the Canadian Center for Applied Research in Cancer Control and used observational data from patients treated in British Columbia.184 This study found that the difference in mean overall survival between patients treated with erlotinib or docetaxel was 1 day, and the mean difference in cost was less than $3000; neither difference was statistically significant. The authors concluded that “erlotinib and docetaxel are statistically equivalent in terms of treatment cost and overall survival.”
The two analyses of the comparing gefitinib to docetaxel both used data from the INTEREST trial for their estimates of effectiveness. One study was supported by two hospitals in Toronto,185 while the other does not state how it was supported.186 The latter study used a Thai payer perspective and concluded the gefitinib is cost-saving compared to docetaxel. The former study used the Canadian health care system perspective, and found an increase in costs with gefitinib use ($5161 per patient). The main driver of cost was the cost of the drug. No incremental cost-effectiveness ratio, in terms of life-years or QALYs, was presented. The authors concluded that “the modest increase in cost associated with gefitinib supports its use as an alternative to docetaxel as second-line treatment of advanced NSCLC.”
The two analyses comparing docetaxel to pemetrexed were both supported by the makers of the drugs in question. The analysis supported by the maker of docetaxel concluded that “second line treatment for NSCLC is more cost-effective with docetaxel that with pemetrexed.”187 The analysis supported by the maker of pemetrexed concluded that “pemetrexed as a second-line treatment option for patients with a predominantly non-squamous histology in NSCLC is a cost-effective alternative to docetaxel.”183
Key Sub-question 4.4. Which approach is more cost-effective: switch maintenance with pemetrexed, continuation maintenance therapy with pemetrexed or erlotinib?
Only one cost-effectiveness analysis was included in the review by Bongers and colleagues, published in 2010, and we also include it in our assessment here.188 We identified 6 cost-effectiveness analyses of maintenance therapy published since 2010. Of these, 2 analyses assessed erlotinib,189, 190 2 assessed pemetrexed,188, 191 and 2 assessed erlotinib compared to pemetrexed.192, 193 All but one study were supported by the manufacturers of either erlotinib or pemetrexed. The two studies of erlotinib funded by the makers of erlotinib concluded that “erlotinib is a cost-effective treatment option when used as first-line maintenance therapy for locally advanced or metastatic NSCLC”189 and “the overall budget impact to a health plan of expanding the use of erlotinib from the second/third line advanced NSCLC setting to include the maintenance setting was relatively small”.190 The one analysis supported by the maker of pemetrexed concluded that “compared with observation and other agents used and/or reimbursed for maintenance therapy in advanced NCSLC, pemetrexed may be considered cost-effective, particularly in patients with non-squamous cell histology.”188 The two analyses comparing erlotinib to pemetrexed, both of which were supported by the maker of erlotinib, concluded “erlotinib appears to be a cost-saving treatment alternative to pemetrexed, producing comparable survival benefits, based on indirect comparisons, at a lower cost”193 and “erlotinib maintenance therapy in patients with advanced NSCLC causes lower adverse event management costs than pemetrexed maintenance therapy indicating a potentially superior tolerability profile”.192
The one study that was not industry supported came from Swiss researchers and assessed the incremental cost-effectiveness ratio of adding pemetrexed to best supportive care, compared to best supportive care alone.191 Data came from the study by Ciuleanu.164 The model used a life-time cost horizon using a Swiss health care perspective. No discount rate was used. The principal results were that the addition of pemetrexed resulted in an incremental cost-effectiveness ratio of 106,202 Euros/QALY (approximately $145,000/QALY). The results were most sensitive to assumptions about the utility of being in the stable disease state and then the costs of best supportive care in the pemetrexed-treated patients. The authors concluded that “switch maintenance therapy with pemetrexed in patients with advanced nonsquamous-cell lung cancer after standard first-line chemotherapy is not cost-effective.”191
Additional evidence to consider for this sub-question comes from that reported in Key Question 3 in this report, about maintenance therapy. A systematic review and meta-analysis by Zhang and colleagues3 was unable to support clinically important differences between the switch and the continuous approach to maintenance therapy, and equally unable to support clinically important differences between switch therapy with cytotoxic agents and therapy with tyrosine kinase inhibitors. Of note, the number of original trials for assessment are small, and there are no published head-to-head comparisons of maintenance therapy with erlotinib compared to pemetrexed, meaning any conclusions must be made on indirect comparisons.
Summary of Key Question 4
There are a large number of published cost-effectiveness analyses, but approximately two-thirds of such studies are supported by the makers of the drugs being assessed. Invariably, studies supported by the makers concluded that their drug was cost-effective. Of the cost-effectiveness analyses not supported by industry, the addition of bevacizumab to first-line therapy was found in one study to be not cost-effective, erlotinib was found in one study to be marginally cost-effective, and the differences between erlotinib and docetaxel maintenance therapy were slight in another study (GRADE=low).
- LITERATURE FLOW
- For patients with metastatic non-small cell lung cancer (NSCLC) what is the comparative effectiveness of the different recommended (e.g. NCCN guidelines) first line chemotherapy regimens?
- For patients with metastatic NSCLC what is the comparative effectiveness of the different recommended (e.g. NCCN guidelines) second line chemotherapy regimens?
- For patients with metastatic NSCLC what is the benefit of maintenance therapy following first line chemotherapy regimens compared with no maintenance therapy?
- What is the relative cost and cost-effectiveness of the different approaches in Key Questions 1–3?
- RESULTS - Treatment of Metastatic Non-Small Cell Lung Cancer: A Systematic Revie...RESULTS - Treatment of Metastatic Non-Small Cell Lung Cancer: A Systematic Review of Comparative Effectiveness and Cost-Effectiveness
Your browsing activity is empty.
Activity recording is turned off.
See more...