By agreement with the publisher, this book is accessible by the search feature, but cannot be browsed.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Gilbert SF. Developmental Biology. 6th edition. Sunderland (MA): Sinauer Associates; 2000.

Developmental Biology. 6th edition.
Show detailsAn individual gains knowledge of its environment through its sensory organs. The major sensory organs of the head develop from the interactions of the neural tube with a series of epidermal thickenings called the cranial ectodermal placodes. The most anterior of these are the two olfactory placodes that form the ganglia for the olfactory nerves, which are responsible for the sense of smell. The auditory placodes similarly invaginate to form the inner ear labyrinth, whose neurons form the acoustic ganglion, which enables us to hear. In this section, we will focus on the eye.
The dynamics of optic development
The induction of the eye was discussed in Chapter 6, and will only be summarized here (Figure 12.27). At gastrulation, the involuting endoderm and mesoderm interact with the adjacent prospective head ectoderm to give the head ectoderm a lens-forming bias (Saha et al. 1989). But not all parts of the head ectoderm eventually form lenses, and the lens must have a precise spatial relationship with the retina. The activation of the head ectoderm's latent lens-forming ability and the positioning of the lens in relation to the retina is accomplished by the optic vesicle. It extends from the diencephalon, and when it meets the head ectoderm, it induces the formation of a lens placode, which then invaginates to form the lens. The optic vesicle becomes the two-walled optic cup, whose two layers differentiate in different directions. The cells of the outer layer produce melanin pigment (being one of the few tissues other than the neural crest cells that can form this pigment) and ultimately become the pigmented retina. The cells of the inner layer proliferate rapidly and generate a variety of glia, ganglion cells, interneurons, and light-sensitive photoreceptor neurons. Collectively, these cells constitute the neural retina. The retinal ganglion cells are neurons whose axons send electrical impulses to the brain. Their axons meet at the base of the eye and travel down the optic stalk. This stalk is then called the optic nerve.

Figure 12.27
Development of the vertebrate eye (see also Figure 6.5). (A) The optic vesicle evaginates from the brain and contacts the overlying ectoderm, inducing a lens placode. (B, C) The overlying ectoderm differentiates into lens cells as the optic vesicle folds (more...)
But how is it that a specific region of neural ectoderm is informed that it will become the optic vesicle? It appears that a group of transcription factors—Six3, Pax6, and Rx1—are expressed together in the most anterior tip of the neural plate. This single domain will later split into the bilateral regions that will form the optic vesicles. Again, we see the similarities between the Drosophila and the vertebrate nervous system, for these three proteins are also necessary for the formation of the Drosophila eye. As discussed in Chapters 4 and 5, the Pax6 protein appears to be especially important in the development of the lens and retina. Indeed, it appears to be a common denominator for photoreceptive cells in all phyla. If the mouse Pax6 gene is inserted into the Drosophila genome and activated randomly, Drosophila eyes form in those cells where the mouse Pax6 is being expressed (see Chapter 22; Halder et al. 1995)! While Pax6 is also expressed in the murine forebrain, hindbrain, and nasal placodes, the eyes seem to be most sensitive to its absence. In humans and mice, Pax6 heterozygotes have small eyes, while homozygotic mice and humans (and Drosophila) lack eyes altogether (Jordan et al. 1992; Glaser et al. 1994; Quiring et al. 1994).
The separation of the single eye field into two bilateral fields depends upon the secretion of Sonic hedgehog. If the sonic hedgehog gene is mutated, or if the processing of this protein is inhibited, the single median eye field will not split. The result is cyclopia—a single eye in the center of the face (and usually below the nose) (Figure 12.28; see also Figure 6.25; Chiang et al. 1996; Kelley et al. 1996; Roessler et al. 1996; Li et al. 1997). It is thought that Sonic hedgehog protein from the prechordal plate suppresses Pax6 expression in the center of the embryo, dividing the field in two.
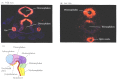
Figure 12.28
Brain defects in embryos lacking sonic hedgehog. (A) A wild-type mouse and (B) a 12.5-day embryo lacking sonic hedgehog. The expression of the otx-2 gene is seen in red to highlight certain regions. In the mutant, no midline forms, and there is a single, (more...)
WEBSITE
12.8 Human cyclopia. Mutations in sonic hedgehog have been implicated in causing cyclopia in humans. Moreover, mutations of genes involved in cholesterol synthesis have also been implicated. http://www.devbio.com/chap12/link1208.shtml
Neural retina differentiation
Like the cerebral and cerebellar cortices, the neural retina develops into a layered array of different neuronal types (Figure 12.29). These layers include the light- and color-sensitive photoreceptor (rod and cone) cells, the cell bodies of the ganglion cells, and the bipolar interneurons that transmit electrical stimuli from the rods and cones to the ganglion cells. In addition, there are numerous Müller glial cells, which maintain the integrity of the retina, as well as amacrine neurons (which lack large axons) and horizontal neurons that transmit electrical impulses in the plane of the retina.

Figure 12.29
Development of the human retina. Retinal neurons sort out into functional layers during development. (A, B) Initial separation of neuroblasts within the retina. (C) The three layers of neurons in the adult retina and the synaptic layers between them. (more...)
In the early stages of retinal development, cell division in a germinal layer and the migration and differential death of the resulting cells form the striated, laminar pattern of the neural retina. The formation of this highly structured tissue is one of the most intensely studied problems of developmental neurobiology. It has been shown (Turner and Cepko 1987) that a single neuroblast precursor cell from the retinal germinal layer can give rise to at least three types of neurons or to two types of neurons and a glial cell. This analysis was performed using an ingenious technique to label the cells generated by one particular neuroblast precursor cell. Newborn rats (whose retinas are still developing) were injected in the back of their eyes with a virus that can integrate into their DNA. This virus contained a β-galactosidase gene (not present in rat retina) that would be expressed only in the infected cells. A month after the rats' eyes were infected, the retinas were removed and stained for the presence of β-galactosidase. Only the progeny of the infected cells should have stained blue. Figure 12.30 shows one of the strips of cells derived from an infected precursor cell. The stain can be seen in five rods, a bipolar neuron, and a retinal (Müller) glial cell.

Figure 12.30
Determination of the lineage of a neuroblast in the rat retina. (A) Technique whereby a virus containing a functional β-galactosidase gene is injected into the back of the eye of a newborn rat to infect some of the retinal precursor cells. After (more...)
Lens and cornea differentiation
During its continued development into a lens, the lens placode rounds up and contacts the new overlying ectoderm. The lens vesicle then induces the ectoderm to form the transparent cornea. Here, physical parameters play an important role in the development of the eye. Intraocular fluid pressure is necessary for the correct curvature of the cornea so that light can be focused on the retina. The importance of this pressure can be demonstrated experimentally: the cornea will not develop its characteristic curve when a small glass tube is inserted through the wall of a developing chick eye to drain away the intraocular fluid (Coulombre 1956, 1965). Intraocular pressure is sustained by a ring of scleral bones (probably derived from the neural crest), which acts as an inelastic restraint.
The differentiation of the lens tissue into a transparent membrane capable of directing light onto the retina involves changes in cell structure and shape as well as the synthesis of transparent, lens-specific proteins called crystallins (Figure 12.31). The cells at the inner portion of the lens vesicle elongate and, under the influence of the neural retina, become the lens fibers (Piatigorsky 1981). As these fibers continue to grow, they synthesize crystallins, which eventually fill up the cell and cause the extrusion of the nucleus. The crystallin-synthesizing fibers continue to grow and eventually fill the space between the two layers of the lens vesicle. The anterior cells of the lens vesicle constitute a germinal epithelium, which keeps dividing. These dividing cells move toward the equator of the vesicle, and as they pass through the equatorial region, they, too, begin to elongate (Figure 12.31D). Thus, the lens contains three regions: an anterior zone of dividing epithelial cells, an equatorial zone of cellular elongation, and a posterior and central zone of crystallin-containing fiber cells. This arrangement persists throughout the lifetime of the animal as fibers are continuously being laid down. In the adult chicken, the process of differentiation from an epithelial cell to a lens fiber takes 2 years (Papaconstantinou 1967).
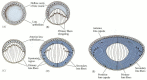
Figure 12.31
Differentiation of the lens cells. (A) Lens vesicle as shown in Figure 12.27. (B) Elongation of the interior cells, producing lens fibers. (C) Lens filled with crystallin-synthesizing cells. (D) New lens cells are derived from anterior lens epithelium. (more...)
Directly in front of the lens is a pigmented and muscular tissue called the iris. The iris muscles control the size of the pupil (and give an individual his or her characteristic eye color). Unlike the other muscles of the body (which are derived from the mesoderm), part of the iris is derived from the ectodermal layer. Specifically, this region of the iris develops from a portion of the optic cup that is continuous with the neural retina, but does not make photoreceptors.
WEBSITE
12.9 Why babies don't see well. The retinal photoreceptors are not fully developed at birth. As one gets older, the density of photoreceptors increases, allowing far better discrimination and nearly 350 times the light-absorbing capacity. http://www.devbio.com/chap12/link1209.shtml
- Development of the Vertebrate Eye - Developmental BiologyDevelopment of the Vertebrate Eye - Developmental Biology
Your browsing activity is empty.
Activity recording is turned off.
See more...