TOPIC DEVELOPMENT
This project was nominated by Leonard Pogach, MD, National Program Director for Diabetes. The scope of the report and key questions were refined with input from a technical expert panel.
The key questions, as shown in the analytic framework in Figure 1, were as follows:
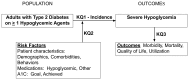
Figure 1
Analytic Framework.
- Key Question #1.
What is the incidence of severe hypoglycemia in adults with type 2 diabetes on one or more hypoglycemic agents?
- Key Question #2.
What are the risk factors for severe hypoglycemia in adults with type 2 diabetes on one or more hypoglycemic agents (e.g., demographics, co-morbidities, diabetes treatment regimen, other medication use, goal and achieved HbA1c)?
- Key Question #3.
What is the effect of severe hypoglycemia on other outcomes in adults with type 2 diabetes on one or more hypoglycemic agents (e.g., quality of life, mortality, morbidity, utilization)?
Extension of Key Question #1: In order to gain a more population-based perspective on hypoglycemia incidence (as recommended by our technical expert panel November 1, 2011) we re-reviewed all the abstracts identified through the initial search strategy (through November, 2011) might contain data from more representative groups that had not met the initial inclusion criteria.
SEARCH STRATEGY
We searched MEDLINE (OVID) for clinical trials and systematic reviews from 1950 to December 2010 using standard search terms. The search was updated in November 2011. We limited the search to articles involving adult, human subjects and published in the English language. Search terms included: hypoglycemia, hypoglycaemia, and diabetes mellitus, type 2. The full MEDLINE search strategy is presented in Appendix A.
We obtained additional articles from a search of the Cochrane Library, other systematic reviews, reference lists of pertinent studies, reviews, editorials, and by consulting experts. We also searched the following Web sites: Centers for Disease Control, ClinicalTrials.gov, Department of Transportation, Framingham Heart Study, National Health and Nutrition Examination Survey, National Institute of Diabetes and Digestive and Kidney Diseases, and Occupational Safety and Health Administration.
STUDY SELECTION
Investigators and research assistants trained in the critical analysis of literature assessed for relevance the abstracts of citations identified from literature searches. Full-text articles of potentially relevant abstracts were retrieved for further review.
Specific exclusion criteria for Key Questions #1 and #2 were as follows:
- Population: exclude if animal study, age less than 18 years, inpatients, type 1 diabetes, patient on dialysis, gestational diabetes, or fasting populations.
- Publication type: exclude case reports, narrative reviews, case series, letters, editorials, commentaries, book chapters, dissertations, other summaries, duplicate publications.
- Outcomes: exclude if no outcomes of interest. Outcomes of interest are incidence of severe hypoglycemia and risk factors for severe hypoglycemia. Exclude if severe hypoglycemia not reported or defined.
- Study duration: exclude if study is less than 6 months in duration.
- Sample size: exclude if study enrolled fewer than 500 patients.
- Intervention: exclude if study only includes patients on one or more non-FDA approved hypoglycemic agent (vildagliptin, algogliptin, taspoglutide, giclazide, troglitazone, exubera, any inhaled insulin) or on continuous insulin infusion.
For Key Question #1 – Extension, we employed the same exclusion criteria with the following modifications: we included population or clinic-based studies that may have enrolled fewer than 500 patients or had fewer than 6 months of follow-up; in which the definition of severe hypoglycemia may not have been rigorously defined but included some definition of symptomatic hypoglycemia; and in which there may not have been true incidence data (e.g., cross-sectional patient surveys). From this search we identified 16 articles (see Figure 2, shaded boxes).
For Key Question #3, we placed no restriction on sample size or study duration. The study had to report an association between severe hypoglycemia and outcomes of interest. Outcomes of interest included all-cause mortality, non-fatal myocardial infarction, non-fatal stroke, neurological events (other than stroke), hospitalizations, emergency department visits, accidents/trauma, quality of life, cognitive function, productivity, and other health resource utilization.
DATA ABSTRACTION
We abstracted the following data for each included study (as appropriate based on study design): study design, definition of severe hypoglycemia, length of follow-up, population characteristics, subject inclusion and exclusion criteria, intervention(s), comparison(s), length of follow-up, and outcome(s).
QUALITY ASSESSMENT
We assessed study quality for randomized controlled trials using the criteria recommended by the Cochrane Collaboration to assess the risk of bias of studies included in a systematic review:13 1) adequate allocation concealment, based on the approach by Schulz and Grimes;14 2) blinding methods (participant, investigator, or outcome assessor); 3) how incomplete data were addressed (did the study analyze the data based on the intention-to-treat principle, i.e., were all subjects who were randomized included in the outcomes analyses), 4) reasons for dropouts/attrition reported. Studies were rated good, fair or of poor quality. A rating of good generally indicated that the trial reported adequate allocation concealment, blinding, analysis by intent-to-treat, and reasons for dropouts/attrition were reported. Studies were generally rated poor if the method of allocation concealment was inadequate, blinding was not defined, analysis by intent-to-treat was not utilized and reasons for dropouts/attrition were not reported and/or there was a high rate of attrition.
Quality assessment for non-randomized studies was based on: 1) population, 2) outcomes, 3) measurement, 4) confounding, and 5) intervention (if applicable). We assessed whether the study fulfilled the descriptive characteristics for each element (see Appendix B). Studies were considered to be of higher quality and more applicable if they were prospective, explicitly defined severe hypoglycemia, used multivariate analysis and included patients representative of typical patients with type 2 diabetes.
DATA SYNTHESIS
We constructed evidence tables showing the study characteristics for all included studies. Outcomes tables were organized by key question. We critically analyzed studies to compare their characteristics, methods, and findings. We compiled a summary of findings for each key question and drew conclusions based on qualitative synthesis of the findings or pooled results, where appropriate.
For Key Question #1, data were pooled and analyzed in Comprehensive Meta-Analysis software© (Biostat, Inc., Englewood, NJ). Risk ratios (RR) were calculated using a random-effects model if substantial heterogeneity was present. Statistical heterogeneity between trials was assessed using the I2 test with a score of 50% or greater suggesting moderate to substantial heterogeneity among studies.
PEER REVIEW
A draft version of this report was reviewed by technical experts as well as clinical leadership. Their comments and our responses are shown in Appendix C.
Publication Details
Copyright
Publisher
Department of Veterans Affairs (US), Washington (DC)
NLM Citation
Bloomfield HE, Greer N, Newman D, et al. Predictors and Consequences of Severe Hypoglycemia in Adults with Diabetes - A Systematic Review of the Evidence [Internet]. Washington (DC): Department of Veterans Affairs (US); 2012 Apr. METHODS.
