NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Dwivedi Y, editor. The Neurobiological Basis of Suicide. Boca Raton (FL): CRC Press/Taylor & Francis; 2012.
9.1. OVERVIEW OF NEUROIMAGING STRATEGIES
Neuroimaging methods provide a great opportunity to improve our understanding of the pathway between adverse environmental conditions and suicide (see Figure 9.1). Since neuroimaging is completed in vivo, it is tailored to investigate the intermediary links between neuropathology and the symptoms or traits associated with suicide since such symptoms and traits may be measured at the time of brain scanning. As well, links between environmental conditions and neuropathology associated with suicide may be investigated since neuroimaging may be conducted during different environmental conditions.
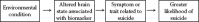
FIGURE 9.1
The strength of neuroimaging methods is to elucidate steps as outlined in the above diagram. In vivo imaging offers the opportunity to link environmental conditions with biomarker changes and in turn link these biomarkers with symptoms or traits implicated (more...)
In this chapter, the neurobiological targets related to suicide presented include serotonin 5-HT2A receptors, serotonin transporters (5-HTT), monoamine oxidase A, D2 receptor binding, and μ-opioid receptors. Recent discoveries in the area of 5-HT2A receptor, serotonin transporter, and MAO-A imaging have led to a pathophysiological understanding for the mechanism of pessimism in major depressive disorder (MDD). Neuroimaging of MAO-A, D2 and μ-opioid imaging are leading toward better understanding of mechanisms of impulsivity. An important ongoing direction is determining how environmental conditions lead to alterations in brain neurochemistry that can increase the risk for suicide and this will be illustrated in an example of neuroimaging MAO-A in early postpartum.
9.2. 5-HT2ARECEPTORS AND SUICIDE
9.2.1. Greater Prefrontal Cortex 5-HT2a Binding: Most Frequent Abnormality in Suicide
In the year 2000, it could be argued that the most consistent postmortem biological abnormality of suicide was increased serotonin2A receptor binding in the prefrontal cortex. Beginning in 1984, a number of investigations examined serotonin2A receptor binding in suicide victims and it was often reported that this binding was increased in the prefrontal cortex, most commonly in Brodmann area 9.1–11 At the time of study, these were reported as alterations in serotonin2 receptor binding but it is generally believed that these studies represented investigations of serotonin2A receptors given that ligand binding to 5-HT2C receptors in cortex is extremely low12,13 and mRNA of 5-HT2B receptors is extremely low in cortex.14 These findings were reported in studies in which diagnosis of the suicide victim was unrestricted. However, these findings were more consistent in the subsample of studies of depressed suicide victims and medication-free suicide victims.4,7
It was unclear whether this finding was associated with a specific diagnosis, a symptom cluster associated with suicide, or represented a direct marker of suicidal ideation. It had been proposed that this finding was somewhat more common in studies of suicide victims who died of violent means,1,3,5,6,15,16 but it was unclear how this explanation linked this abnormality to symptoms, life events, or personality traits. For example, it could be argued that selection of violent means of suicide represented greater planning of suicide or a predisposition toward violent behavior. However, one psychological autopsy investigation suggested that the latter association was possible.17 Since it is much easier to recruit subjects with specific diagnoses in vivo, most neuroimaging studies of 5-HT2A receptors focused upon three diagnostic areas in relation to 5-HT2A receptors: MDD, borderline personality disorder, and aggressive behavior.
9.2.2. Greater Prefrontal Cortex 5-HT2a Binding: A Diagnostic and Symptom-Specific Finding
If one examines the list of neuroimaging studies of 5-HT2A receptors that purely compare depressed and healthy samples as found in Table 9.1, they appear to contradict postmortem findings as many of these between-group comparisons reported a regional decrease in 5-HT2A binding. The discrepancy can be resolved substantially through the observation that studies in which subjects recently received selective serotonin reuptake inhibitor (SSRI) treatment usually report decreased regional 5-HT2A receptor binding.18–20,23,26,29,30 Since the initial studies in the field sampled people with recent antidepressant treatment, the initial impression derived from this work was that regional 5-HT2A binding tended to be reduced in MDD.
TABLE 9.1
Imaging Studies of 5-HT2A Receptors in Major Depressive Disorder.
However, the first two studies not sampling subjects who recently had antidepressant treatment found no difference between depressed and healthy subjects.21,22 The first study of medication-free subjects by Meyer et al. applied [18F]setoperone. [18F] Setoperone is a very good radioligand for imaging 5-HT2A receptors owing to its specific binding in cortex, reversibility, and favorable ratio of specific binding to free and nonspecific binding31–35 (see Meyer for a review of neuroimaging radioligands that bind to 5-HT2A receptor28). The study by Meyer et al. sampled medication-free (>6 months) subjects in the midst of a major depressive episode (MDE) from early onset MDD. Subjects also had no comorbid psychiatric illnesses. No difference in prefrontal cortex 5-HT2A binding was found as compared to healthy controls.21 The second study of medication-free subjects applied [18F]altanserin positron emission tomography (PET) in older depressed subjects and found no difference in 5-HT2A binding between patients and healthy controls.22 [18F]Altanserin is a reasonable technique as it has high specific binding ratio but there are radioactive metabolites that cross the blood–brain barrier, so groups that use this technique currently apply it with a bolus plus infusion approach.36 After considering medication-free status, there still was a discrepancy, but a lesser one, between studies of suicide victims and neuroimaging studies of MDEs: 5-HT2A density was often elevated in prefrontal cortex in postmortem studies of suicide victims yet 5-HT2A binding in prefrontal cortex was unchanged in medication-free depressed subjects.
One interpretation of the lack of difference in prefrontal cortex 5-HT2A binding between medication-free depressed and control subjects is that there is no relationship of this analysis to the investigations of suicide victims. This seems unlikely given that half of suicide victims have a diagnosis of MDD37,38 and that some of the postmortem studies that reported elevated 5-HT2A density in prefrontal cortex sampled subjects with MDD.37,38
An alternative perspective is that a subgroup of MDE subjects have the biological abnormality reported in suicide victims. To investigate this question, one could take advantage of the issue that 5-HT2A receptor density has an inverse relationship with extracellular serotonin levels such that the density of 5-HT2A receptors in cortex increases after chronic serotonin depletion and decreases after chronically raising extracellular serotonin.39–42 Based upon this relationship, the candidate subgroup are depressed subjects suspected of having low extracellular serotonin in prefrontal cortex.
The symptom used to identify this subgroup was the elevated pessimism (dysfunctional attitudes) observed during MDEs. There is a modest level of dysfunctional attitudes in health, which increase to a variable extent during depressive episodes.43–45 Greater pessimism during MDEs is an important symptom that contributes to the generation of sad mood and is targeted by cognitive therapy.43–45 This symptom also abates when SSRI treatment is successful.44,45 The rationale for choosing this symptom of elevated dysfunctional attitudes is that raising extracellular serotonin after administration of intravenous d-fenfluramine is associated with a strong shift in dysfunctional attitudes toward optimism 1 h later in healthy individuals.25 This argues that among the many roles of serotonin, one of them is to modulate dysfunctional attitudes in humans. Both the anterior cingulate cortex and subregions of prefrontal cortex (dorsolateral and medial prefrontal cortex) participate in functions related to optimism and pessimism.46–49 It is convenient that dysfunctional attitudes can be measured with the dysfunctional attitudes scale (DAS), a measure sensitive for detecting negativistic thinking in the midst of depressive episodes,50,51 with very good internal consistency (Cronbach’s α = 0.85–0.87)52,53 and high test–retest reliability.43,53
A strong correlation was observed between severity of dysfunctional attitudes (pessimism) and the elevation in cortex 5-HT2A binding potential reflecting specific binding relative to free and nonspecific binding (BPND) was discovered. Moreover, cortex 5-HT2A BPND was significantly elevated in subjects with MDE and severe pessimism.25 For example, in the prefrontal cortex region centered on Brodmann area 9, 5-HT2A BPND was elevated 29% in depression subjects with dysfunctional attitude scores higher (more pessimistic) than the median for the group. There was a strong, significant correlation between severity of pessimism and prefrontal cortex 5-HT2A BPND (see Figure 9.2). A recent study by Bhagwagar et al. replicated this relationship between dysfunctional attitudes severity and prefrontal cortex 5-HT2A BPND in recovered depressed subjects.27 In a separate study of a large sample of healthy subjects, two personality facets related to pessimism, vulnerability, and anxiety were also positively correlated with prefrontal cortex, temporal cortex, and left insula 5-HT2A BPND.54
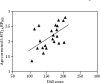
FIGURE 9.2
5-HT2A Binding potential in prefrontal cortex is associated with dysfunctional attitudes in depressed subjects. Age corrected 5-HT2A receptor binding potential (5-HT2A BPND) within bilateral prefrontal cortex (Brodmann area 9) in depressed subjects is (more...)
The investigations correlating severity of dysfunctional attitudes with greater 5-HT2A BPND 25,27 are highly consistent with postmortem investigations reporting greater 5-HT2A receptor density in the prefrontal cortex of suicide victims. Fifty percent of suicide victims have MDD.37,38 The DAS is highly correlated with hopelessness measured with the Beck Hopelessness Scale.55–58 Given that hopelessness is a risk factor for suicide,59,60 it is plausible that investigations of suicide victims reporting increased 5-HT2A BPND sampled depressed subjects with greater severity of pessimism. See Figure 9.3 that represents the relationship of sampling studies for MDD, dysfunctional attitudes, and suicide.

FIGURE 9.3
Elevated prefrontal cortex 5-HT2A density in suicide reflects sampling from major depressive disorder with pessimism and from aggressive behavior but not from borderline personality disorder.
Two other conditions that may be sampled in the studies of suicide victims are borderline personality and antisocial personality disorder.37,38,61 In two investigations of 5-HT2A binding in medication-free borderline personality disorder, no difference was found in the prefrontal cortex, although the second study reported an increase in 5-HT2A binding in hippocampus in a post hoc analysis.25,62 Meyer et al. investigated that 5-HT2A binding in aggressive individuals found no group difference in any region, but did report an age interaction such that 5-HT2A binding tended to increase considerably more with age in individuals with aggression.63 Some theories of aggression and impulsivity consider the mechanisms of inadequate pruning and it is possible that this age-related effect might represent an index of differential change in pyramidal cell loss, since most 5-HT2A receptors are found in apical dendrites of pyramidal cell neurons and the pattern of loss of pyramidal cells matches the loss in 5-HT2A receptors with age.64–67 A recent study of 5-HT2A receptors by Rosell et al. reported greater binding in orbitofrontal cortex in a group of aggressive individuals who met current criteria for intermittent explosive personality disorder, some of whom had personality disorders.68 A limitation of the latter study is that study subjects did not receive urine screening for substance abuse, a condition commonly comorbid with aggression, and such substances may bias 5-HT2A receptor binding.69,70 Hence, among the investigations of 5-HT2A binding there is support for increased binding in older individuals with aggression throughout cortex and in orbitofrontal cortex in people with aggression.
The findings of greater 5-HT2A density in the prefrontal cortex of suicide victims in postmortem study can be reinterpreted in light of diagnostic and symptom-specific findings from neuroimaging studies of 5-HT2A binding. MDD with high levels of pessimism is associated with greater prefrontal cortex 5-HT2A binding, is sampled heavily in studies of suicide victims because half of the suicide victims have MDD and hopelessness (a strong correlate of pessimism), which is associated with the risk of suicide.37,38,55–60 The diagnosis of borderline personality disorder does not contribute to the finding of greater prefrontal cortex 5-HT2A density in suicide victims since no change in prefrontal 5-HT2A BPND was found in this group.25,62 In those with aggressive behavior, at least a subgroup of subjects older than age 34 show a similar neurobiological finding and would be expected to also contribute to the original findings of greater prefrontal 5-HT2A density in suicide victims.63,68 See Figure 9.3 for a Venn diagram that represents the sampling contributions of the various diagnoses and symptoms to the finding of greater 5-HT2A density in suicide victims.
9.3. SEROTONIN TRANSPORTER BINDING AND SUICIDE
9.3.1. Discovery of Quantitative Method to Measure Serotonin Transporter Binding
In 2000, after 20 years of attempts, a major discovery in the field of serotonin transporter neuroimaging occurred with the development of [11C]-3-amino-4-(2-dimethylaminomethyl-phenylsulfanyl)-benzonitrile ([11C]DASB). The two previous radiotracers applied for in vivo imaging had limitations: 2-β-carbomethoxy-3-β-(4-iodophenyl)-tropane (β-CIT) single photon emission tomography (SPECT) was the first technique developed and the specific binding signal could be differentiated in the midbrain,71–73 but it has almost equal affinity for the dopamine transporter as compared to the serotonin transporter.74,75 As dopamine transporter density is high in the substantia nigra,76 one cannot determine the relative contributions of dopamine and serotonin transporter binding in midbrain (the location where this radiotracer technique is applied for an index of serotonin transporter binding). The other early radiotracer, [11C](+)McN5652 has a low ratio of specific binding relative to free and nonspecific binding, which in combination with modest reversibility makes valid and reliable quantitation difficult in regions other than the thalamus, and impossible in human cortex.77–80 The radiotracer [11C] (DASB, 3-amino-4-(2-dimethylaminomethylphenylsulfanyl)-benzonitrile) was a major advance owing to its selectivity, reversibility, greater specific binding relative to free and nonspecific binding, and reliability.81–89 This radiotracer was three orders of magnitude more selective for the 5-HTT over other monoamine transporters and highly selective for the 5-HTT in comparison to a number of other targets screened.88,89 Selectivity was further confirmed as 92%–95% of the specific binding to 5-HTT was displaceable by 5-HTT binding medications in animal models.88,89 [11C]DASB has very good brain uptake in humans.81,82 In humans, its ratio of specific binding relative to free and nonspecific is good and its free and nonspecific binding has low between-subject variability.81,83 Multiple brain regions may be assessed with noninvasive methods81–89 and reliability of regional 5-HTT BPND measures is very good.85–87,90 The 5-HTT BPND measures are low in cortex, but with standardized region of interest methods, very good reliability of 5-HTT BPND in human cortex was achieved.85–87,90 In summary, [11C]DASB PET imaging was a discovery that created a new opportunity for quantifying 5-HTT binding in humans.
Given that depleting serotonin in humans can lead to sad mood, as evidenced in the tryptophan depletion paradigm,91–95 that 5-HT2A receptor binding is often elevated in samples of suicide victims1–11 and during pessimism with MDD,25,27 and that 5-HT2A receptor binding shows an inverse relationship to extracellular serotonin,39–42 understanding whether extracellular serotonin levels may be lower in suicide is an important question. Direct evidence that serotonin is low in suicide is difficult to obtain: brain serotonin cannot be directly measured in vivo and it is likely, based upon animal simulations of postmortem delay, that serotonin levels are very unstable, even within 24 h of death.96 Moreover, postmortem investigations (previously listed by Mann et al.97 and Stockmeier11) have difficultly sampling medication-free subjects.
9.3.2. Serotonin Transporter Binding and Disease Models
Measurement of serotonin transporter binding has considerable value in several different models of disease that may affect extracellular serotonin levels. There are four plausible models to consider in regard to how serotonin transporter binding could be altered in a disease that lowers extracellular brain serotonin.70 These are referred to here as models one through four. Model one is a lesion model that reduces monoamine-releasing neurons. In a lesion model, reductions in binding occur. Model two is a model of secondary change in serotonin transporter binding consequent to serotonin lowering via a different process. Model three is increased clearance of extracellular monoamine via greater monoamine transporter density. In model three, greater available serotonin transporter binding leads to greater clearance of monoamines from extracellular locations. Model four is endogenous displacement and is dependent upon the properties of the radioligand. Endogenous displacement is the property of a few radioligands to express different binding after short-term manipulations of their endogenous neurotransmitter. Abnormalities in serotonin transporter binding during MDEs may be discussed in the context of these models.
Model two is unlikely to be relevant for serotonin transporter binding. Acute reductions in serotonin have repeatedly shown reductions in 5-HTT mRNA.98–100 However, longer-term reductions or elevations in serotonin typically show no effect upon regional 5-HTT density.101–103 However, it is worth noting that available evidence suggests that the different monoamine transporters do not regulate in the same fashion after chronic depletion of their endogenous monoamine. In contrast, for dopamine transporters in striatum, the evidence to support a relationship between long-term reductions in extracellular dopamine and a lowering of striatal dopamine transporter density is fairly strong.104–107 It is reported that norepinephrine density decreases in the locus coeruleus after chronic norepinephrine depletion.108
Model four is unlikely for [11C]DASB, but it is unclear whether this model would complicate interpretation of other serotonin transporter binding radiotracers because it has not been investigated for other serotonin transporter binding radiotracers. Endogenous displacement refers to the property, found in a minority of PET radiotracers under physiological conditions, to have increased binding potential measures after an acute reduction in endogenous neurotransmitter.109 The phenomenon described is that the neurotransmitter itself prevents access of the radiotracer to receptors through competition. For [11C]DASB, endogenous displacement may occur with exceptionally large magnitude changes in extracellular 5-HT but this would not be expected to occur with extracellular 5-HT changes that are physiologically tolerable for humans. In an animal study with [11C]DASB, after an intraperitoneal injection of 10 mg/kg of the MAO-A/B inhibitor tranylcypromine, which raises extracellular serotonin several hundred to thousand percent,110–112 a reduction in 5-HTT BPND was observed.113 This has been replicated in animals with similar doses of tranylcypromine.114 However, humans cannot tolerate one-tenth of this dose of tranylcypromine even with lengthy titrations and oral administration. Thus, this magnitude of serotonin change likely exceeds what is physiologically tolerable in humans. In accord with this perspective, we found no effect of tryptophan depletion upon 5-HTT BPND in 14 humans, demonstrating that endogenous serotonin occupancy is unlikely to appreciably influence [11C]DASB binding under physiologically tolerable conditions.87 Talbot et al. reported similar results in eight humans.115 For other PET radiotracers such as [11C]-N,N-dimethyl-2-(2′-amino-4′-hydroxymethylphenylthio)benzylamine ([11C]HOMADAM), [11C] MADAM, trans-1,2,3,5,6,10-β-Hexahydro-6-[4-(methylthio) phenyl]-pyrrolo-[2,1-a]-isoquinoline ([11C](+)McN5652), or SPECT radiotracers, single photon emission tomography 2-beta-carbomethoxy-3-beta-(4-iodophenyl)-tropane (β-CIT SPECT), or (2-([2-([dimethylamino]methyl)phenyl]thio)-5-123I-iodophenylamine) ([123I]ADAM), it is it is unknown whether endogenous levels of serotonin influence binding levels. This fourth model is unlikely to apply to PET imaging studies with [11C]DASB in humans but it is unclear as to whether this model applies to other serotonin transporter radiotracers since the question has not been tested.
9.3.3. Relationship of Serotonin Transporter Imaging to Dysfunctional Attitudes and Major Depressive Disorder
Dysfunctional attitude is an important symptom of MDD, particularly because it is strongly related to suicide. Hopelessness59,60 (and difficulty seeing positive reasons for living116) is an important risk factor for suicide and it has been clearly established in four separate samples of depressed subjects that greater hopelessness is associated with greater severity of dysfunctional attitudes as measured with the DAS.55–58 The DAS43 is a sensitive measure for detecting pessimistic thinking in the midst of MDE50,51 that has very good internal consistency (Cronbach’s α = 0.85–0.87)52,53 and high test–retest reliability.43,53
Low extracellular serotonin in prefrontal and anterior cingulate cortex during MDD is a highly plausible explanation for the strong correlation between prefrontal and anterior cingulate cortex 5-HT2A receptor binding and dysfunctional attitudes. Key subregions of prefrontal cortex (particularly the medial prefrontal cortex and dorsolateral prefrontal cortex) as well as the anterior cingulate cortex participate in cognitive functions related to optimism/pessimism.46–49
Modulation of extracellular serotonin can shift dysfunctional attitudes: Elevating extracellular serotonin abruptly in healthy humans via intravenous d-fenfluramine administration (in contrast to control condition) shifted perspective toward optimism as measured by the DAS.25 The interpretation of this shift toward optimism after d-fenfluramine was that one of the functions of extracellular serotonin in humans is to reduce pessimism.25 A property of 5-HT2A receptors is that 5-HT2A receptor density has an inverse relationship with extracellular serotonin levels such that the density of 5-HT2A receptors in cortex increases after chronic serotonin depletion and decreases after chronically raising extracellular serotonin.39–42 Therefore, an interpretation of the finding that prefrontal and anterior cingulate cortex 5-HT2A BPND was significantly elevated in subjects with MDE and severe pessimism,25 and that 5-HT2A BPND in these regions is positively associated with severity of dysfunctional attitudes,25,27 is that extracellular serotonin is low in MDD with severe pessimism.
There are only two postmortem investigations of 5-HTT density in subjects with recent symptoms of depressive episodes.117,118 In these investigations, one of which concurrently sampled suicide victims reported no changes in 5-HTT density in the dorsal raphe or the locus coeruleus. Other postmortem investigations of 5-HTT density sampled subjects with a history of a depressive episode and these investigations usually studied the prefrontal cortex and/or dorsal raphe nucleus. Findings ranged from decreased 5-HTT density119–123 to no difference in 5-HTT density.124–128 In several of these studies, subjects were medication free,121,124,125 and for many of these investigations, average postmortem delay was less than a day.117–119,121,123,127 For greater detail, the reader is referred to the review of Stockmeier.11 Other sampling issues that may influence postmortem investigations are effects of additionally sampling patients with bipolar disorder and possible differences between early versus late onset MDD. None of the postmortem studies investigated the relationship between 5-HTT binding to indices of hopelessness and pessimism.
The first application of [11C]DASB PET imaging to investigate MDD investigated the relationship of 5-HTT BPND to severity of dysfunctional attitudes and presence of a MDE. In this study, Meyer et al. sampled 20 subjects with MDEs (from early onset MDD) and 20 healthy controls.84 Subjects were medication free for at least 3 months, had no other comorbid axis I illnesses, were nonsmoking, and had early onset depression. There was no evidence for a difference in regional 5-HTT BPND in the group with MDEs and those in the midst of health. However, subjects with MDEs and severely pessimistic dysfunctional attitudes had significantly higher 5-HTT BPND, compared to healthy in brain regions sampling serotonin nerve terminals (dorsolateral and medial prefrontal cortex, anterior cingulated cortex, thalamus, bilateral caudate, and bilateral putamen). On average, 5-HTT BPND was 21% greater in these regions in depressed subjects with severely pessimistic dysfunctional attitudes. Moreover, within the MDE group, greater 5-HTT BPND was strongly associated with more negativistic dysfunctional attitudes in the same brain regions (see Figure 9.4). The interpretation was that serotonin transporters have an important role in influencing extracellular serotonin during MDEs: Greater regional 5-HTT levels can provide greater vulnerability to low extracellular 5-HT and symptoms of extremely negativistic dysfunctional attitudes. This interpretation, in subjects with high levels of pessimism during MDE, corresponds to model number three (see earlier under Section 9.3.2).
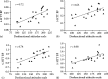
FIGURE 9.4
Correlations between dysfunctional attitudes scale (DAS) and serotonin transporter binding potential (5-HTT BP) in some of the larger regions in depressed subjects. Highly significant correlations were found: (a) dorsolateral prefrontal cortex (p = 0.0004), (more...)
In general, neuroimaging studies that sample subjects who have early onset MDD, are medication free for greater than 2 months, are nonsmoking, and do not have comorbid axis I disorders and apply better quality radiotracer technology tend to find either no change in regional 5-HTT binding or an increase in regional 5-HTT binding.84,129–131 Investigations that include sampling of subjects with late onset MDD, comorbid axis I psychiatric disorders, recent antidepressant use, and current cigarette smoking and do not apply a selective radiotracer are more likely to report a reduction in regional 5-HTT binding.132–136 Only the first study of [11C]DASB PET concurrently investigated a measure of pessimism or hopelessness.
9.3.4. Relationship of Serotonin Transporter Imaging to Postmortem Investigations of Suicide Victims
In addition to the series of postmortem studies of 5-HTT binding in depression (with or without concurrent suicide), a pair of studies, both from the same group, reported decreased 5-HTT binding in the ventral prefrontal cortex associated with suicide, independent of diagnosis.121,137 In the larger study, a subanalysis was carried out comparing the 43 suicide victims with a history of depression to 20 suicide victims without a history of depression and significantly lower 5-HTT binding in ventral prefrontal cortex was found in the suicide victims.121 As well, the entire group of 82 suicide victims had lower ventral prefrontal cortex 5-HTT binding as compared to nonsuicide controls.121
Subsequent neuroimaging studies have identified the conditions associated with decreased global 5-HTT binding and a recent study describes a region-specific pattern that matches of a predominant lower of ventral prefrontal cortex binding. Global reductions in 5-HTT binding may occur after antidepressant treatment,78,85,86,138 or in winter season relative to summer season.139,140 After ecstasy abuse, particularly if the abuse occurred within the past 4 months, there is a reduction in 5-HTT binding with a preferential effect in cortex regions as compared to subcortical regions (striatum and midbrain).141–143 One of two recent investigations of obsessive–compulsive disorder also report a region-specific effect such that the lowest 5-HTT binding is in ventral prefrontal cortex.144,145 In general, substance abuse and anxiety disorders are associated with greater risk for suicide; therefore, it is theoretically possible that ecstasy abuse from several months earlier, or presence of obsessive–compulsive disorder, could contribute to reduced ventral prefrontal cortex 5-HTT binding in suicide victims.121
Another possibility that requires further investigation is whether 5-HTT binding in impulsivity disorder or other aspects related to risk for suicide is related to the reduction in 5-HTT binding. Interestingly, a loss of 5-HTT binding occurred in peer-reared rhesus monkeys with early maternal separation (who later develop aggressiveness and impulsivity), but the pattern of 5-HTT binding loss, which included midbrain, thalamus, caudate, putamen, and anterior cingulate cortex, did not extend into the prefrontal cortex.146 Takano et al. reported that neuroticism, which can include impulsivity, was positively associated with 5-HTT binding in the thalamus and Kalbitzer et al. reported a negative correlation between neuroticism and openness to values, the latter which then correlated negatively with 5-HTT binding in most subcortical (but not cortical) regions.147,148 There is a study of 5-HTT binding in aggression applying [11C]McN5652 PET reporting decreased 5-HTT binding in the anterior cingulate cortex149 and a study of [123I]ADAM reporting greater 5-HTT binding in brain stem of people with borderline personality disorder,150 but, unfortunately, neither of these two radiotracers has sufficient specific binding signal to assess whether such binding changes occur in prefrontal cortex. A recent study of 5-HTT binding in alcohol abuse was negative.151 To date, neuroimaging studies of 5-HTT binding in impulsivity (or conditions related to impulsivity) have not matched the ventral prefrontal cortex pattern of 5-HTT binding loss described in the two postmortem studies that specifically focused upon this region.121,137
9.4. MONOAMINE OXIDASE A AND SUICIDALITY
Monoamine oxidase A (MAO-A) is a target of interest in research of suicidality given its role in metabolizing monoamines and its widespread distribution throughout the brain. In the brain, the predominant location for this enzyme is on the outer mitochondria membranes in neurons,152 and monoamine oxidase A density is highest in brain stem (locus coeruleus), lower in the hippocampus, cortex, striatum, and minimal in white matter tissue.152,153 Monoamine oxidase A metabolizes serotonin given that serotonin is a high affinity substrate for MAO-A,154–157 MAO-A is detectable in serotonin-releasing neurons,158,159 and that MAO-A clearly influences extracellular serotonin as administration of MAO-A inhibitors increase extracellular serotonin from 20% to 200%, depending upon drug, dose, and region.110,160–165 Norepinephrine157,166 and dopamine154–156 are also high affinity substrates for MAO-A,157,166 and there is evidence that under MAO-A inhibition, extracellular concentrations of these monoamines160,167–171 are raised. MAO-A is easily detectable in cells that synthesize norepinephrine152,158,159,172 but more difficult to detect in dopamine-synthesizing neurons.159,173 In knockout models of MAO-A, extracellular serotonin, norepinephrine, and dopamine are also raised substantively (100%–200%) in prefrontal cortex, hippocampus, and superior raphe nuclei.174
As of 2005, based upon postmortem study, it was unknown as to whether brain MAO-A binding or activity is abnormal during at risk states for suicide such as MDEs because each previous investigation of brain MAO-A had at least two important limitations that created significant heterogeneity in the samples175–180 such as nonspecificity of technique for MAO-A versus monoamine oxidase B, enrollment of subjects who recently took medication, unclear diagnosis of suicide victims, small sample size, and lack of categorizing between early onset depression and late onset depression. In contrast to the common, early onset depression prior to age 40, late onset depression likely has a different pathophysiology attributable to lesions and/or degenerative disease.181
Neuroimaging radioligands to measure an index of MAO-A levels in humans include [11C]clorgyline, deuterium-labeled [11C]clorgyline, [11C]harmine, and [11C] befloxatone.182–187 The latter two have an useful advantage in terms of having more rapid kinetics and greater reversibility. [11C]Harmine was also modeled in humans and it possesses high affinity for the MAO-A site, as well as high selectivity, excellent specific binding relative to free and nonspecific binding ratios, and high brain uptake.184,186,188–190 Given these properties and validation, it is the lead radiotracer for quantitating brain MAO-A binding in humans at this time.
9.4.1. Monoamine Oxidase A and Major Depressive Disorder
Prior to 2006, there were no studies of MAO-A density, activity, or mRNA in early onset MDD. In 2006, MAO-A VS, an index of MAO-A density, was measured in 17 MDE and 17 healthy subjects with [11C]harmine PET.190 All subjects were otherwise healthy. Depressed subjects had early onset depression (before age 40) and were drug free for at least 5 months although most were antidepressant naïve. Depressed subjects were aged 18–50, met DSM-IV diagnosis of current MDE and MDD verified by SCID for DSM IV,191 were nonsmoking and had a reasonable severity of depression with a score greater than 17 on the 17-item HDRS. The MAO-A VS was highly significantly elevated (p < 0.001 each region, average magnitude 34% [or two standard deviations]) in the depressed subjects (see Figure 9.5). The study was considered definitive for showing that MAO-A binding is elevated in early onset depression because the magnitude was large, the sample was carefully defined, and the method is selective for MAO-A.

FIGURE 9.5
Comparison of MAO-A DVs between depressed and healthy subjects. On average MAO-A DVs was elevated by 34%, or two standard deviations, in depressed individuals. Differences between groups were highly significant in each region: *p = 0.001, **p < (more...)
This finding was subsequently replicated by the same group and the relationship between MAO-A binding and state of MDD was also investigated.192 During remission from MDD, MAO-A binding was elevated in most brain regions such as prefrontal cortex, anterior cingulate cortex, striatum, hippocampus, thalamus, and midbrain. Elevated MAO-A binding may be viewed as an index of a monoamine-lowering process and a historical set of observations were known that had linked chronic monoamine-lowering processes with subsequent MDEs: During the development of reserpine-based antihypertensives in the 1950s, subsequent onset of MDE occurred typically 2 weeks to 4 months later.193 Consistent with this line of observation, those recovered MDD subjects who had recurrence of their MDEs in the subsequent 6 months had the highest levels MAO-A binding in the prefrontal cortex and anterior cingulate cortex at the time of scanning.192
The raised MAO-A binding during MDEs adds to a pathophysiological model of extracellular serotonin loss contributing to pessimism during MDEs. Greater MAO-A binding can be viewed as an index of greater MAO-A levels. When MAO-A levels are greater in cell lines or in brain homogenates, MAO-A activity shows a positively correlated parallel, and typically linear relationship.153,194–196 Hence, the index of greater MAO-A binding may be viewed as a mechanism of excessive serotonin removal. The inverse relationship between extracellular serotonin levels and dysfunctional attitudes as demonstrated by the shift in dysfunctional attitudes after intravenous d-fenfluramine and the reduction in dysfunctional attitudes after SSRI treatment suggests a role for extracellular serotonin in modulating optimism/pessimism.25 Brain regions most strongly associated for a role in optimism/pessimism include the anterior cingulate cortex, and the medial and dorsolateral subdivisions of the prefrontal cortex.46,197 5-HT2A receptor density has an inverse relationship to extracellular serotonin levels,39–42 and greater 5-HT2A receptor binding was observed in these regions during MDD with higher levels of pessimism.25,27 5-HTT has a role in clearing extracellular serotonin, and a positive correlation between total 5-HTT binding and severity of dysfunctional attitudes was found in the medial prefrontal cortex, the dorsolateral prefrontal cortex, and the anterior cingulate cortex.84 Collectively, the findings suggest that pessimism in the anterior cingulate cortex and prefrontal cortex is associated with greater 5-HT2A binding, which may reflect ongoing loss of extracellular serotonin in these regions, and potential sources of loss are excessive 5-HTT binding, and elevated MAO-A levels (see Figure 9.6).
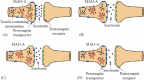
FIGURE 9.6
(See color insert.) Modern model of excessive extracellular serotonin loss during major depressive disorder (A) serotonin release in a synapse in health. (B) During a major depressive episode, monoamine oxidase A (MAO-A) density is elevated resulting (more...)
9.4.2. Postpartum, Suicide, and Monoamine Oxidase A
On average, during pregnancy, the risk of suicide is lower and it elevates during postpartum, yet is still less than other times in life.198 However, when psychiatric illness such as postpartum depression (PPD) occurs, the risk of suicide elevates >10-fold.199,200 This issue, coupled with the fact that the general risk of a MDE during the postpartum period is 13%,201–205 illustrates the importance of investigating the neurobiology of early postpartum related to the risk for MDE and suicide.
The most likely psychiatric disturbances in the postnatal period affect mood and there are three types. One is the “baby blues” or maternity blues, which is common (occurring up to 75% of the time) and transient, usually finishing within the first week postpartum.206,207 The second is MDE with postpartum onset (“postpartum depression”), which is defined as a MDE that occurs within the first 4 weeks after delivery.208 Twenty percent of women with maternity blues will go on to have a MDE with postpartum onset.207,209 The third is postpartum psychosis (which may be accompanied by depressive symptoms). The third condition is rare occurring in 0.1%–0.2% of deliveries and is strongly associated with the presence of bipolar disorder.210,211
A profound change that occurs during the first week postpartum, when “postpartum blues” are common, is the enormous reduction in 17β-estradiol and estriol levels. It is well known that estradiol and estriol are produced by the placenta during pregnancy and that plasma levels raise 100-fold and 1000-fold, respectively.212 It is also well known that estradiol and estriol levels decrease abruptly during postpartum with loss of the placenta. Most of the decline is in the first 4 days with a modest decline thereafter.207,213,214 It is also a time of reduction in progesterone levels.
Declines in estrogen and progesterone have been implicated in generating sad mood. In an experimental paradigm involving a reduction in estrogen and progesterone, Bloch et al.215 found that women with a history of PPD had lowering of mood. In this paradigm, eight women with a history of PPD and eight women without a history of PPD were given a gonadotrophin-releasing agonist for 8 weeks and then the agonist was withdrawn (to simulate delivery and loss of placenta). Five of the eight women who had a history of PPD had sustained lowered mood, whereas none of the women who had no history of PPD had sustained lowered mood. Additional support for an inverse relationship between estrogen and mood was supported by the fact that estrogen (17β-estradiol) administration has reduced mood symptoms in some preliminary clinical trials as a treatment for MDE with postpartum onset and MDE in postmenopausal women.216–223 These findings implicate an inverse relationship between estrogen change and mood; however, it was unclear how this might link to the pathophysiology of MDD.
A candidate link between estrogen loss and the pathophysiology of MDD was the relationship between changes in estrogen levels and changes in MAO-A density. Within regions of high MAO-A density, repeated (increased) estrogen administration is associated with reductions in MAO-A density, mRNA, and/or activity in contrast to estrogen depletion, which is associated with increases in MAO-A density, mRNA, and/or activity: Estradiol administration for 1–3 weeks consistently lowers MAO-A activity in high density areas (amygdala and hypothalamus) in ovariectomized rats.224–226 In ovariectomized macaque monkeys, 1 month of estradiol administration reduced MAO-A mRNA in the dorsal raphe nucleus.227 In a separate study of ovariectomized macaque monkeys, 1 month of estradiol administration reduced MAO-A protein in the dorsal raphe region by ∼50%.228 Repeated estradiol administration is associated with a reduction in MAO-A activity in neuroblastoma cell lines. In a neuroblastoma cell line that expresses the human estrogen receptor, exposure to 17β-estradiol for 12 days lowers MAO-A activity.229 This finding was replicated with exposure to physiological levels of 17β-estradiol for 10 days.230 Although changes in several indices of MAO-A levels had been reported in relation to estrogen loss, MAO-A binding, mRNA, or activity had never been studied in any species in early postpartum.
Given the 100- to 1000-fold decline in estrogen over the first 4 days postpartum,201,213,214 the relationship between estrogen decline and elevation in MAO-A synthesis, and the link between greater MAO-A levels and lower mood, there was a strong rationale to neuroimage MAO-A binding in brain regions involved in affect regulation during early postpartum (during days 4–6). Applying [11C]harmine PET neuroimaging, we completed the first investigation of MAO-A binding in any species during the immediate postpartum period. The main finding is a significant elevation of MAO-A binding postpartum (magnitude of 43%) throughout all brain regions assayed (prefrontal cortex, anterior cingulate cortex, hippocampus, striatum, and thalamus) in the immediate postpartum period in healthy women (see Figure 9.7). A voxel-based analysis confirmed that the elevation in MAO-A binding was present throughout the gray matter of the brain (MAO-A density is minimal in white matter152,153).
FIGURE 9.7
Monoamine oxidase A binding in the immediate postpartum period. MAO-A VT is an index of harmine binding to tissue at equilibrium and is an index of MAO-A level. On average regional MAO-A, VT was elevated by 43% during the early postpartum period. Differences (more...)
This discovery provides a neurobiological model of postpartum blues in humans, involving a rapid decline in estrogen, followed by a rapid rise in MAO-A levels in affect-modulating structures in the brain, with subsequent sad mood and symptoms of postpartum blues. As mentioned earlier, estrogen removal has been associated with a rise in indexes of MAO-A levels,224,227,228,230,231 and the findings shown in Figure 9.8 argue that this relationship applies strongly during the immediate postpartum period in humans. Consistent with this neurobiological model, MAO-A binding was highest on day 5 postpartum, the day most strongly associated with the lowest mood in most investigations of postpartum blues.233 MAO-A VT measured in early postpartum can be viewed as an index for MAO-A levels. Changes in levels of MAO-A density parallel changes in MAO-A activity during paradigms of hormone administration.196 The acute rise in MAO-A levels in the early postpartum period can be interpreted as an important monoamine-lowering process and monoamine-lowering is associated with sad mood.91,92,95,233–237
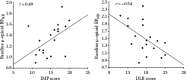
FIGURE 9.8
Association between impulsiveness (IMP) and deliberation (DLB) scores and baseline μ-opioid receptor availability. Areas in which significant differences in μ-receptor availability in vivo were observed between individuals with high and (more...)
A therapeutic implication of this neurobiological model is that one may target the proposed pathway of estrogen decline, rise in MAO-A levels, excessive metabolism of monoamines, and subsequent sad mood/distress. In order to prevent PPD, it may be useful to attenuate the severity of postpartum blues because greater severity of postpartum blues is associated with greater risk for PPD.207,238 Given the need to develop treatments that are compatible with breast-feeding and widespread use, dietary supplements of monoamine precursors in early postpartum may be a promising strategy to maintain a sufficient balance of monoamines during this time. For example, the administration of precursor supplements, such as the amino acids tryptophan for serotonin and tyrosine for norepinephrine and dopamine, could be investigated for the prevention of severe postpartum blues and PPD. Other strategies for prophylaxis in high risk groups could include inhibiting MAO-A, or raising multiple monoamines (that are metabolized by MAO-A with antidepressants). Less invasive strategies such as administration of amino acid precursors have potential for women at low risk of developing PPD, whereas interventions such as MAO-A inhibitors could be considered for those at high risk for PPD.
9.5. NEUROCHEMICAL ABNORMALITIES ASSOCIATED WITH PERSONALITY TRAITS OF IMPULSIVITY
Since the etiology of suicidal behavioral is multifactorial, one useful contribution from neuroimaging is the ability to quantitate the neurochemistry associated with specific risk factors for suicide. Earlier in this chapter, the neurochemistry of dysfunctional attitudes, a construct related closely to hopelessness, was presented. Similarly, greater impulsivity represents another factor associated with greater risk for death by suicide. Personality assessment instruments have been applied in combination with brain imaging and to date have identified strong relationships between impulsivity and MAO-A binding (in all brain regions assessed), striatal dopamine release, and ventral striatal μ-opioid binding.
9.5.1. Monoamine Oxidase A, Impulsivity, and Deliberation
There is a significant body of work to argue that reductions in MAO-A function during development lead to impulsivity in rodents. In rodents, both MAO-A KO and MAO-A inhibitor administration during development are associated with greater aggression, decreased latency to attack in the resident–intruder paradigm, and enhanced fear conditioning.239–243 MAO-A-deficient animals also show less positive social exploration and fewer investigative behaviors such as time spent exploring novelty, nose-poking, and social investigations of cage intruders.240,242,243
It has been argued that excess serotonin during neurodevelopment, which may occur during reduced MAO-A function,110,154–157,160–165 is important in developing impulsive behavior. Excess brain serotonin (5-HT) that occurs during development disrupts the organization of somatosensory thalamocortical afferents and retinal afferents in the dorsal lateral geniculate nucleus and superior colliculus.244,245 The abnormal development of thalamocortical afferents is also associated with abnormalities in the somatosensory cortex barrel fields.245 It has been proposed that impulsive aggression observed in MAO-A KO mice is consequent to impairment in organizing sensory input secondary to the loss of barrel field structure in the somatosensory cortex.246,247
Although experimentally induced reductions in MAO-A function during development lead to impulsivity in animals, it need not necessarily be an influential mechanism in humans. However, there was reason to suspect that these animal models have relevance for some subpopulations of people. Men who are deficient in MAO-A, consequent to an X-chromosome defect at the vicinity of the MAO-A gene, often show aggressive behavior.248 Also, a genotype associated with low levels of MAO-A from in vitro assay was associated with greater risk of developing antisocial behavior.249,250 Low 5-hydroxyindoleacetic acid (5-HIAA) levels in cerebrospinal fluid (CSF) are associated with greater likelihood of developing antisocial personality disorder and aggressive criminal behavior.251–253 While this dataset has been traditionally interpreted as a crude surrogate of extracellular serotonin levels, it is possible that it represents a reduction in MAO-A levels since lower 5-HIAA levels can occur when MAO-A is pharmacologically inhibited.254,255
With respect to phenotype, it was shown that reductions in MAO-A function during development are associated with greater impulsivity in animals, and it was shown that in rare cases in humans when there is a severe deficiency in MAO-A there is aggressive behavior.248 However, this does not necessarily mean that the phenotypic relationship applies frequently, that is, it does not necessarily hold that MAO-A levels have a relationship with impulsivity levels in the general population. Two neuroimaging studies have investigated this question and demonstrated that there is a strong relationship between reductions in MAO-A binding and levels of aggression and impulsivity. In the first study, applying [11C]clorgyline PET, Alia-Klein et al. demonstrated that reduced prefrontal cortex MAO-A binding is associated with self-reported aggression in males.256 The second study investigated both genders, and in addition to replicating the relationship between prefrontal cortex MAO-A binding and the impulsive/aggressive facets of personality, it also identified a second covariation between MAO-A and deliberation, such that lower levels of deliberation were associated with lower MAO-A binding. In the latter study, 38% of the variance in prefrontal cortex MAO-A binding was accounted for by the personality facets of angry hostility and deliberation, clearly implicating a strong relationship between these two personality facets and MAO-A binding.257 In both studies, the correlations between the personality traits and MAO-A binding were present throughout the brain regions assessed; however, this is probably due to the issue that MAO-A binding in different brain regions covaries and it is suspected that MAO-A binding in particular brain regions that participate in impulsivity and planning, such as the orbitofrontal cortex and dorsolateral prefrontal cortex,258 is where the relationship between these two measures is most important.
9.5.2. Dopamine and Impulsivity
Dopamine release in the nucleus accumbens is associated with exposures to rewarding stimuli259–261 and it was postulated that a greater biological tendency toward the reward from salient stimuli contributes toward impulsivity.262 In a recent study applying the D2/D3 radiotracer [18F]fallypride with PET, Buckholtz et al. discovered that trait impulsivity inversely associated with D2/D3 binding in the substantia nigra/ventral tegmental area.263 They also found that trait impulsivity positively associated with striatal responsivity to d-amphetamine. In an earlier study, Dalley et al. found that reduced ventral striatal D2/D3 binding with [18F]fallypride PET was also associated with greater levels of impulsivity.262 Since reduced D2/D3 binding may occur when extracellular dopamine is increased, one interpretation of these findings is that in humans, lesser availability of autoreceptors in the substantia nigra/ventral tegmental area leads to lesser inhibitory function predisposing toward dopamine release, which, in the context of salient stimuli, leads to impulsiveness.
9.5.3. μ-Opioid Receptors and Impulsivity
Some behavioral studies in humans and rodents have supported a link between pharmacological challenges upon the μ-opioid receptor and impulsive behavior. Some studies report greater impulsive behavior in the context of rewarding circumstances when agonists are present and reduced impulsive behavior in similar context with some antagonists.264–266 Furthermore, motivation and positive behavioral responses to rewards are enhanced by administration of μ-opioid agonists in the ventral striatum/nucleus accumbens.268 Love et al. recently reported that high impulsiveness and low deliberation, two orthogonal facets of the NEO Personality Inventory Revised,268 were associated with higher μ-opioid receptor binding in the ventral striatum, as well as in the anterior cingulate cortex and medial prefrontal cortex.269 In addition, in this study, low deliberation was associated with greater right prefrontal cortex and thalamus binding. There is one study of μ-opioid binding, applying [3H]DAGO autoradiography in suicide victims reporting increased binding in the inferior prefrontal cortex, cingulate gyrus, postcentral gyrus, and medial temporal gyrus, although binding was increased in every region assessed, and at least by 20%.270 Given these findings, it would be interesting to assess in future neuroimaging work whether μ-opioid receptor binding and impulsivity are related in samples with psychiatric illnesses that have both impulsivity and greater risk for suicide.
9.6. CONCLUSIONS
While neuroimaging is limited by the range in biomarkers available, its ability for in vivo measurement enables discoveries bridging neurochemistry to specific clinic states. For example, the finding of greater 5-HT2A binding in the dorsolateral prefrontal cortex of suicide victims can be translated into diagnostic and symptom specificity: 5-HT2A binding is greater throughout the prefrontal cortex in MDD with high levels of pessimism (a symptom that creates risk for suicide).25,27 The mechanism for this finding is best explained by excessive serotonin loss consequent to greater levels of functional serotonin transporter and high levels of monoamine oxidase A (leading to greater metabolism).190,84 Greater monoamine oxidase A binding occurs throughout the brain in early postpartum and may explain the vulnerability to psychiatric illness, in particular MDD, which occurs in postpartum.271 Since MDD during early postpartum is associated with more than a 10-fold greater risk of suicide, preventing PPD is important.199,200 Potential biological strategies for prevention of PPD may involve suppressing the pathway between estrogen decline and MAO-A rise or dietary compensations for high MAO-A.
The findings of reduced ventral prefrontal 5-HTT binding in suicide victims are also present with similar regional specificity in a neuroimaging study of obsessive–compulsive disorder,144,145 but reduced 5-HTT binding may also be present in this region in ecstasy abuse within the previous 4 months, and in the winter relative to summer.139–143 Neuroimaging of impulsive macaque monkeys shows a pattern of brain 5-HTT loss that spares the ventral prefrontal region.146 Further neuroimaging studies are needed to understand the diagnostic and symptom specificity of 5-HTT change related to suicide.
Another advantage of in vivo imaging measurement is that it can be related to clinical measures associated with suicide, that are also sampled in vivo, proximal to the time of brain scanning. For example, impulsivity is associated with particular neurochemical findings: The association of greater impulsivity with reduced regional brain MAO-A binding in humans256,257 is implicated in altered neurodevelopment leading to altered neural architecture and organization of sensory input. Impulsivity in humans is also associated with neurochemical markers implicated in greater responsivity to reward such as enhanced ventral striatal dopamine release, reduced D2/D3 autoreceptor binding,263 and greater ventral striatal μ-opioid receptor binding.269 In the future, it is possible that these findings will represent new therapeutic targets for impulsivity-reducing treatments so as to reduce the risk of suicide.
REFERENCES
- 1.
- Arango V, Ernsberger P, Marzuk PM. et al. Autoradiographic demonstration of increased serotonin 5-HT2 and beta-adrenergic receptor binding sites in the brain of suicide victims. Arch Gen Psychiatry. 1990;47(11):1038–1047. [PubMed: 2173513]
- 2.
- Arango V, Underwood M, Mann J. Alterations in monoamine receptors in the brain of suicide victims. J Clin Psychopharm 199212(2)8S–12S. [PubMed: 1315808]
- 3.
- Arora RC, Meltzer HY. Serotonergic measures in the brains of suicide victims: 5-HT2 binding sites in the frontal cortex of suicide victims and control subjects. Am J Psychiatry. 1989;146(6):730–736. [PubMed: 2729424]
- 4.
- Hrdina P, Vu T. Chronic fluoxetine treatment upregulates 5-HT uptake sites and 5-HT2 receptors in rat brain: An autoradiographic study. Synapse. 1993;14:324–331. [PubMed: 8248854]
- 5.
- Mann JJ, Stanley M, McBride PA, McEwen BS. Increased serotonin2 and beta-adrenergic receptor binding in the frontal cortices of suicide victims. Arch Gen Psychiatry. 1986;43(10):954–959. [PubMed: 3019268]
- 6.
- Stanley M, Mann JJ. Increased serotonin-2 binding sites in frontal cortex of suicide victims. Lancet. 1983;1(8318):214–216. [PubMed: 6130248]
- 7.
- Yates M, Leake A, Candy JM, Fairbairn AF, McKeith IG, Ferrier IN. 5HT2 receptor changes in major depression. Biol Psychiatry. 1990;27(5):489–496. [PubMed: 2310804]
- 8.
- Stockmeier CA, Dilley GE, Shapiro LA, Overholser JC, Thompson PA, Meltzer HY. Serotonin receptors in suicide victims with major depression. Neuropsychopharmacology. 1997;16(2):162–173. [PubMed: 9015799]
- 9.
- Turecki G, Briere R, Dewar K. et al. Prediction of level of serotonin 2A receptor binding by serotonin receptor 2A genetic variation in postmortem brain samples from subjects who did or did not commit suicide. Am J Psychiatry. 1999;156(9):1456–1458. [PubMed: 10484964]
- 10.
- Pandey GN, Dwivedi Y, Rizavi HS. et al. Higher expression of serotonin 5-HT(2A) receptors in the postmortem brains of teenage suicide victims. Am J Psychiatry. 2002;159(3):419–429. [PubMed: 11870006]
- 11.
- Stockmeier CA. Involvement of serotonin in depression: Evidence from postmortem and imaging studies of serotonin receptors and the serotonin transporter. J Psychiatr Res. 2003;37(5):357–373. [PubMed: 12849929]
- 12.
- Hoyer D, Pazos A, Probst A, Palacios JM. Serotonin receptors in the human brain. II. Characterization and autoradiographic localization of 5-HT1C and 5-HT2 recognition sites. Brain Res. 1986;376(1):97–107. [PubMed: 2941113]
- 13.
- Marazziti D, Rossi A, Giannaccini G. et al. Distribution and characterization of [3H] mesulergine binding in human brain postmortem. Eur Neuropsychopharmacol. 1999;10(1):21–26. [PubMed: 10647092]
- 14.
- Schmuck K, Ullmer C, Engels P, Lubbert H. Cloning and functional characterization of the human 5-HT2B serotonin receptor. FEBS Lett. 1994;342(1):85–90. [PubMed: 8143856]
- 15.
- Mann J. Postmortem studies of suicide victims. In: Watson S, editor. Biology of Schizophrenia and Affective Disease. London, U.K.: American Psychiatric Press Inc; 1996. pp. 197–221.
- 16.
- Hrdina PD, Demeter E, Vu TB, Sotonyi P, Palkovits M. 5-HT uptake sites and 5-HT2 receptors in brain of antidepressant-free suicide victims/depressives: Increase in 5-HT2 sites in cortex and amygdala. Brain Res. 1993;614(1–2):37–44. [PubMed: 8348328]
- 17.
- Dumais A, Lesage AD, Lalovic A. et al. Is violent method of suicide a behavioral marker of lifetime aggression? Am J Psychiatry. 2005;162(7):1375–1378. [PubMed: 15994723]
- 18.
- Dhaenen H, Bossuyt A, Mertens J, Bossuyt-Piron C, Gijesmans M, Kaufman L. SPECT imaging of serotonin2 receptors in depression. Psychiatry Res Neuroimaging. 1992;45:227–237. [PubMed: 1292040]
- 19.
- Biver F, Wikler D, Lotstra F, Damhaut P, Goldman S, Mendlewicz J. Serotonin 5-HT2 receptor imaging in major depression: Focal changes in orbito-insular cortex. Br J Psychiatry. 1997;171:444–448. [PubMed: 9463603]
- 20.
- Attar-Levy D, Martinot J-L, Blin J. et al. The cortical serotonin2 receptors studied with positron emission tomography and [18F]-setoperone during depressive illness and anti-depressant treatment with clomipramine. Biol Psychiatry. 1999;45:180–186. [PubMed: 9951565]
- 21.
- Meyer J, Kapur S, Houle S. et al. Prefrontal cortex 5-HT2 receptors in depression: A [18F] setoperone PET imaging study. Am J Psychiatry. 1999;156:1029–1034. [PubMed: 10401447]
- 22.
- Meltzer C, Price J, Mathis C. et al. PET imaging of serotonin type 2A receptors in late-life neuropsychiatric disorders. Am J Psychiatry. 1999;156(12):1871–1878. [PubMed: 10588399]
- 23.
- Yatham LN, Liddle PF, Shiah I. et al. Brain serotonin2 receptors in major depression: A positron emission tomography study. Arch Gen Psychiatry. 2000;57(9):850–858. [PubMed: 10986548]
- 24.
- Messa C, Colombo C, Moresco R. et al. 5-HT(2A) receptor binding is reduced in drug-naive and unchanged in SSRI-responder depressed patients compared to healthy controls: A PET study. Psychopharmacology (Berl). 2003;167(1):72–78. [PubMed: 12632246]
- 25.
- Meyer JH, McMain S, Kennedy SH. et al. Dysfunctional attitudes and 5-HT(2) receptors during depression and self-harm. Am J Psychiatry. 2003;160(1):90–99. [PubMed: 12505806]
- 26.
- Mintun MA, Sheline YI, Moerlein SM, Vlassenko AG, Huang Y, Snyder AZ. Decreased hippocampal 5-HT2A receptor binding in major depressive disorder: In vivo measurement with [18F]altanserin positron emission tomography. Biol Psychiatry. 2004;55(3):217–224. [PubMed: 14744461]
- 27.
- Bhagwagar Z, Hinz R, Taylor M, Fancy S, Cowen P, Grasby P. Increased 5-HT(2A) receptor binding in euthymic, medication-free patients recovered from depression: A positron emission study with [(11)C]MDL 100,907. Am J Psychiatry. 2006;163(9):1580–1587. [PubMed: 16946184]
- 28.
- Meyer JH. Applying neuroimaging ligands to study major depressive disorder. Semin Nucl Med. 2008;38(4):287–304. [PubMed: 18514084]
- 29.
- van Heeringen C, Audenaert K, Van Laere K, et al. Prefrontal 5-HT2a receptor binding index, hopelessness and personality characteristics in attempted suicide. J Affect Disord. 2003;74(2):149–158. [PubMed: 12706516]
- 30.
- Audenaert K, Van Laere K, Dumont F. et al. Decreased frontal serotonin 5-HT 2a receptor binding index in deliberate self-harm patients. Eur J Nucl Med. 2001;28(2):175–182. [PubMed: 11303887]
- 31.
- Blin J, Pappata S, Kiyosawa M, Crouzel C, Baron J. [18F]Setoperone: A new high-affinity ligand for positron emission tomography study of the serotonin-2 receptors in baboon brain in vivo. Eur J Pharmacol. 1988;147:73–82. [PubMed: 3259509]
- 32.
- Blin J, Sette G, Fiorelli M. et al. A method for the in vivo investigation of the serotonergic 5-HT2 receptors in the human cerebral cortex using positron emission tomography and 18F-labeled setoperone. J Neurochem. 1990;54(5):1744–1754. [PubMed: 2182776]
- 33.
- Petit-Taboue MC, Landeau B, Osmont A, Tillet I, Barre L, Baron JC. Estimation of neocortical serotonin-2 receptor binding potential by single-dose fluorine-18-setoperone kinetic PET data analysis. J Nucl Med. 1996;37(1):95–104. [PubMed: 8544011]
- 34.
- Maziere B, Crouzel C, Venet M. et al. Synthesis, affinity and specificity of 18F-setoperone, a potential ligand for in-vivo imaging of cortical serotonin receptors. Nucl Med Biol. 1988;15(4):463–468. [PubMed: 3255742]
- 35.
- Fischman A, Bonab A, Babich J. et al. Positron emission tomographic analysis of central 5-hydroxytryptamine 2 receptor occupancy in healthy volunteers treated with the novel antipsychotic agent ziprasidone. J Pharmacol Exp Ther. 1996;279(2):939–947. [PubMed: 8930203]
- 36.
- Pinborg LH, Adams KH, Svarer C. et al. Quantification of 5-HT2A receptors in the human brain using [18F]altanserin-PET and the bolus/infusion approach. J Cereb Blood Flow Metab. 2003;23(8):985–996. [PubMed: 12902843]
- 37.
- Barraclough B, Bunch J, Nelson B, Sainsbury P. A hundred cases of suicide: Clinical aspects. Br J Psychiatry. 1974;125:355–373. [PubMed: 4425774]
- 38.
- Robins E, Murphy G, Wilkinson R, Gassner S, Kayes J. Some clinical considerations in the prevention of suicide based on a study of 134 successful suicides. Am J Public Health. 1959;49(7):888–899. [PMC free article: PMC1372909] [PubMed: 13661481]
- 39.
- O’Regan D, Kwok RP, Yu PH, Bailey BA, Greenshaw AJ, Boulton AA. A behavioural and neurochemical analysis of chronic and selective monoamine oxidase inhibition. Psychopharmacology. 1987;92(1):42–47. [PubMed: 3110827]
- 40.
- Roth B, McLean S, Zhu X, Chuang D. Characterization of two [3H]ketanserin recognition sites in rat striatum. J Neurochem. 1987;49(6):1833–1838. [PubMed: 2960784]
- 41.
- Stockmeier CA, Kellar KJ. In vivo regulation of the serotonin-2 receptor in rat brain. Life Sci. 1986;38(2):117–127. [PubMed: 2935693]
- 42.
- Todd KG, McManus DJ, Baker GB. Chronic administration of the antidepressants phenelzine, desipramine, clomipramine, or maprotiline decreases binding to 5-hydroxytryptamine2A receptors without affecting benzodiazepine binding sites in rat brain. Cell Mol Neurobiol. 1995;15(3):361–370. [PubMed: 7553735]
- 43.
- Weissman A. The dysfunctional attitude scale: A validation study. Dissert Abstr Int. 1979;40:1389B–1390B.
- 44.
- Simons AD, Murphy GE, Levine JL, Wetzel RD. Cognitive therapy and pharmacotherapy for depression. Sustained improvement over one year. Arch Gen Psychiatry. 1986;43(1):43–48. [PubMed: 3942473]
- 45.
- Fava M, Bless E, Otto M, Pava J, Rosenbaum J. Dysfunctional attitudes in major depression changes with pharmacotherapy. J Nerv Ment Dis. 1994;182(1):45–49. [PubMed: 8277301]
- 46.
- Sharot T, Riccardi AM, Raio CM, Phelps EA. Neural mechanisms mediating optimism bias. Nature. 2007;450(7166):102–105. [PubMed: 17960136]
- 47.
- Mitterschiffthaler MT, Williams SC, Walsh ND. et al. Neural basis of the emotional Stroop interference effect in major depression. Psychol Med. 2008;38(2):247–256. [PubMed: 17825123]
- 48.
- Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ. The neural basis of mood-congruent processing biases in depression. Arch Gen Psychiatry. 2002;59(7):597–604. [PubMed: 12090812]
- 49.
- Taylor Tavares JV, Clark L, Furey ML, Williams GB, Sahakian BJ, Drevets WC. Neural basis of abnormal response to negative feedback in unmedicated mood disorders. Neuroimage. 2008;42(3):1118–1126. [PMC free article: PMC2745889] [PubMed: 18586109]
- 50.
- Dohr K, Rush A, Bernstein I. Cognitive biases and depression. J Abnorm Psychol. 1989;98(3):263–267. [PubMed: 2788667]
- 51.
- Simons AD, Garfield SL, Murphy GE. The process of change in cognitive therapy and pharmacotherapy for depression. Changes in mood and cognition. Arch Gen Psychiatry. 1984;41(1):45–51. [PubMed: 6691784]
- 52.
- Cane D, Olinger L, Gotlib I, Kuiper N. Factor structure of the dysfunctional attitude scale in a student population. J Clin Psychol. 1986;42:307–309.
- 53.
- Oliver J, Baumgart E. The dysfunctional attitude scale: Psychometric properties and relation to depression in an unselected adult population. Cog Ther Res. 1985;9:161–167.
- 54.
- Frokjaer VG, Mortensen EL, Nielsen FA. et al. Frontolimbic serotonin 2A receptor binding in healthy subjects is associated with personality risk factors for affective disorder. Biol Psychiatry. 2008;63(6):569–576. [PubMed: 17884017]
- 55.
- Bouvard M, Charles S, Guerin J, Aimard G, Cottraux J. [Study of Beck’s hopelessness scale. Validation and factor analysis] Encephale. 1992;18(3):237–240. [PubMed: 1299593]
- 56.
- Cannon B, Mulroy R, Otto MW, Rosenbaum JF, Fava M, Nierenberg AA. Dysfunctional attitudes and poor problem solving skills predict hopelessness in major depression. J Affect Disord. 1999;55(1):45–49. [PubMed: 10512605]
- 57.
- DeRubeis RJ, Evans MD, Hollon SD, Garvey MJ, Grove WM, Tuason VB. How does cognitive therapy work? Cognitive change and symptom change in cognitive therapy and pharmacotherapy for depression. J Consult Clin Psychol. 1990;58(6):862–869. [PubMed: 2292637]
- 58.
- Norman W, Miller I, Dow M. Characteristics of depressed patients with elevated levels of dysfunctional cognitions. Cog Ther Res. 1988;12:39–51.
- 59.
- Beck A, Steer R, Kovacs M, Garrison B. Hopelessness and eventual suicide: A 10-year prospective study of patients hospitalized with suicidal ideation. Am J Psychiatry. 1985;142(5):559–563. [PubMed: 3985195]
- 60.
- Beck AT, Brown G, Steer RA. Prediction of eventual suicide in psychiatric inpatients by clinical ratings of hopelessness. J Consult Clin Psychol. 1989;57(2):309–310. [PubMed: 2708621]
- 61.
- Brodsky BS, Malone KM, Ellis SP, Dulit RA, Mann JJ. Characteristics of borderline personality disorder associated with suicidal behavior. Am J Psychiatry. 1997;154(12):1715–1719. [PubMed: 9396951]
- 62.
- Soloff PH, Price JC, Meltzer CC, Fabio A, Frank GK, Kaye WH. 5HT2A receptor binding is increased in borderline personality disorder. Biol Psychiatry. 2007;62(6):580–587. [PubMed: 17448449]
- 63.
- Meyer JH, Wilson AA, Rusjan P. et al. Serotonin2A receptor binding potential in people with aggressive and violent behaviour. J Psychiatry Neurosci. 2008;33(6):499–508. [PMC free article: PMC2575762] [PubMed: 18982172]
- 64.
- Jakab R, Goldman-Rakic P. 5-Hydroxytryptamine2A serotonin receptors in the primate cerebral cortex: Possible site of action of hallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. Proc Natl Acad Sci U. S. A. 1998;95:735–740. [PMC free article: PMC18490] [PubMed: 9435262]
- 65.
- Santana N, Bortolozzi A, Serrats J, Mengod G, Artigas F. Expression of serotonin1A and serotonin2A receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb Cortex. 2004;14(10):1100–1109. [PubMed: 15115744]
- 66.
- Wu C, Singh SK, Dias P, Kumar S, Mann DM. Activated astrocytes display increased 5-HT2a receptor expression in pathological states. Exp Neurol. 1999;158(2):529–533. [PubMed: 10415157]
- 67.
- Jacobs B, Driscoll L, Schall M. Life-span dendritic and spine changes in areas 10 and 18 of human cortex: A quantitative Golgi study. J Comp Neurol. 1997;386(4):661–680. [PubMed: 9378859]
- 68.
- Rosell DR, Thompson JL, Slifstein M. et al. Increased serotonin 2A receptor availability in the orbitofrontal cortex of physically aggressive personality disordered patients. Biol Psychiatry. 2010;67(12):1154–1162. [PMC free article: PMC3091264] [PubMed: 20434136]
- 69.
- Bubar MJ, Cunningham KA. Prospects for serotonin 5-HT2R pharmacotherapy in psychostimulant abuse. Prog Brain Res. 2008;172:319–346. [PubMed: 18772040]
- 70.
- Meyer JH. Imaging the serotonin transporter during major depressive disorder and antidepressant treatment. J Psychiatry Neurosci. 2007;32(2):86–102. [PMC free article: PMC1810585] [PubMed: 17353938]
- 71.
- Brucke T, Kornhuber J, Angelberger P, Asenbaum S, Frassine H, Podreka I. SPECT imaging of dopamine and serotonin transporters with [123I]beta-CIT. Binding kinetics in the human brain. J Neural Transm Gen Sect. 1993;94(2):137–146. [PubMed: 8110440]
- 72.
- Innis RB, Seibyl JP, Scanley BE. et al. Single photon emission computed tomographic imaging demonstrates loss of striatal dopamine transporters in Parkinson disease. Proc Natl Acad Sci U. S. A. 1993;90(24):11965–11969. [PMC free article: PMC48106] [PubMed: 8265656]
- 73.
- Kuikka JT, Bergstrom KA, Vanninen E, Laulumaa V, Hartikainen P, Lansimies E. Initial experience with single-photon emission tomography using iodine-123-labelled 2 beta-carbomethoxy-3 beta-(4-iodophenyl) tropane in human brain. Eur J Nucl Med. 1993;20(9):783–786. [PubMed: 8223773]
- 74.
- Carroll FI, Kotian P, Dehghani A. et al. Cocaine and 3 beta-(4′-substituted phenyl)tropane-2 beta-carboxylic acid ester and amide analogues. New high-affinity and selective compounds for the dopamine transporter. J Med Chem. 1995;38(2):379–388. [PubMed: 7830281]
- 75.
- Laruelle M, Giddings SS, Zea-Ponce Y, et al. Methyl 3 beta-(4-[125I]iodophenyl)tropane-2 beta-carboxylate in vitro binding to dopamine and serotonin transporters under “physiological” conditions. J Neurochem. 1994;62(3):978–986. [PubMed: 8113817]
- 76.
- Ciliax BJ, Drash GW, Staley JK. et al. Immunocytochemical localization of the dopamine transporter in human brain. J Comp Neurol. 1999;409(1):38–56. [PubMed: 10363710]
- 77.
- Parsey RV, Kegeles LS, Hwang DR. et al. In vivo quantification of brain serotonin transporters in humans using [11C]McN 5652. J Nucl Med. 2000;41(9):1465–1477. [PubMed: 10994724]
- 78.
- Kent JM, Coplan JD, Lombardo I. et al. Occupancy of brain serotonin transporters during treatment with paroxetine in patients with social phobia: A positron emission tomography study with 11C McN 5652. Psychopharmacology (Berl). 2002;164(4):341–348. [PubMed: 12457263]
- 79.
- Ikoma Y, Suhara T, Toyama H. et al. Quantitative analysis for estimating binding potential of the brain serotonin transporter with [11C]McN5652. J Cereb Blood Flow Metab. 2002;22(4):490–501. [PubMed: 11919520]
- 80.
- Buck A, Gucker PM, Schonbachler RD. et al. Evaluation of serotonergic transporters using PET and [11C](+)McN-5652: Assessment of methods. J Cereb Blood Flow Metab. 2000;20(2):253–262. [PubMed: 10698061]
- 81.
- Ginovart N, Wilson AA, Meyer JH, Hussey D, Houle S. Positron emission tomography quantification of [(11)C]-DASB binding to the human serotonin transporter: Modeling strategies. J Cereb Blood Flow Metab. 2001;21(11):1342–1353. [PubMed: 11702049]
- 82.
- Houle S, Ginovart N, Hussey D, Meyer J, Wilson A. Imaging the serotonin transporter with positron emission tomography: Initial human studies with [11C]DAPP and [11C] DASB. Eur J Nucl Med. 2000;27(11):1719–1722. [PubMed: 11105830]
- 83.
- Ichise M, Liow JS, Lu JQ. et al. Linearized reference tissue parametric imaging methods: Application to [11C]DASB positron emission tomography studies of the serotonin transporter in human brain. J Cereb Blood Flow Metab. 2003;23(9):1096–1112. [PubMed: 12973026]
- 84.
- Meyer JH, Houle S, Sagrati S. et al. Brain serotonin transporter binding potential measured with carbon 11-labeled DASB positron emission tomography: Effects of major depressive episodes and severity of dysfunctional attitudes. Arch Gen Psychiatry. 2004;61(12):1271–1279. [PubMed: 15583118]
- 85.
- Meyer JH, Wilson AA, Ginovart N. et al. Occupancy of serotonin transporters by paroxetine and citalopram during treatment of depression: A [(11)C]DASB PET imaging study. Am J Psychiatry. 2001;158(11):1843–1849. [PubMed: 11691690]
- 86.
- Meyer JH, Wilson AA, Sagrati S. et al. Serotonin transporter occupancy of five selective serotonin reuptake inhibitors at different doses: An [11C]DASB positron emission tomography study. Am J Psychiatry. 2004;161(5):826–835. [PubMed: 15121647]
- 87.
- Praschak-Rieder N, Wilson AA, Hussey D. et al. Effects of tryptophan depletion on the serotonin transporter in healthy humans. Biol Psychiatry. 2005;58(10):825–830. [PubMed: 16026765]
- 88.
- Wilson A, Schmidt M, Ginovart N, Meyer J, Houle S. Novel radiotracers for imaging the serotonin transporter by positron emission tomography: Synthesis, radiosynthesis, in vitro and ex vivo evaluation of [11C]-labelled 2-(phenylthio) araalkylamines. J Med Chem. 2000;43(16):3103–3110. [PubMed: 10956218]
- 89.
- Wilson AA, Ginovart N, Hussey D, Meyer J, Houle S. In vitro and in vivo characterisation of [11C]-DASB: A probe for in vivo measurements of the serotonin transporter by positron emission tomography. Nucl Med Biol. 2002;29(5):509–515. [PubMed: 12088720]
- 90.
- Takano A, Suhara T, Ichimiya T, Yasuno F, Suzuki K. Time course of in vivo 5-HTT transporter occupancy by fluvoxamine. J Clin Psychopharmacol. 2006;26(2):188–191. [PubMed: 16633150]
- 91.
- Leyton M, Young SN, Blier P. et al. The effect of tryptophan depletion on mood in medication-free, former patients with major affective disorder. Neuropsychopharmacology. 1997;16(4):294–297. [PubMed: 9094147]
- 92.
- Neumeister A, Nugent AC, Waldeck T. et al. Neural and behavioral responses to tryptophan depletion in unmedicated patients with remitted major depressive disorder and controls. Arch Gen Psychiatry. 2004;61(8):765–773. [PubMed: 15289275]
- 93.
- Neumeister A, Praschak-Rieder N, Hesselmann B. et al. Effects of tryptophan depletion in drug-free depressed patients who responded to total sleep deprivation. Arch Gen Psychiatry. 1998;55(2):167–172. [PubMed: 9477931]
- 94.
- Smith K, Clifford E, Hockney R, Clark M, Cowen P. Effect of tryptophan depletion on mood in male and female volunteers: A pilot study. Hum Psychopharm. 1997;12:111–117.
- 95.
- Young SN, Smith SE, Pihl RO, Ervin FR. Tryptophan depletion causes a rapid lowering of mood in normal males. Psychopharmacology. 1985;87(2):173–177. [PubMed: 3931142]
- 96.
- Kontur PJ, al-Tikriti M, Innis RB, Roth RH. Postmortem stability of monoamines, their metabolites, and receptor binding in rat brain regions. J Neurochem. 1994;62(1):282–290. [PubMed: 7505313]
- 97.
- Mann J, Underwood M, Arango V. Postmortem studies of suicide victims. Washington, DC: American Psychiatric Press; Biology of Schizophrenia and Affective Disorders. 1996:197–221.
- 98.
- Linnet K, Koed K, Wiborg O, Gregersen N. Serotonin depletion decreases serotonin transporter mRNA levels in rat brain. Brain Res. 1995;697(1–2):251–253. [PubMed: 8593584]
- 99.
- Xiao Q, Pawlyk A, Tejani-Butt SM. Reserpine modulates serotonin transporter mRNA levels in the rat brain. Life Sci. 1999;64(1):63–68. [PubMed: 10027743]
- 100.
- Yu A, Yang J, Pawlyk AC, Tejani-Butt SM. Acute depletion of serotonin down-regulates serotonin transporter mRNA in raphe neurons. Brain Res. 1995;688(1–2):209–212. [PubMed: 8542310]
- 101.
- Benmansour S, Cecchi M, Morilak D. et al. Effects of chronic antidepressant treatments on serotonin transporter function, density and mRNA level. J Neurosci. 1999;19(23):10494–10501. [PMC free article: PMC6782424] [PubMed: 10575045]
- 102.
- Dewar KM, Grondin L, Carli M, Lima L, Reader TA. [3H]Paroxetine binding and serotonin content of rat cortical areas, hippocampus, neostriatum, ventral mesencephalic tegmentum, and midbrain raphe nuclei region following p-chlorophenylalanine and p-chloroamphetamine treatment. J Neurochem. 1992;58(1):250–257. [PubMed: 1370077]
- 103.
- Graham D, Tahraoui L, Langer SZ. Effect of chronic treatment with selective monoamine oxidase inhibitors and specific 5-hydroxytryptamine uptake inhibitors on [3H] paroxetine binding to cerebral cortical membranes of the rat. Neuropharmacology. 1987;26(8):1087–1092. [PubMed: 2958720]
- 104.
- Gordon I, Weizman R, Rehavi M. Modulatory effect of agents active in the presynaptic dopaminergic system on the striatal dopamine transporter. Eur J Pharmacol. 1996;298(1):27–30. [PubMed: 8867915]
- 105.
- Han S, Rowell PP, Carr LA. D2 Autoreceptors are not involved in the down-regulation of the striatal dopamine transporter caused by alpha-methyl-p-tyrosine. Res Commun Mol Pathol Pharmacol. 1999;104(3):331–338. [PubMed: 10741383]
- 106.
- Ikawa K, Watanabe A, Kaneno S, Toru M. Modulation of [3H]mazindol binding sites in rat striatum by dopaminergic agents. Eur J Pharmacol. 1993;250(2):261–266. [PubMed: 7906653]
- 107.
- Kilbourn MR, Sherman PS, Pisani T. Repeated reserpine administration reduces in vivo [18F]GBR 13119 binding to the dopamine uptake site. Eur J Pharmacol. 1992;216(1):109–112. [PubMed: 1526249]
- 108.
- Lee CM, Javitch JA, Snyder SH. Recognition sites for norepinephrine uptake: Regulation by neurotransmitter. Science. 1983;220(4597):626–629. [PubMed: 6301013]
- 109.
- Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: A critical review. J Cereb Blood Flow Metab. 2000;20(3):423–451. [PubMed: 10724107]
- 110.
- Celada P, Artigas F. Monoamine oxidase inhibitors increase preferentially extracellular 5-hydroxytryptamine in the midbrain raphe nuclei. A brain microdialysis study in the awake rat. Naunyn Schmiedebergs Arch Pharmacol. 1993;347(6):583–590. [PubMed: 7689703]
- 111.
- Malyszko J, Urano T, Serizawa K. et al. Serotonergic measures in blood and brain and their correlations in rats treated with tranylcypromine, a monoamine oxidase inhibitor. Jpn J Physiol. 1993;43(5):613–626. [PubMed: 7511710]
- 112.
- Ferrer A, Artigas F. Effects of single and chronic treatment with tranylcypromine on extracellular serotonin in rat brain. Eur J Pharmacol. 1994;263(3):227–234. [PubMed: 7531152]
- 113.
- Ginovart N, Wilson AA, Meyer JH, Hussey D, Houle S. [11C]-DASB, a tool for in vivo measurement of SSRI-induced occupancy of the serotonin transporter: PET characterization and evaluation in cats. Synapse. 2003;47(2):123–133. [PubMed: 12454950]
- 114.
- Lundquist P, Wilking H, Hoglund AU. et al. Potential of [11C]DASB for measuring endogenous serotonin with PET: Binding studies. Nucl Med Biol. 2005;32(2):129–136. [PubMed: 15721758]
- 115.
- Talbot PS, Frankle WG, Hwang DR. et al. Effects of reduced endogenous 5-HT on the in vivo binding of the serotonin transporter radioligand 11C-DASB in healthy humans. Synapse. 2005;55(3):164–175. [PubMed: 15605360]
- 116.
- Malone KM, Oquendo MA, Haas GL, Ellis SP, Li S, Mann JJ. Protective factors against suicidal acts in major depression: Reasons for living. Am J Psychiatry. 2000;157(7):1084–1088. [PubMed: 10873915]
- 117.
- Bligh-Glover W, Kolli TN, Shapiro-Kulnane L. et al. The serotonin transporter in the midbrain of suicide victims with major depression. Biol Psychiatry. 2000;47(12):1015–1024. [PubMed: 10862800]
- 118.
- Klimek V, Roberson G, Stockmeier CA, Ordway GA. Serotonin transporter and MAO-B levels in monoamine nuclei of the human brainstem are normal in major depression. J Psychiatr Res. 2003;37(5):387–397. [PubMed: 12849931]
- 119.
- Perry EK, Marshall EF, Blessed G, Tomlinson BE, Perry RH. Decreased imipramine binding in the brains of patients with depressive illness. Br J Psychiatry. 1983;142:188–192. [PubMed: 6839075]
- 120.
- Crow TJ, Cross AJ, Cooper SJ. et al. Neurotransmitter receptors and monoamine metabolites in the brains of patients with Alzheimer-type dementia and depression, and suicides. Neuropharmacology. 1984;23(12B):1561–1569. [PubMed: 6084823]
- 121.
- Mann JJ, Huang YY, Underwood MD. et al. A serotonin transporter gene promoter polymorphism (5-HTTLPR) and prefrontal cortical binding in major depression and suicide [see comments] Arch Gen Psychiatry. 2000;57(8):729–738. [PubMed: 10920459]
- 122.
- Arango V, Underwood MD, Boldrini M. et al. Serotonin 1A receptors, serotonin transporter binding and serotonin transporter mRNA expression in the brainstem of depressed suicide victims. Neuropsychopharmacology. 2001;25(6):892–903. [PubMed: 11750182]
- 123.
- Austin MC, Whitehead RE, Edgar CL, Janosky JE, Lewis DA. Localized decrease in serotonin transporter-immunoreactive axons in the prefrontal cortex of depressed subjects committing suicide. Neuroscience. 2002;114(3):807–815. [PubMed: 12220580]
- 124.
- Hrdina P, Foy B, Hepner A. Summers R. Antidepressant binding sites in brain: Autoradiographic comparison of [3H]paroxetine and [3H]imipramine localization and relationship to serotonin transporter. J Pharm Exp Ther. 1990;252(1):410–418. [PubMed: 2137177]
- 125.
- Lawrence KM, De Paermentier F, Cheetham SC, Crompton MR, Katona CL, Horton RW. Brain 5-HT uptake sites, labelled with [3H]paroxetine, in antidepressant-free depressed suicides. Brain Res. 1990;526(1):17–22. [PubMed: 2150340]
- 126.
- Leake A, Fairbairn AF, McKeith IG, Ferrier IN. Studies on the serotonin uptake binding site in major depressive disorder and control post-mortem brain: Neurochemical and clinical correlates. Psychiatry Res. 1991;39(2):155–165. [PubMed: 1798816]
- 127.
- Little KY, McLauglin DP, Ranc J. et al. Serotonin transporter binding sites and mRNA levels in depressed persons committing suicide. Biol Psychiatry. 1997;41(12):1156–1164. [PubMed: 9171906]
- 128.
- Hendricksen M, Thomas AJ, Ferrier IN, Ince P, O’Brien JT. Neuropathological study of the dorsal raphe nuclei in late-life depression and Alzheimer’s disease with and without depression. Am J Psychiatry. 2004;161(6):1096–1102. [PubMed: 15169699]
- 129.
- Herold N, Uebelhack K, Franke L. et al. Imaging of serotonin transporters and its blockade by citalopram in patients with major depression using a novel SPECT ligand [(123) I]-ADAM. J Neural Transm. 2006;113(5):659–670. [PubMed: 16465456]
- 130.
- Ichimiya T, Suhara T, Sudo Y. et al. Serotonin transporter binding in patients with mood disorders: A PET study with [11C](+)McN5652. Biol Psychiatry. 2002;51(9):715–722. [PubMed: 11983185]
- 131.
- Cannon DM, Ichise M, Rollis D. et al. Elevated serotonin transporter binding in major depressive disorder assessed using positron emission tomography and [(11)C]DASB; Comparison with bipolar disorder. Biol Psychiatry. 2007;62(8):870–877. [PubMed: 17678634]
- 132.
- Malison RT, Price LH, Berman R. et al. Reduced brain serotonin transporter availability in major depression as measured by [123I]-2beta-carbomethoxy-3beta-(4-iodophenyl)tropane and single photon emission computed tomography [see comments] Biol Psychiatry. 1998;44(11):1090–1098. [PubMed: 9836013]
- 133.
- Newberg AB, Amsterdam JD, Wintering N. et al. 123I-ADAM binding to serotonin transporters in patients with major depression and healthy controls: A preliminary study. J Nucl Med. 2005;46(6):973–977. [PubMed: 15937308]
- 134.
- Parsey RV, Hastings RS, Oquendo MA. et al. Lower serotonin transporter binding potential in the human brain during major depressive episodes. Am J Psychiatry. 2006;163(1):52–58. [PubMed: 16390889]
- 135.
- Selvaraj S, Venkatesha Murthy N, Bhagwagar Z. et al. Diminished brain 5-HT transporter binding in major depression: A positron emission tomography study with [(11)C] DASB. Psychopharmacology (Berl). 2011;213(2–3):555–562. [PubMed: 19756523]
- 136.
- Joensuu M, Tolmunen T, Saarinen PI. et al. Reduced midbrain serotonin transporter availability in drug-naive patients with depression measured by SERT-specific [(123)I] nor-beta-CIT SPECT imaging. Psychiatry Res. 2007;154(2):125–131. [PubMed: 17289353]
- 137.
- Arango V, Underwood MD, Gubbi AV, Mann JJ. Localized alterations in pre- and postsynaptic serotonin binding sites in the ventrolateral prefrontal cortex of suicide victims. Brain Res. 1995;688(1–2):121–133. [PubMed: 8542298]
- 138.
- Suhara T, Takano A, Sudo Y. et al. High levels of serotonin transporter occupancy with low-dose clomipramine in comparative occupancy study with fluvoxamine using positron emission tomography. Arch Gen Psychiatry. 2003;60(4):386–391. [PubMed: 12695316]
- 139.
- Kalbitzer J, Erritzoe D, Holst KK. et al. Seasonal changes in brain serotonin transporter binding in short serotonin transporter linked polymorphic region-allele carriers but not in long-allele homozygotes. Biol Psychiatry. 2010;67(11):1033–1039. [PubMed: 20110086]
- 140.
- Praschak-Rieder N, Willeit M, Wilson AA, Houle S, Meyer JH. Seasonal variation in human brain serotonin transporter binding. Arch Gen Psychiatry. 2008;65(9):1072–1078. [PubMed: 18762593]
- 141.
- McCann UD, Szabo Z, Seckin E. et al. Quantitative PET studies of the serotonin transporter in MDMA users and controls using [11C]McN5652 and [11C]DASB. Neuropsychopharmacology. 2005;30(9):1741–1750. [PMC free article: PMC2034411] [PubMed: 15841106]
- 142.
- Selvaraj S, Hoshi R, Bhagwagar Z. et al. Brain serotonin transporter binding in former users of MDMA (‘ecstasy’). Br J Psychiatry. 2009;194(4):355–359. [PubMed: 19336788]
- 143.
- Kish SJ, Lerch J, Furukawa Y. et al. Decreased cerebral cortical serotonin transporter binding in ecstasy users: A positron emission tomography/[(11)C]DASB and structural brain imaging study. Brain. 2010;133(Pt 6):1779–1797. [PMC free article: PMC2912692] [PubMed: 20483717]
- 144.
- Matsumoto R, Ichise M, Ito H. et al. Reduced serotonin transporter binding in the insular cortex in patients with obsessive–compulsive disorder: A [11C]DASB PET study. Neuroimage. 2010;49(1):121–126. [PubMed: 19660554]
- 145.
- Reimold M, Smolka MN, Zimmer A. et al. Reduced availability of serotonin transporters in obsessive–compulsive disorder correlates with symptom severity—A [11C]DASB PET study. J Neural Transm. 2007;114(12):1603–1609. [PubMed: 17713719]
- 146.
- Ichise M, Vines DC, Gura T. et al. Effects of early life stress on [11C]DASB positron emission tomography imaging of serotonin transporters in adolescent peer- and mother-reared rhesus monkeys. J Neurosci. 2006;26(17):4638–4643. [PMC free article: PMC6674071] [PubMed: 16641244]
- 147.
- Takano A, Arakawa R, Hayashi M, Takahashi H, Ito H, Suhara T. Relationship between neuroticism personality trait and serotonin transporter binding. Biol Psychiatry. 2007;62(6):588–592. [PubMed: 17336939]
- 148.
- Kalbitzer J, Frokjaer VG, Erritzoe D. et al. The personality trait openness is related to cerebral 5-HTT levels. Neuroimage. 2009;45(2):280–285. [PubMed: 19135154]
- 149.
- Frankle WG, Lombardo I, New AS. et al. Brain serotonin transporter distribution in subjects with impulsive aggressivity: A positron emission study with [11C]McN 5652. Am J Psychiatry. 2005;162(5):915–923. [PubMed: 15863793]
- 150.
- Koch W, Schaaff N, Popperl G. et al. [I-123]ADAM and SPECT in patients with borderline personality disorder and healthy control subjects. J Psychiatry Neurosci. 2007;32(4):234–240. [PMC free article: PMC1911193] [PubMed: 17653291]
- 151.
- Brown AK, George DT, Fujita M. et al. PET [11C]DASB imaging of serotonin transporters in patients with alcoholism. Alcohol Clin Exp Res. 2007;31(1):28–32. [PubMed: 17207098]
- 152.
- Saura J, Bleuel Z, Ulrich J. et al. Molecular neuroanatomy of human monoamine oxidases A and B revealed by quantitative enzyme radioautography and in situ hybridization histochemistry. Neuroscience. 1996;70(3):755–774. [PubMed: 9045087]
- 153.
- Saura J, Kettler R, Da Prada M, Richards JG. Quantitative enzyme radioautography with 3H-Ro 41-1049 and 3H-Ro 19-6327 in vitro: Localization and abundance of MAO-A and MAO-B in rat CNS, peripheral organs, and human brain. J Neurosci. 1992;12(5):1977–1999. [PMC free article: PMC6575899] [PubMed: 1578281]
- 154.
- Fowler C, Oreland L. Singer T, Von Korff R, Murphy D, editors. Monoamine Oxidase: Structure, Function, and Altered Functions; NewYork:Academic Press, Inc.: Substrate-selective interaction between monoamine oxidase and oxygen. 1979:145–151.
- 155.
- Kinemuchi H, Fowler C, Tipton K. Tipton K, Dostert P, Strolin-Benedetti M, editors. Monoamine Oxidase and Disease: Prospects for Therapy with Reversible Inhibitors; New York: Academic Press, Inc.: Substrate specificities of the two forms of monoamine oxidase. 1984:53–62.
- 156.
- Schoepp DD, Azzaro AJ. Specificity of endogenous substrates for types A and B monoamine oxidase in rat striatum. J Neurochem. 1981;36(6):2025–2031. [PubMed: 6787175]
- 157.
- White H, Tansik R. Characterization of multiple substrate binding sites of MAO. Singer T, Von Korff R, Murphy D, editors. Monoamine Oxidase: Structure, Function and Altered Functions; New York: Academic Press, Inc.: 1979:129–144.
- 158.
- Konradi C, Svoma E, Jellinger K, Riederer P, Denney R, Thibault J. Topographic immuno cytochemicalmappingofmonoamineoxidase-A, mono amineoxidase-Bandtyrosine hydroxylase in human post mortem brain stem. Neuroscience. 1988;26(3):791–802. [PubMed: 2904662]
- 159.
- Luque JM, Kwan SW, Abell CW, Da Prada M, Richards JG. Cellular expression of mRNAs encoding monoamine oxidases A and B in the rat central nervous system. J Comp Neurol. 1995;363(4):665–680. [PubMed: 8847423]
- 160.
- Fagervall I, Ross SB. A and B forms of monoamine oxidase within the monoaminergic neurons of the rat brain. J Neurochem. 1986;47(2):569–576. [PubMed: 3734795]
- 161.
- Adell A, Biggs TA, Myers RD. Action of harman (1-methyl-beta-carboline) on the brain: Body temperature and in vivo efflux of 5-HT from hippocampus of the rat. Neuropharmacology. 1996;35(8):1101–1107. [PubMed: 9121613]
- 162.
- Bel N, Artigas F. In vivo evidence for the reversible action of the monoamine oxidase inhibitor brofaromine on 5-hydroxytryptamine release in rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1995;351(5):475–482. [PubMed: 7543977]
- 163.
- Celada P, Bel N, Artigas F. The effects of brofaromine, a reversible MAO-A inhibitor, on extracellular serotonin in the raphe nuclei and frontal cortex of freely moving rats. J Neural Transm Suppl. 1994;41:357–363. [PubMed: 7931251]
- 164.
- Curet O, Damoiseau-Ovens G, Sauvage C. et al. Preclinical profile of befloxatone, a new reversible MAO-A inhibitor. J Affect Disord. 1998;51(3):287–303. [PubMed: 10333983]
- 165.
- Haefely W, Burkard WP, Cesura AM. et al. Biochemistry and pharmacology of moclobemide, a prototype RIMA. Psychopharmacology (Berl). 1992;106 (Suppl):S6–S14. [PubMed: 1546143]
- 166.
- Houslay MD, Tipton KF. A kinetic evaluation of monoamine oxidase activity in rat liver mitochondrial outer membranes. Biochem J. 1974;139(3):645–652. [PMC free article: PMC1166328] [PubMed: 4850215]
- 167.
- Finberg JP, Pacak K, Goldstein DS, Kopin IJ. Modification of cerebral cortical noradrenaline release by chronic inhibition of MAO-A. J Neural Transm Suppl. 1994;41:123–125. [PubMed: 7931217]
- 168.
- Finberg JP, Pacak K, Kopin IJ, Goldstein DS. Chronic inhibition of monoamine oxidase type A increases noradrenaline release in rat frontal cortex. Naunyn Schmiedebergs Arch Pharmacol. 1993;347(5):500–505. [PubMed: 8391652]
- 169.
- Colzi A, d’Agostini F, Kettler R, Borroni E, Da Prada M. Effect of selective and reversible MAO inhibitors on dopamine outflow in rat striatum: A microdialysis study. J Neural Transm Suppl. 1990;32:79–84. [PubMed: 2089114]
- 170.
- Arbuthnott GW, Fairbrother IS, Butcher SP. Dopamine release and metabolism in the rat striatum: An analysis by ‘in vivo’ brain microdialysis. Pharmacol Ther. 1990;48(3):281–293. [PubMed: 2128125]
- 171.
- Butcher SP, Fairbrother IS, Kelly JS, Arbuthnott GW. Effects of selective monoamine oxidase inhibitors on the in vivo release and metabolism of dopamine in the rat striatum. J Neurochem. 1990;55(3):981–988. [PubMed: 2117053]
- 172.
- Konradi C, Kornhuber J, Froelich L. et al. Demonstration of monoamine oxidase-A and -B in the human brainstem by a histochemical technique. Neuroscience. 1989;33(2):383–400. [PubMed: 2622533]
- 173.
- Moll G, Moll R, Riederer P, Gsell W, Heinsen H, Denney RM. Immunofluorescence cytochemistry on thin frozen sections of human substantia nigra for staining of monoamine oxidase A and monoamine oxidase B: A pilot study. J Neural Transm Suppl. 1990;32:67–77. [PubMed: 2089113]
- 174.
- Evrard A, Malagie I, Laporte AM. et al. Altered regulation of the 5-HT system in the brain of MAO-A knock-out mice. Eur J Neurosci. 2002;15(5):841–851. [PubMed: 11906526]
- 175.
- Grote SS, Moses SG, Robins E, Hudgens RW, Croninger AB. A study of selected catecholamine metabolizing enzymes: A comparison of depressive suicides and alcoholic suicides with controls. J Neurochem. 1974;23(4):791–802. [PubMed: 4154358]
- 176.
- Gottfries CG, Oreland L, Wiberg A, Winblad B. Lowered monoamine oxidase activity in brains from alcoholic suicides. J Neurochem. 1975;25(5):667–673. [PubMed: 1194922]
- 177.
- Mann JJ, Stanley M. Postmortem monoamine oxidase enzyme kinetics in the frontal cortex of suicide victims and controls. Acta Psychiatr Scand. 1984;69(2):135–139. [PubMed: 6702476]
- 178.
- Sherif F, Marcusson J, Oreland L. Brain gamma-aminobutyrate transaminase and monoamine oxidase activities in suicide victims. Eur Arch Psychiatry Clin Neurosci. 1991;241(3):139–144. [PubMed: 1790159]
- 179.
- Ordway GA, Farley JT, Dilley GE. et al. Quantitative distribution of monoamine oxidase A in brainstem monoamine nuclei is normal in major depression. Brain Res. 1999;847(1):71–79. [PubMed: 10564737]
- 180.
- Galva MD, Bondiolotti GP, Olasmaa M, Picotti GB. Effect of aging on lazabemide binding, monoamine oxidase activity and monoamine metabolites in human frontal cortex. J Neural Transm Gen Sect. 1995;101(1–3):83–94. [PubMed: 8695059]
- 181.
- Krishnan KR. Biological risk factors in late life depression. Biol Psychiatry. 2002;52(3):185–192. [PubMed: 12182925]
- 182.
- Fowler JS, Volkow ND, Wang GJ. et al. Brain monoamine oxidase A inhibition in cigarette smokers. Proc Natl Acad Sci U. S. A. 1996;93(24):14065–14069. [PMC free article: PMC19495] [PubMed: 8943061]
- 183.
- Fowler JS, Logan J, Ding YS. et al. Non-MAO A binding of clorgyline in white matter in human brain. J Neurochem. 2001;79(5):1039–1046. [PubMed: 11739617]
- 184.
- Bergstrom M, Westerberg G, Nemeth G. et al. MAO-A inhibition in brain after dosing with esuprone, moclobemide and placebo in healthy volunteers: In vivo studies with positron emission tomography. Eur J Clin Pharmacol. 1997;52(2):121–128. [PubMed: 9174681]
- 185.
- Bottlaender M, Dolle F, Guenther I. et al. Mapping the cerebral monoamine oxidase type A: Positron emission tomography characterization of the reversible selective inhibitor [11C]befloxatone. J Pharmacol Exp Ther. 2003;305(2):467–473. [PubMed: 12606609]
- 186.
- Ginovart N, Meyer JH, Boovariwala A. et al. Positron emission tomography quantification of [11C]-harmine binding to monoamine oxidase-A in the human brain. J Cereb Blood Flow Metab. 2006;26(3):330–344. [PubMed: 16079787]
- 187.
- Dolle F, Valette H, Bramoulle Y. et al. Synthesis and in vivo imaging properties of [11C] befloxatone: A novel highly potent positron emission tomography ligand for monoamine oxidase-A. Bioorg Med Chem Lett. 2003;13(10):1771–1775. [PubMed: 12729662]
- 188.
- Bergstrom M, Westerberg G, Langstrom B. 11C-Harmine as a tracer for monoamine oxidase A (MAO-A): In vitro and in vivo studies. Nucl Med Biol. 1997;24(4):287–293. [PubMed: 9257326]
- 189.
- Tweedie DJ, Burke MD. Metabolism of the beta-carbolines, harmine and harmol, by liver microsomes from phenobarbitone- or 3-methylcholanthrene-treated mice. Identification and quantitation of two novel harmine metabolites. Drug Metab Dispos. 1987;15(1):74–81. [PubMed: 2881762]
- 190.
- Meyer JH, Ginovart N, Boovariwala A. et al. Elevated monoamine oxidase a levels in the brain: An explanation for the monoamine imbalance of major depression. Arch Gen Psychiatry. 2006;63(11):1209–1216. [PubMed: 17088501]
- 191.
- First M, Spitzer R, Williams J, Gibbon M. Washington, DC: American Psychiatric Press: Structured Clinical Interview for DSM-IV-Non-Patient Edition (SCID-NP, Version 1.0). 1995
- 192.
- Meyer JH, Wilson AA, Sagrati S. et al. Brain monoamine oxidase A binding in major depressive disorder: Relationship to selective serotonin reuptake inhibitor treatment, recovery, and recurrence. Arch Gen Psychiatry. 2009;66(12):1304–1312. [PubMed: 19996035]
- 193.
- Freis ED. Mental depression in hypertensive patients treated for long periods with large doses of reserpine. N Engl J Med. 1954;251(25):1006–1008. [PubMed: 13214379]
- 194.
- Nelson DL, Herbet A, Glowinski J, Hamon M. [3H]Harmaline as a specific ligand of MAO A—II. Measurement of the turnover rates of MAO A during ontogenesis in the rat brain. J Neurochem. 1979;32(6):1829–1836. [PubMed: 448371]
- 195.
- Nelson DL, Herbet A, Petillot Y, Pichat L, Glowinski J, Hamon M. [3H]Harmaline as a specific ligand of MAO A—I. Properties of the active site of MAO A from rat and bovine brains. J Neurochem. 1979;32(6):1817–1827. [PubMed: 448370]
- 196.
- Edelstein SB, Breakefield XO. Monoamine oxidases A and B are differentially regulated by glucocorticoids and “aging” in human skin fibroblasts. Cell Mol Neurobiol. 1986;6(2):121–150. [PubMed: 3731213]
- 197.
- Knutson B, Taylor J, Kaufman M, Peterson R, Glover G. Distributed neural representation of expected value. J Neurosci. 2005;25(19):4806–4812. [PMC free article: PMC6724773] [PubMed: 15888656]
- 198.
- Samandari G, Martin SL, Kupper LL, Schiro S, Norwood T, Avery M. Are pregnant and postpartum women: At increased risk for violent death? Suicide and homicide findings from North Carolina. Matern Child Health J. 2011;15(5):660–669. [PubMed: 20549551]
- 199.
- Comtois KA, Schiff MA, Grossman DC. Psychiatric risk factors associated with postpartum suicide attempt in Washington State, 1992–2001 Am J Obstet Gynecol 2008199(2)120.e1–120.e5. [PubMed: 18355781]
- 200.
- Lewis G. London, U.K.: Royal College of Obstetricians and Gynaecologists: Why Mothers Die 1997–1999, Confidential Enquiry in Maternal Deaths. 2001
- 201.
- O’Hara MW, Swain A. Rates and risk of postpartum depression—A meta analysis. Int Rev Psychiatry. 1996;8:37–54.
- 202.
- Steiner M. Perinatal mood disorders: Position paper. Psychopharmacol Bull. 1998;34(3):301–306. [PubMed: 9803759]
- 203.
- Pop VJ, Essed GG, de Geus CA, van Son MM, Komproe IH. Prevalence of post partum depression—Or is it post-puerperium depression? Acta Obstet Gynecol Scand. 1993;72(5):354–358. [PubMed: 8392265]
- 204.
- Carothers AD, Murray L. Estimating psychiatric morbidity by logistic regression: Application to post-natal depression in a community sample. Psychol Med. 1990;20(3):695–702. [PubMed: 2236379]
- 205.
- Kornstein SG. The evaluation and management of depression in women across the life span. J Clin Psychiatry. 2001;62 (Suppl 24):11–17. [PubMed: 11676428]
- 206.
- O’Hara M. New York: Springer-Verlag: Postpartum Depression: Causes and Consequences. 1994
- 207.
- O’Hara MW, Schlechte JA, Lewis DA, Varner MW. Controlled prospective study of postpartum mood disorders: Psychological, environmental, and hormonal variables. J Abnorm Psychol. 1991;100(1):63–73. [PubMed: 2005273]
- 208.
- Association AP. 4th edn. Washington, DC: American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders. 1994
- 209.
- Campbell S, Cohn J, Flanagan C, Popper S, Meyers T. Course and correlates of postpartum depression during the transition to parenthood. Dev Psychopathol. 1992;4:29–47.
- 210.
- Brockington IF, Cernik KF, Schofield EM, Downing AR, Francis AF, Keelan C. Puerperal psychosis. Phenomena and diagnosis. Arch Gen Psychiatry. 1981;38(7):829–833. [PubMed: 7247645]
- 211.
- Kendell RE, Chalmers JC, Platz C. Epidemiology of puerperal psychoses. Br J Psychiatry. 1987;150:662–673. [PubMed: 3651704]
- 212.
- Hendrick V, Altshuler LL, Suri R. Hormonal changes in the postpartum and implications for postpartum depression. Psychosomatics. 1998;39(2):93–101. [PubMed: 9584534]
- 213.
- Nott PN, Franklin M, Armitage C, Gelder MG. Hormonal changes and mood in the puerperium. Br J Psychiatry. 1976;128:379–383. [PubMed: 944062]
- 214.
- O’Hara MW, Schlechte JA, Lewis DA, Wright EJ. Prospective study of postpartum blues. Biologic and psychosocial factors. Arch Gen Psychiatry. 1991;48(9):801–806. [PubMed: 1929770]
- 215.
- Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR. Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry. 2000;157(6):924–930. [PubMed: 10831472]
- 216.
- Sichel DA, Cohen LS, Robertson LM, Ruttenberg A, Rosenbaum JF. Prophylactic estrogen in recurrent postpartum affective disorder. Biol Psychiatry. 1995;38(12):814–818. [PubMed: 8750040]
- 217.
- Gregoire AJ, Kumar R, Everitt B, Henderson AF, Studd JW. Transdermal oestrogen for treatment of severe postnatal depression. Lancet. 1996;347(9006):930–933. [PubMed: 8598756]
- 218.
- Ahokas A, Kaukoranta J, Wahlbeck K, Aito M. Estrogen deficiency in severe postpartum depression: Successful treatment with sublingual physiologic 17beta-estradiol: A preliminary study. J Clin Psychiatry. 2001;62(5):332–336. [PubMed: 11411813]
- 219.
- Schneider LS, Small GW, Hamilton SH, Bystritsky A, Nemeroff CB, Meyers BS. Estrogen replacement and response to fluoxetine in a multicenter geriatric depression trial. Fluoxetine Collaborative Study Group. Am J Geriatr Psychiatry. 1997;5(2):97–106. [PubMed: 9106373]
- 220.
- Rasgon NL, Altshuler LL, Fairbanks LA. et al. Estrogen replacement therapy in the treatment of major depressive disorder in perimenopausal women. J Clin Psychiatry. 2002;63 (Suppl 7):45–48. [PubMed: 11995778]
- 221.
- Schneider LS, Small GW, Clary CM. Estrogen replacement therapy and antidepressant response to sertraline in older depressed women. Am J Geriatr Psychiatry. 2001;9(4):393–399. [PubMed: 11739065]
- 222.
- Amsterdam J, Garcia-Espana F, Fawcett J. et al. Fluoxetine efficacy in menopausal women with and without estrogen replacement. J Affect Disord. 1999;55(1):11–17. [PubMed: 10512601]
- 223.
- Grigoriadis S, Kennedy SH, Srinivisan J, McIntyre RS, Fulton K. Antidepressant augmentation with raloxifene. J Clin Psychopharmacol. 2005;25(1):96–98. [PubMed: 15643109]
- 224.
- Luine VN, McEwen BS. Effect of oestradiol on turnover of type A monoamine oxidase in brain. J Neurochem. 1977;28(6):1221–1227. [PubMed: 874483]
- 225.
- Leung TK, Lai JC, Marr W, Lim L. The activities of the A and B forms of monoamine oxidase in liver, hypothalamus and cerebral cortex of the female rat: Effects of administration of ethinyloestradiol and the progestogens norethisterone acetate and d-norgestrel. Biochem Soc Trans. 1980;8(5):607–608. [PubMed: 7450249]
- 226.
- Holschneider DP, Kumazawa T, Chen K, Shih JC. Tissue-specific effects of estrogen on monoamine oxidase A and B in the rat. Life Sci. 1998;63(3):155–160. [PubMed: 9698044]
- 227.
- Gundlah C, Lu NZ, Bethea CL. Ovarian steroid regulation of monoamine oxidase-A and -B mRNAs in the macaque dorsal raphe and hypothalamic nuclei. Psychopharmacology (Berl). 2002;160(3):271–282. [PubMed: 11889496]
- 228.
- Smith LJ, Henderson JA, Abell CW, Bethea CL. Effects of ovarian steroids and raloxifene on proteins that synthesize, transport, and degrade serotonin in the raphe region of macaques. Neuropsychopharmacology. 2004;29(11):2035–2045. [PubMed: 15199371]
- 229.
- Ma ZQ, Bondiolotti GP, Olasmaa M. et al. Estrogen modulation of catecholamine synthesis and monoamine oxidase A activity in the human neuroblastoma cell line SK-ER3. J Steroid Biochem Mol Biol. 1993;47(1–6):207–211. [PubMed: 7903862]
- 230.
- Ma ZQ, Violani E, Villa F, Picotti GB, Maggi A. Estrogenic control of monoamine oxidase A activity in human neuroblastoma cells expressing physiological concentrations of estrogen receptor. Eur J Pharmacol. 1995;284(1–2):171–176. [PubMed: 8549621]
- 231.
- Chevillard C, Barden N, Saavedra JM. Estradiol treatment decreases type A and increases type B monoamine oxidase in specific brain stem areas and cerebellum of ovariectomized rats. Brain Res. 1981;222(1):177–181. [PubMed: 7296265]
- 232.
- Harris B, Lovett L, Newcombe RG, Read GF, Walker R, Riad-Fahmy D. Maternity blues and major endocrine changes: Cardiff puerperal mood and hormone study II. Br Med J. 1994;308(6934):949–953. [PMC free article: PMC2539811] [PubMed: 8173402]
- 233.
- Neumeister A. Tryptophan depletion, serotonin, and depression: Where do we stand? Psychopharmacol Bull. 2003;37(4):99–115. [PubMed: 15131521]
- 234.
- Leyton M, Young SN, Pihl RO. et al. Effects on mood of acute phenylalanine/tyrosine depletion in healthy women. Neuropsychopharmacology. 2000;22(1):52–63. [PubMed: 10633491]
- 235.
- Verhoeff NP, Kapur S, Hussey D. et al. A simple method to measure baseline occupancy of neostriatal dopamine d(2) receptors by dopamine in vivo in healthy subjects. Neuropsychopharmacology. 2001;25(2):213–223. [PubMed: 11425505]
- 236.
- Laruelle M, D’Souza CD, Baldwin RM. et al. Imaging D2 receptor occupancy by endogenous dopamine in humans. Neuropsychopharmacology. 1997;17(3):162–174. [PubMed: 9272483]
- 237.
- Hasler G, Fromm S, Carlson PJ. et al. Neural response to catecholamine depletion in unmedicated subjects with major depressive disorder in remission and healthy subjects. Arch Gen Psychiatry. 2008;65(5):521–531. [PMC free article: PMC2676777] [PubMed: 18458204]
- 238.
- Adewuya AO. Early postpartum mood as a risk factor for postnatal depression in Nigerian women. Am J Psychiatry. 2006;163(8):1435–1437. [PubMed: 16877659]
- 239.
- Kim JJ, Shih JC, Chen K. et al. Selective enhancement of emotional, but not motor, learning in monoamine oxidase A-deficient mice. Proc Natl Acad Sci U. S. A. 1997;94(11):5929–5933. [PMC free article: PMC20883] [PubMed: 9159177]
- 240.
- Cases O, Seif I, Grimsby J. et al. Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science. 1995;268(5218):1763–1766. [PMC free article: PMC2844866] [PubMed: 7792602]
- 241.
- Shih JC, Ridd MJ, Chen K. et al. Ketanserin and tetrabenazine abolish aggression in mice lacking monoamine oxidase A. Brain Res. 1999;835(2):104–112. [PubMed: 10415365]
- 242.
- Popova NK, Skrinskaya YA, Amstislavskaya TG, Vishnivetskaya GB, Seif I, de Meier E. Behavioral characteristics of mice with genetic knockout of monoamine oxidase type A. Neurosci Behav Physiol. 2001;31(6):597–602. [PubMed: 11766896]
- 243.
- Popova NK, Vishnivetskaya GB, Ivanova EA, Skrinskaya JA, Seif I. Altered behavior and alcohol tolerance in transgenic mice lacking MAO A: A comparison with effects of MAO A inhibitor clorgyline. Pharmacol Biochem Behav. 2000;67(4):719–727. [PubMed: 11166062]
- 244.
- Upton AL, Salichon N, Lebrand C. et al. Excess of serotonin (5-HT) alters the segregation of ipsilateral and contralateral retinal projections in monoamine oxidase A knock-out mice: Possible role of 5-HT uptake in retinal ganglion cells during development. J Neurosci. 1999;19(16):7007–7024. [PMC free article: PMC6782873] [PubMed: 10436056]
- 245.
- Cases O, Vitalis T, Seif I, De Maeyer E, Sotelo C, Gaspar P. Lack of barrels in the somatosensory cortex of monoamine oxidase A-deficient mice: Role of a serotonin excess during the critical period. Neuron. 1996;16(2):297–307. [PubMed: 8789945]
- 246.
- Bortolato M, Chen K, Shih JC, Bortolato M, Chen K, Shih JC. Monoamine oxidase inactivation: From pathophysiology to therapeutics. Adv Drug Deliv Rev. 2008;60(13–14):1527–1533. [PMC free article: PMC2630537] [PubMed: 18652859]
- 247.
- Chen K, Cases O, Rebrin I. et al. Forebrain-specific expression of monoamine oxidase A reduces neurotransmitter levels, restores the brain structure, and rescues aggressive behavior in monoamine oxidase A-deficient mice. J Biol Chem. 2007;282(1):115–123. [PMC free article: PMC2844870] [PubMed: 17090537]
- 248.
- Brunner HG, Nelen M, Breakefield XO, Ropers HH, van Oost BA. Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science. 1993;262(5133):578–580. [PubMed: 8211186]
- 249.
- Caspi A, McClay J, Moffitt TE. et al. Role of genotype in the cycle of violence in maltreated children [see comment] Science. 2002;297(5582):851–854. [PubMed: 12161658]
- 250.
- Kim-Cohen J, Caspi A, Taylor A. et al. MAOA, maltreatment, and gene–environment interaction predicting children’s mental health: New evidence and a meta-analysis. Mol Psychiatry. 2006;11(10):903–913. [PubMed: 16801953]
- 251.
- Brown GL, Goodwin FK, Ballenger JC, Goyer PF, Major LF. Aggression in humans correlates with cerebrospinal fluid amine metabolites. Psychiatry Res. 1979;1(2):131–139. [PubMed: 95232]
- 252.
- Linnoila M, Virkkunen M, Scheinin M, Nuutila A, Rimon R, Goodwin FK. Low cerebrospinal fluid 5-hydroxyindoleacetic acid concentration differentiates impulsive from nonimpulsive violent behavior. Life Sci. 1983;33(26):2609–2614. [PubMed: 6198573]
- 253.
- Linnoila VM, Virkkunen M. Aggression, suicidality, and serotonin. J Clin Psychiatry. 1992;53 (Suppl):46–51. [PubMed: 1385390]
- 254.
- Waldmeier PC, Baumann PA. Effects of CGP 11305 A, a new reversible and selective inhibitor of MAO A, on biogenic amine levels and metabolism in the rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1983;324(1):20–26. [PubMed: 6195533]
- 255.
- Garrick NA, Seppala T, Linnoila M, Murphy DL. Rhesus monkey cerebrospinal fluid amine metabolite changes following treatment with the reversible monoamine oxidase type-A inhibitor cimoxatone. Psychopharmacology (Berl). 1985;86(3):265–269. [PubMed: 2412251]
- 256.
- Alia-Klein N, Goldstein RZ, Kriplani A. et al. Brain monoamine oxidase A activity predicts trait aggression. J Neurosci. 2008;28(19):5099–5104. [PMC free article: PMC2430409] [PubMed: 18463263]
- 257.
- Soliman A, Bagby RM, Wilson AA. et al. Relationship of monoamine oxidase A binding to adaptive and maladaptive personality traits. Psychol Med. 2011;41(5):1051–1060. [PubMed: 20810002]
- 258.
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10(3):295–307. [PubMed: 10731224]
- 259.
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U. S. A. 1988;85(14):5274–5278. [PMC free article: PMC281732] [PubMed: 2899326]
- 260.
- Vaccarino FJ. Nucleus accumbens dopamine–CCK interactions in psychostimulant reward and related behaviors. Neurosci Biobehav Rev. 1994;18(2):207–214. [PubMed: 7914685]
- 261.
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275(5306):1593–1599. [PubMed: 9054347]
- 262.
- Dalley JW, Fryer TD, Brichard L. et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315(5816):1267–1270. [PMC free article: PMC1892797] [PubMed: 17332411]
- 263.
- Buckholtz JW, Treadway MT, Cowan RL. et al. Dopaminergic network differences in human impulsivity Science 30329(5991)532. [PMC free article: PMC3161413] [PubMed: 20671181]
- 264.
- Mitchell JM, Tavares VC, Fields HL, D’Esposito M, Boettiger CA. Endogenous opioid blockade and impulsive responding in alcoholics and healthy controls. Neuropsychopharmacology. 2007;32(2):439–449. [PubMed: 17047667]
- 265.
- Kieres AK, Hausknecht KA, Farrar AM, Acheson A, de Wit H, Richards JB. Effects of morphine and naltrexone on impulsive decision making in rats. Psychopharmacology (Berl). 2004;173(1–2):167–174. [PubMed: 14752586]
- 266.
- Pattij T, Schetters D, Janssen MC, Wiskerke J, Schoffelmeer AN. Acute effects of morphine on distinct forms of impulsive behavior in rats. Psychopharmacology (Berl). 2009;205(3):489–502. [PMC free article: PMC2712067] [PubMed: 19436995]
- 267.
- Pecina S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: Where do mu-opioids cause increased hedonic impact of sweetness? J Neurosci. 2005;25(50):11777–11786. [PMC free article: PMC6726018] [PubMed: 16354936]
- 268.
- Young MS, Schinka JA. Research Validity Scales for the NEO-PI-R: Additional evidence for reliability and validity. J Pers Assess. 2001;76(3):412–420. [PubMed: 11499455]
- 269.
- Love TM, Stohler CS, Zubieta JK. Positron emission tomography measures of endogenous opioid neurotransmission and impulsiveness traits in humans. Arch Gen Psychiatry. 2009;66(10):1124–1134. [PMC free article: PMC3085183] [PubMed: 19805703]
- 270.
- Gabilondo AM, Meana JJ, Garcia-Sevilla JA. Increased density of mu-opioid receptors in the postmortem brain of suicide victims. Brain Res. 1995;682(1–2):245–250. [PubMed: 7552322]
- 271.
- Sacher J, Wilson A, Houle S. et al. Elevated brain monoamine oxidase A binding in early postpartum. Arch Gen Psychiatry. 2010;67(5):468–474. [PubMed: 20439828]
- Review Neurochemical imaging and depressive behaviours.[Curr Top Behav Neurosci. 2013]Review Neurochemical imaging and depressive behaviours.Meyer JH. Curr Top Behav Neurosci. 2013; 14:101-34.
- Increased mRNA expression of alpha2A-adrenoceptors, serotonin receptors and mu-opioid receptors in the brains of suicide victims.[Neuropsychopharmacology. 2004]Increased mRNA expression of alpha2A-adrenoceptors, serotonin receptors and mu-opioid receptors in the brains of suicide victims.Escribá PV, Ozaita A, García-Sevilla JA. Neuropsychopharmacology. 2004 Aug; 29(8):1512-21.
- Review Structural and functional neuroimaging studies of the suicidal brain.[Prog Neuropsychopharmacol Biol...]Review Structural and functional neuroimaging studies of the suicidal brain.Desmyter S, van Heeringen C, Audenaert K. Prog Neuropsychopharmacol Biol Psychiatry. 2011 Jun 1; 35(4):796-808. Epub 2011 Jan 6.
- Review [Posttraumatic stress disorder (PTSD) as a consequence of the interaction between an individual genetic susceptibility, a traumatogenic event and a social context].[Encephale. 2012]Review [Posttraumatic stress disorder (PTSD) as a consequence of the interaction between an individual genetic susceptibility, a traumatogenic event and a social context].Auxéméry Y. Encephale. 2012 Oct; 38(5):373-80. Epub 2012 Jan 24.
- Elevated brain monoamine oxidase A binding in the early postpartum period.[Arch Gen Psychiatry. 2010]Elevated brain monoamine oxidase A binding in the early postpartum period.Sacher J, Wilson AA, Houle S, Rusjan P, Hassan S, Bloomfield PM, Stewart DE, Meyer JH. Arch Gen Psychiatry. 2010 May; 67(5):468-74.
- Neuroimaging High Risk States for Suicide - The Neurobiological Basis of SuicideNeuroimaging High Risk States for Suicide - The Neurobiological Basis of Suicide
Your browsing activity is empty.
Activity recording is turned off.
See more...
